Abstract
High pancreatic cancer mortality and poor prognosis are caused by the difficulty for early diagnosis and extremely low rates of resection because of metastasis. Mesothelin overexpression in pancreatic cancer is a remarkable biomarker for tumor progression, especially for invasion and metastasis. Here, we generated a novel replication-defective recombinant adenovirus 40 (rAd40), whose gene delivery properties are totally different from a conventional rAd5. In this study, we have identified intravenous administration with rAd40 expressing mouse mesothelin (Msln) as an effective prophylactic cancer vaccine against metastatic lesions of pancreatic cancer in mice. Intravenous administration of rAd40 (rAd40 i.v.) achieved transgene delivery in wider range of organs compared to rAd5 i.v., while rAd5 was distributed mainly to the liver, spleen, and lungs. Additionally, rAd40 i.v. showed less transduction of the liver or inflammatory responses, resulted in reduced liver toxicity compared to rAd5 i.v. Also, more robust systemic antigen-specific immune responses were stimulated by rAd40 i.v. Pretreatment with a single ovalbumin-expressing rAd40 i.v. prevented tumor growth in mouse subcutaneous models of ovalbumin-expressing pancreatic cancer. When used with Msln-expressing rAd40 i.v., Msln protein expression and metastases were suppressed in a syngeneic orthotopic mouse model of pancreatic cancer, corresponding to the detection of Msln- and tumor-specific cytotoxic T lymphocyte (CTL). Our novel methods generated antitumor effects against antigen-expressing tumors through antigen- and tumor-specific CTL-mediated immunity. Thus, our results indicate that a rAd40-based intravenous vaccine provides a new strategy for the effective control of metastatic pancreatic cancer and novel therapy against other cancers and infectious diseases.
Keywords: pancreatic cancer, adenovirus, vaccine, mesothelin
Pancreatic cancer is the fourth leading cause of cancer death for both men and women in the United States.1 High pancreatic cancer mortality and poor prognosis are caused by the difficulty for early diagnosis and extremely low rates of resection because of metastasis.2 Mesothelin is a glycosylphosphatidylinositol (GPI)-linked cell surface glycoprotein that is recognized as an immunotherapeutic target in mesothelinoverexpressing cancers, including pancreatic cancer3–5 and is overexpressed in pancreatic ductal adenocarcinoma, although it is not expressed in normal tissue and chronic pancreatitis.6–9 In addition, mesothelin promotes anchorage-independent growth and prevents anoikis via Erk signaling pathway in human breast cancer,10 and induces cell proliferation via Stat3 signaling pathway in human pancreatic cancer.11 These reports indicate that mesothelin is a promising target for pancreatic cancer.
Prophylactic cancer vaccination stimulates the immune system to prevent the tumor growth and metastasis by tumorassociated antigens.12 Such system would be clinically useful as postoperative therapy to avoid distant and/or local relapse of the disease. The vaccination with replication-defective recombinant adenovirus 5 (rAd5) has been tested in several clinical trials.13,14 While rAd5 vaccines elicit robust T cell immunity when administered intravenously as either a prime or boost vaccination, vaccines based on hepatotropic Ad5 may not be optimal for reducing the number of metastasis, probably due to diminished CD8+ T-cell responses in the liver.15,16 Ad40 possesses specific structural features and has a unique tropism, mediated by the virion of two fibers.17 Therefore, rAd40 has a possibility to induce systemic immune responses via intravenous administration, making them prime candidates as a systemic prophylactic cancer vaccine. While Ad40 is called the fastidious virus, as it is extremely difficult to culture in vitro,18,19 we recently have established a method to generate Ad40 vectors, as previously described.20
We hypothesized that rAd40-based mesothelin vaccines could prevent tumor development, especially in the context of metastatic pancreatic cancer, via induction of systemic immune reactions. Here, we report a novel strategy for controlling mesothelin-expressing metastatic pancreatic cancers.
Material and Methods
Animal and cell lines
Six- to seven-week-old female C57BL/6 (B6) mice (Harlan Laboratories, Madison, WI) were studied, which was approved by the Institutional Animal Care and Use Committee, University of Minnesota. The B6 mouse-drived pancreatic adenocarcinoma (Pan02, obtained from National Cancer Institute, Frederick, MD) or thyroid lymphoma (EL4, TIB-39; ATCC) cells were used. DNA fragments encoding chicken ovalbumin (Ova) or mouse mesothelin (Msln, 1878 bp between 109 and 1986 of GenBank accession sequence NM018857) were first amplified with reverse-transcription (RT)-PCR using RNA samples from E.G7 or Pan02, respectively. Ova, fusion reporter gene of firefly luciferase (Luc) and GFP (LuciGFP), and Msln were cloned into pMX-puro plasmid (pMX-Ova-puro, pMX-LuciGFP-puro and pMXMsln-puro). Ova-, LuciGFP- or Msln protein-expressing Pan02 cell lines (Pan02-Ova, Pan02-LuciGFP or Pan02-Msln) were generated by transfecting those plasmids by using Lipofectamine 2000 (Invitrogen, Grand Island, NY), and selected and maintained by puromycin (2 µg mL1).
Construction of replication-defective recombinant Ad40 vectors
The partially E1-deleted replication-defective recombinant Ad40 vectors expressing Luc cassette and CMV-promoterdriven Luc, Ova or Msln cassette (rAd40pΔE1-Luc and rAd40pΔE1-CMV-Luc, Ova or Msln) were generated by the homologous recombination between a ClaI-linearized plasmid coding wild-type Ad40 (Ad40wt) and a rescue plasmid with homologous regions of Ad40-left and right-ends for recombination as well as Luc, cytomegalovirus (CMV)-promoter (pCEP4, Clontech Laboratories, Mountain View, CA)-driven Luc (pGL3-Basic Vector, Promega, Madison, WI), Ova or Msln cassette in E. coli BJ5183 (Stratagene, La Jolla, CA; Supporting Information Fig 1). Viruses were generated with the vector coding plasmids after linearization with AsiSI, and subsequently amplified and purified as previously described.
The rAd5-CMV-Luc or rAd5-CMV-Ova was built by the homologous recombination between pShuttle, which included CMV-promoter driven Luc (Ova) and pAdEasy-1 (Stratagene) as previously described.23.
Intravenous administration
The 100 µL phosphate-buffer saline (PBS, control), rAd40 pΔE1-CMV-Luc, rAd40 pΔE1-CMV-Ova, rAd40 pΔE1CMV-Msln, rAd5-CMV-Luc or rAd5-CMV-Ova (1010 VP/100 µl PBS) was injected intravenously by using a 291/2-gauge needle into tail veins.
In vivo biodistribution
Six hours after rAd i.v., brain, thymus, lung, heart, liver, spleen, kidney, jejunum (10 cm median part of the small intestine without macroscopically visiable Peyer’s patch), ileum (10 cm upstream of the cecum), colon (10 cm downstream of the cecum), mesenteric lymph nodes (MLNs), bone marrow and peripheral blood were isolated from B6 mice. DNA was purified by a QIAamp DNA blood mini kit (Qiagen) and relative luciferase copy numbers (Luc DNA copies per GAPDH DNA copies) in the tissues were analyzed by real-time PCR by SYBR green method. PCR-cloned Luc and mouse GAPDH fragments were used as copy number standards. The lower limit of relative Luc DNA was determined to be one Luc DNA copy per 103 GAPDH DNA copies based on control results. After 48-hr post rAd i.v., luciferase activity in tissues of B6 mice were assessed by luciferase activity assays (Promega) and normalized with total protein concentration as determined by DC protein assay (Bio-Rad, Hercules, CA).
Analysis of plasma levels of cytokines and amino transferases
Blood samples were obtained by cardiac puncture in heparincoated syringes immediately following sacrifice. After centrifugation for 15 min at 10,000 rpm at 4°C, the plasma was stored at −80°C. To analyze cytokine levels, mouse IL-6 and TNF-α ELISA (eBioscience, San Diego, CA) were used. Plasma transamine level was determined by ALT activity assay kit (BioVision, Milpitas, CA).
Intracellular cytokine staining and flow cytometry
Cell lines or tumor cells harvested from mice were analyzed by PE-labeled anti-Msln antibody (295D, Molecular and Biological Laboratories, Nagoya, Japan) followed by dead cell staining with 7-AAD (BD Biosciences, San Jose, CA).
Splenocytes were prepared by passing through 100-µm strainer. Erythrocytes were removed using red-blood cells lysis buffer for 5 min followed by washes in PBS and passing through 40- µm strainer. Tumor cells were cultured in RPMI1640 with 200 U mL−1 collagenase D (Invitrogen) and 10 U mL−1 RNase-free DNase (Promega) for 2 hr 30 min by agitating with scissors and passing through 100- and 40-µm strainers. Viable splenocytes were counted using a hemacytometer and trypan blue.
Splenocytes (>1 × 106 ) were directly analyzed by flow cytometry (FCM) or placed in culture, depending on the analyses. Ova-specific CD8+ T cells were analyzed after pro5 MHC Pentamer H-2Kb -SIINFEKL (Proimmune, Sarasota, FL) followed by surface staining with anti-CD8a and -CD19.
Prior to intracellular cytokine staining, splenocytes (2 × 106 /mL) were incubated with 10 µg mL−1 of Ova peptide (SIINFKL, H-2Kb ; Proimmune), Pan02-Ova, -LuciGFP, or - Msln cell lysate (generated by four freeze-thaw cycles, passed through a 0.22-µm filter and total protein concentration as determined by DC protein assay), or 0.1% DMSO and PBS (control) in RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate and 100 IU mL−1 penicillin and 100 µg mL−1 streptomycin for 6 hr. BD GolgiPlug (BD Biosciences) was added for the last 4 hr of ex vivo culture. After surface staining with anti-CD3e, -CD4, -CD8a, or -NK1.1 (BD Biosciences), cells were stained with anti-interferon (IFN)-γ, -IL-4 and -IL-10 using Cytofix/Cytoperm kit (BD Biosciences).
Over 2 × 105 events were aquired on FACSCalibur or LSRII (Becton Dikinson, Franklin Lakes, NJ). Data were analyzed using CellQuest (Becton Dikinson) and FlowJo (TreeStar, Ashland, OR) software. FSC/SSC gates were used for analysis for the 7-AAD-negative population for cell lines or splenocytes. As a negative control, cells were stained with isotype controls.
In vivo cytotoxic T-cell assay
B6 splenocytes (5 × 106 /mL) were incubated for 90 min at 37°C with or without 1 µg mL−1 of Ova peptide and labeled for 10 min at 37°C with carboxyflurescein diacetate succinimidyl diester (CFSE, Invitrogen) at 5 µM (CFSEhigh, peptidelabeled splenocytes) or 0.5 µM (CFSElow, splenocytes without peptide), respectively. B6 mice were injected intravenously with 2.5 × 106 cells of each fraction at 14 days after intravenous administration with either PBS or 1010 VP of rAd40 pΔE1-CMV-Ova or rAd5-CMV-Ova. Splenocytes were isolated 20 hr later and analyzed by FCM. The ratio (CFSEhigh/CFSElow) between the percentage of pulsed (CFSEhigh) and unpulsed (CFSElow) cells was calculated to determine cell cytotoxicity. Over 1 ×106 events were aquired on FACSCalibur (Becton Dikinson). CD3e+CD8a+ cells in the light scatter-based lymphocyte gate were used for analysis.
Tumor challenge study in subcutaneous tumor models
At 14 days after intravenous administration with either PBS or 1010 VP of rAd40 pAE1-CMV-Ova or rAd5-CMV-Ova, Pan02-Ova or Pan02-LuciGFP (5 × 105 viable cells per mouse) was inoculated subcutaneously into the flank of B6 mice. Tumor size was measured with fine calipers and tumor volumes were estimated from two-dimensional measurements: tumor volumes (mm3) = (A × B2)/2, where A and B are the tumor length and width in (mm), respectively. The larger diameter is considered the length, and the smaller diameter is considered the width. Thirty days after tumor inoculation, mice were sacrificed, and peripheral blood, tumor and splenocytes were extracted.
Tumor challenge study in orthotopic tumor models
At 14 days after intravenous administration with PBS or 1010 VP of rAd40 pΔE1-CMV-Ova or rAd5-CMV-Ova, Pan02-LuciGFP (2.5 × 105 viable cells per mouse) cells at the same passage were inoculated from the tail of the pancreas as previously described. Tumor growth was monitored weekly with bioluminescence imaging after tumor inoculation. Because every mouse in our orthotopic Pan02-LuciGFP tumor model was dead until 5 weeks, 4 weeks after tumor inoculation, mice were sacrificed and peripheral blood, as well as tumors and tissues (i.e., pancreas, spleen, lymph nodes (LNs), gastrointestinal tract, liver and peritoneum), was extracted.
Immunofluorescence staining for mouse mesothelin expression
Cultured cells for immunofluorescence and frozen tissues for hematoxylin and eosin (H & E) staining were fixed with 4% paraformaldehyde/PBS at room temperature for 10 min. Frozen sections (6–10 um) were prepared for staining experiments. Sections for immunofluorescence were fixed with cold acetone (−20°C) for 10 mim. Cells or tissues were rinsed with PBS, blocked with 1% BSA in 0.1% Tween20-PBS, incubated with the primary goat polyclonal anti-Msln antibody (1:50, D-16; Santa Cruz Biotechnology, Santa Cruz, CA) overnight and followed by incubation with a Tetramethyl Rhodamine Isothiocyanate-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hr. Finally, DAPI (Vector labs, Burlingame, CA) was used as a nuclear counterstaining. Images were acquired using confocal spectral imaging microscope systems (Nikon, Melville, CA). The unstained and secondary antibody only control sections were used to determine the level of background tissue auto-fluorescence and to identify any potential nonspecific binding of the secondary antibodies.
Statistical analysis
Data are presented as mean values of three to six independent experiments and error bars indicate 95% CI. Continuous variables were compared by Mann–Whitney U-test. All p values were two-sided, and the type I error rate was fixed at p < 0.05. In all figures, asterisks denote statistical significance when comparing indicated groups (*p < 0.05, **p < 0.01, ***p < 0.001). The picture shown is representative of at least three independent experiments.
Results
Biodistribution of luciferase expression vectors in vivo
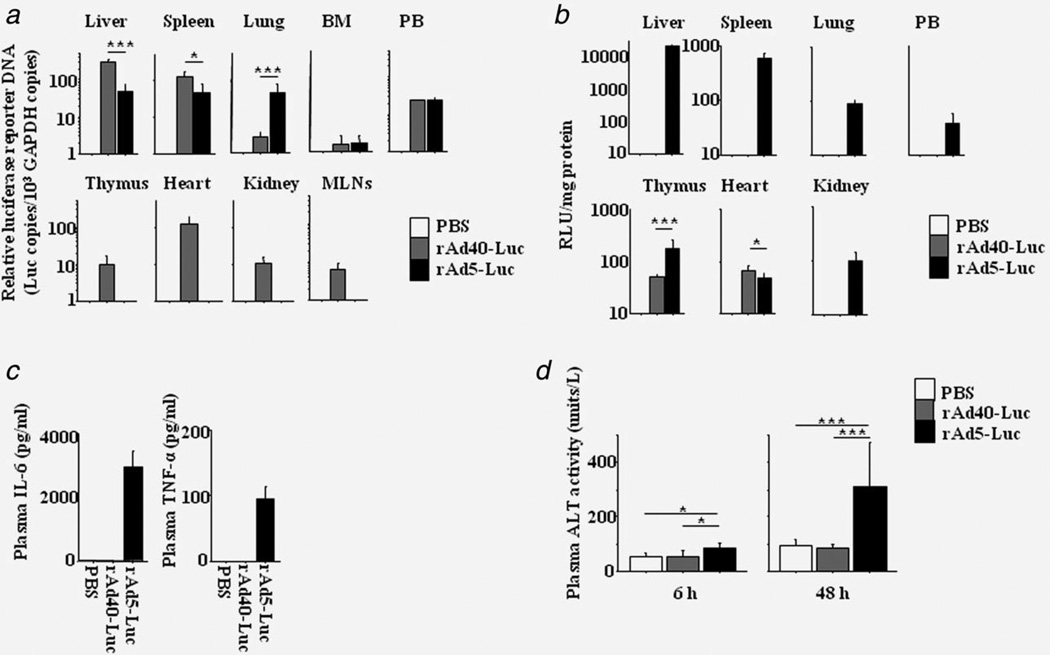
Six hours after intravenous (i.v.) administration in B6 mice, i.v. administration of rAd40 (rAd40 i.v.) achieved transgene delivery in wider range of organs compared to rAd5 i.v., while rAd5 was distributed mainly to the liver, spleen, and lungs (Fig. 1a). The distribution of rAd40pDE1-CMV-Luc (rAd40-Luc) in the liver and spleen was significantly higher than those of rAd5-CMV-Luc (rAd5-Luc). rAd40-Luc was detected in the thymus, heart, kidney, and MLNs. The distribution of rAd5-Luc was higher only in the lung compared to rAd40-Luc. There was no difference in the distribution between rAd40-Luc and rAd5-Luc in the bone marrow and peripheral blood.
Figure 1.
Vector biodistribution and host responses after intravenous administration. Following intravenous administration into B6 mice, in vivo biodistribution of rAd40-Luc and rAd5-Luc) were analyzed. (a) The relative copy numbers of Luc (Luc DNA per mouse GAPDH DNA) 6 hr after rAd i.v. (b) The luciferase activities at 48 hr. (c) Plasma IL-6 and TNF-α levels at 6 hr. (d) Plasma ALT activities at 48 hr. MLNs, mesenteric lymph nodes; BM, bone marrow; PB, peripheral blood; RLU, relative luminescence units. Each group included three to six mice. *p < 0.05, **p < 0.01, ***p < 0.001.
Forty-eight hours after i.v. administration, luciferase activity in the liver was not detected by rAd40-Luc i.v., while strong activity was observed after rAd5-Luc i.v. (Fig. 1b). Luciferase activity of rAd40-Luc was higher than that of rAd5-Luc in the heart, whereas the activity of rAd5-Luc was higher than that of rAd40-Luc in the thymus and was found in the spleen, lung, kidney, and peripheral blood.
Six hours and 48 hr after i.v. administration, rAd40 i.v.-induced less-inflammatory responses and reduced liver toxicity compared to rAd5-Luc i.v. (Fig. 1c and Fig. 1d). The upregulations of plasma IL-6 and TNF-α levels at 6 hr were observed only in rAd5-Luc-pretreated mice. Higher ALT activity levels at 6 and 48 hr were found in rAd5-Luc-pretreated mice compared to PBS (control)- or rAd40-Luc-pretreated mice.
Immune responses of ovalbumin expression vectors
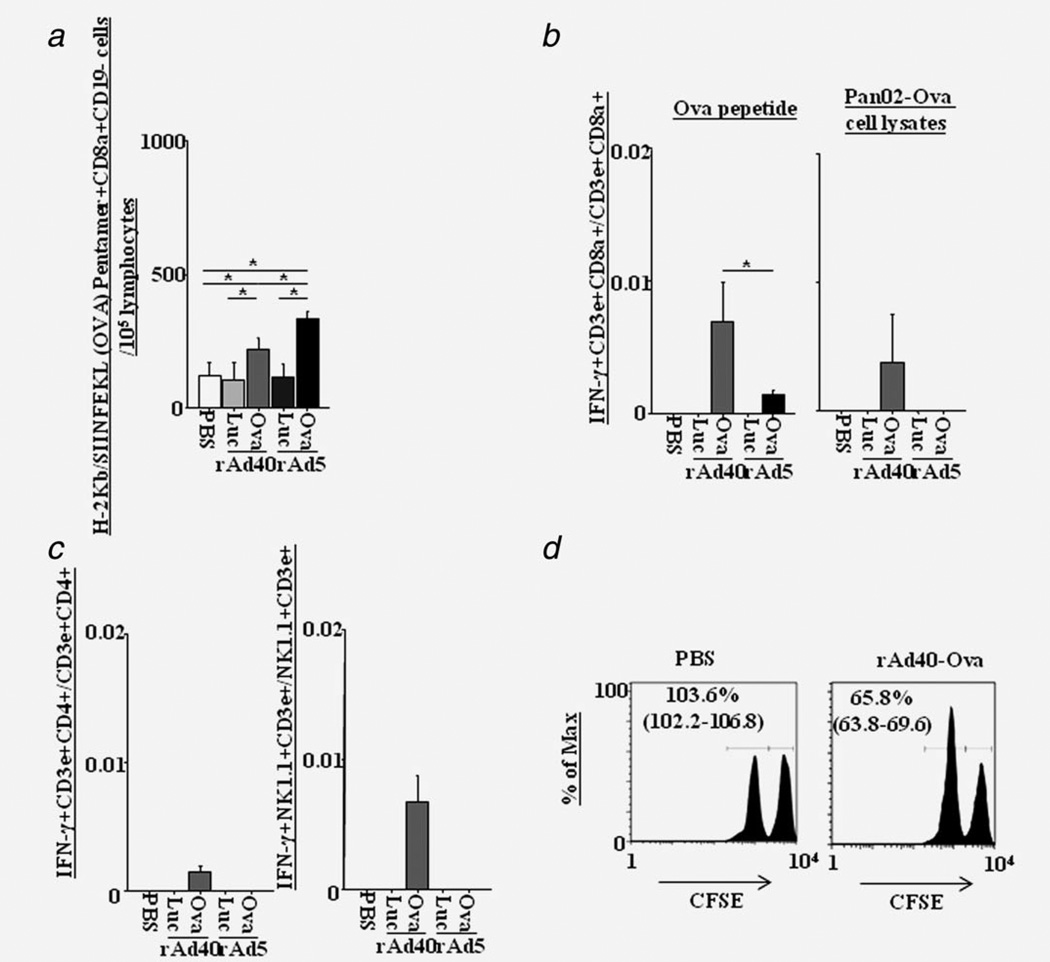
We determined the in vivo immune response to Ova expressed by rAd in B6 mice, because Ova is considered to be an inert antigen with low capacity to modulate the immune response and its wide use as a model antigen. The numbers of Ova-specific cytotoxic T lymphocytes (CTLs, Pentamer H-2Kb-SIINFEKL+CD8a+CD19-) increased in the spleen at 14 days after rAd40-Ova and rAd5-Ova i.v. (Fig. 2a). CTL numbers in the rAd40-Ova-pretreated mice were lower than those in the rAd5-Ova-pretreated mice. However, the number of IFN-γ-secreting CD8+ T cells (IFN-γ+CD3e+CD8a+) stimulated by Ova-peptide in rAd40-Ova-pretreated mice was higher than that in rAd5-Ova-pretreated mice (Fig. 2b). Additionally, the number of IFN-γ-secreting CD8+ T cells stimulated by Pan02-Ova cell lysates was increased only by rAd40-Ova. Also, IFN-γ-secreting CD4+ T (IFN-#x003B3;+CD3e+CD4+) and NK1.1+ T (IFN-γ+NK1.1+CD3e+) cells stimulated by Pan02-Ova cell lysates were detected only in rAd40-Ova groups (Fig. 2c). These observation revealed that only rAd40-Ova can induce functional Pan02-Ova-specific systemic immunity in the direction of CTL- and T helper 1 (Th1)-cell-dependent manners. An in vivo CTL assay was performed to directly measure the killing capacity of antigen-specific CTL responses and rAd40-Ova induced Ova-peptide-specific CTL response in vivo (Fig. 2d), indicating the presence of in vivo functionality of Ova-specific CTLs in rAd40-Ova-pretreated mice.
Figure 2.
Systemic immune responses induced by a single intravenous rAd administration. (a) The number of H-2Kb-SIINFEKL (Ova)-specific CD8a+CD19- cells in the spleen at 14 days after intravenous administration with PBS, rAd40-Luc, rAd40-Ova, rAd5-Luc, or rAd5-Ova. (b) The ratio of Ova peptide- or Pan02-Ova cell lysates-specific IFN-γ-secreting CD8+ T cells in the spleen at 14 days after rAd i.v. (c) The ratio of Pan02-Ova cell lysates-specific IFN-γ-secreting CD4+ T cells and NK1.1+ T cells in the spleen at 14 days after rAd i.v. (d) In vivo evaluation of cytotoxic activity after rAd i.v. Mice after 14 days of intravenous administration with PBS or rAd40-Ova were injected intravenously with CFSE labeled splenocytes pused with the Ova peptide (CFSEhigh) and unpulsed (CFSElow). Splenocytes were recovered 20 hr after transfer and CD8+ T lymphocytes were analyzed for CFSE intensity in a FCM. The ratio (CFSEhigh/CFSElow) of pulsed and unpulsed CD8+ T lymphocytes were calculated.
Antitumor immune responses by ovalbumin expression vectors
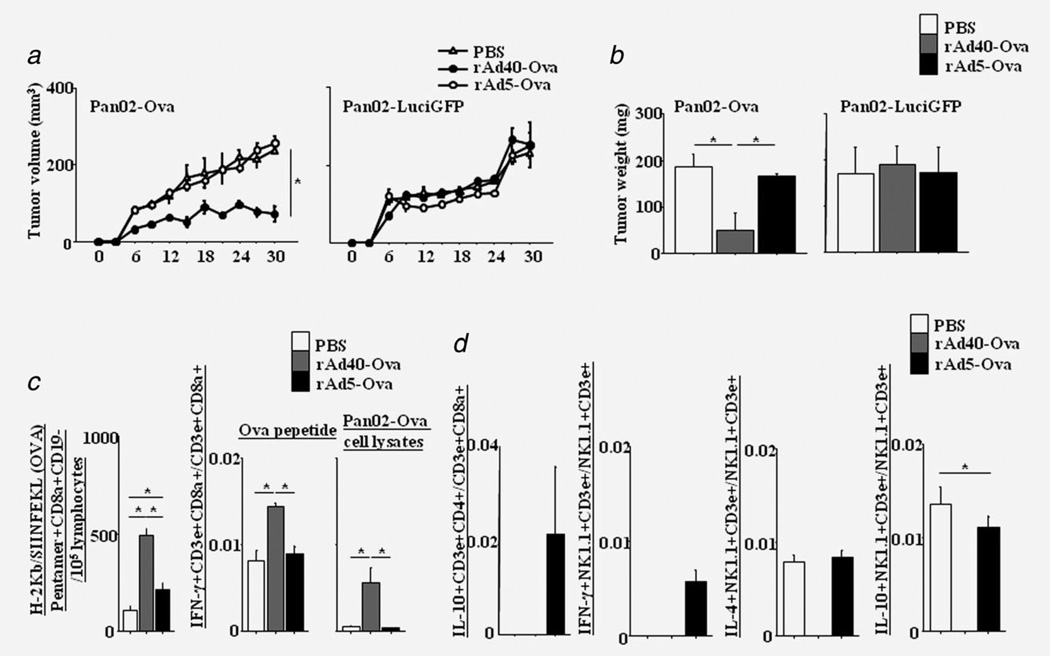
To evaluate antitumor effects against Ova-expressing tumor, we used subcutaneous mouse models with Ova-expressing cells (Pan02-Ova). At 14 days after intravenous administration with Ova-expressing rAds, Pan02-Ova or -LuciGFP (negative control of Pan02-Ova) were inoculated subcutaneously into B6 mice (5 × 105 cells/mouse). As early as 6 days after tumor challenge, statistical differences were observed in tumor volumes between PBS and rAd40-Ova, and rAd5-Ova and rAd40-Ova (Fig. 3a and Fig. 3b). rAd40-Ova i.v. suppressed Pan02-Ova tumor growth until the experiment was terminated at day 30, but did not inhibit Pan02-LuciGFP tumor progression. Regarding Pan02-Ova tumor models at 30 days after tumor challenge, the numbers of Ova-specific CTLs in the spleen of rAd40-Ova-pretreated mice were higher than those of PBS- and rAd5-Ova-pretreated mice (Fig. 3c). Additionally, the numbers of IFN-γ-secreting CD8+ T cells stimulated by Ova-peptide and Pan02-Ova cell lysates in rAd40-Ova-pretreated mice were higher than those in PBS- and rAd5-Ova-pretreated mice. In contrast, Ova-specific IL-10-secreting CD4+ T (IL-10+CD3e+CD4+) cells were detected by rAd5-Ova-pretreatment in both Pan02-Ova and Pan02-LuciGFP tumor models (Fig. 3d, Supporting Information Fig. S4b). Regarding Ova-specific NKT cells (NK1.1+CD3e+-) in rAd5-Ova-pretreated mice, IFN-γ-secreting NKT cells were detected after Pan02-Ova inoculation, and IL-4- and IL-10-secreting NKT cells were detected after Pan02-Ova and Pan02-LuciGFP inoculation. These results suggest the possibility that rAd5-Ova may induce systemic tolerance by liver stimulation. Ova-specific IL-4- and IL-10-secreting NKT cells were detected by PBS- and rAd5-Ova-pretreatment after Pan02-Ova inoculation, showing the possibility of tumor-induced tolerance.
Figure 3.
Tumor growth inhibiton achieved by a single intravenous administration in subcutaneous pancreatic cancer models. (a) Fourteen days after intravenous administration with PBS, rAd40-Ova, or rAd5-Ova, mice were subcutaneously implanted with either 5 × 105 Pan02-Ova cells expressing Ova, or Pan02-LuciGFP cells lacking Ova. Tumor progression was measured by calliper every 3 days for up to 30 days after tumor inoculation. (b) Tumors were excised at 30 days after tumor inoculation, and weighted. Splenocytes were extracted at 30 days after Pan02-Ova tumor inoculation and assessed for Ova-specific cellular immunity. (c) The number of H-2Kb-SIINFEKL (Ova)-specific CD8a+CD19− cells in the spleen of PBS-, rAd40-Ova-, or rAd5-Ova-pretreated mice and the ratio of Ova peptide- or Pan02-Ova cell lysates-specific IFN-γ-secreting CD8+ T cells in the spleen. (d) The ratio of Pan02-Ova cell lysates-specific IL-10-secreting CD4+ T cells, and IFN-γ-, IL-4- or IL-10-secreting NKT cells.
In vivo mesothelin expression in syngenic pancreatic cancer mouse model
In vivo Msln protein expression of Pan02-LuciGFP was examined to determine its expression differences in subcutaneous and orthotopic mouse models. Before in vivo inoculation, Pan02-LuciGFP lacks Msln protein expression and has high luciferase activity as well as GFP expression in vitro (Supporting Information Figs. 5b and 6b). First, subcutaneous mouse pancreatic models were established by Pan02-Ova and Pan02-LuciGFP, and followed up to 30 days. Upon autopsy, tumors had histological features consistent with pancreatic carcinoma visualized by routine H & E staining (Supporting Information Fig. S6c). Msln protein expression was not detected in subcutaneous tumors of Pan02-Ova and Pan02-LuciGFP. None of the mice showed any metastatic diseases by macroscopic views, H & E staining, and in vivo bioluminescense (Supporting Information Fig. S6d, only for Pan02-LuciGFP). There was no statistical difference with bioluminescence of subcutaneous Pan02-LuciGFP tumors in PBS-, rAd40-Ova-, or rAd5-Ova-pretreated mice. To determine the responses of inflammatory markers in subcutaneous models, plasma IL-6 levels were measured (data not shown). There was no IL-6 upregulation in subcutaneous models. Next, orthotopic pancreatic cancer models with Pan02-LuciGFP were evaluated weekly by in vivo bioluminescence up to 28 days. Orthotopically transplanted Pan02-LuciGFP tumors rapidly produced extensive loco-regional and disseminated lesions at day 28 post-transplant by in vivo bioluminescense (abdominal bioluminescent flux mean, 1.28 × 109 photons/sec; 95% CI, 0.94–1.38 × 109). All mice showed metastatic disease in the spleen, and periportal and intestinal LNs. Pancreatic and metastatic tumors showed pathological features of pancreatic carcinoma by H & E staining. However, Msln protein expression was restricted to the metastatic diseases (i.e., spleen and LNs by immunofluorescence (IF, data not shown)). By FCM, Msln and GFP double-positive cells (Supporting Information Fig. S7) were detected in both pancreatic and metastatic diseases. Msln-negative and GFP-positive cells were found only in the pancreas. Fourteen days of ex vivo culture in puromycin-containing medium turned all cells into Msln-negative and GFP-positive. These observations demonstrate the reversibility of Msln protein expression in orthotopic pancreatic cancer models.
Antimetastatic effects induced by mesothelin expression vectors
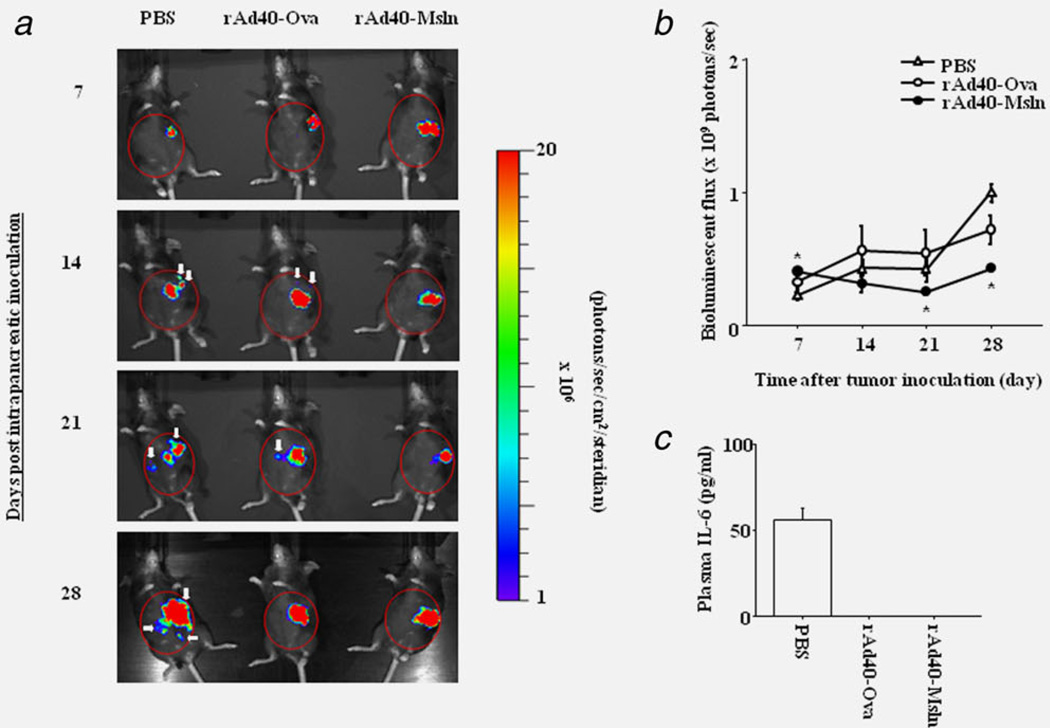
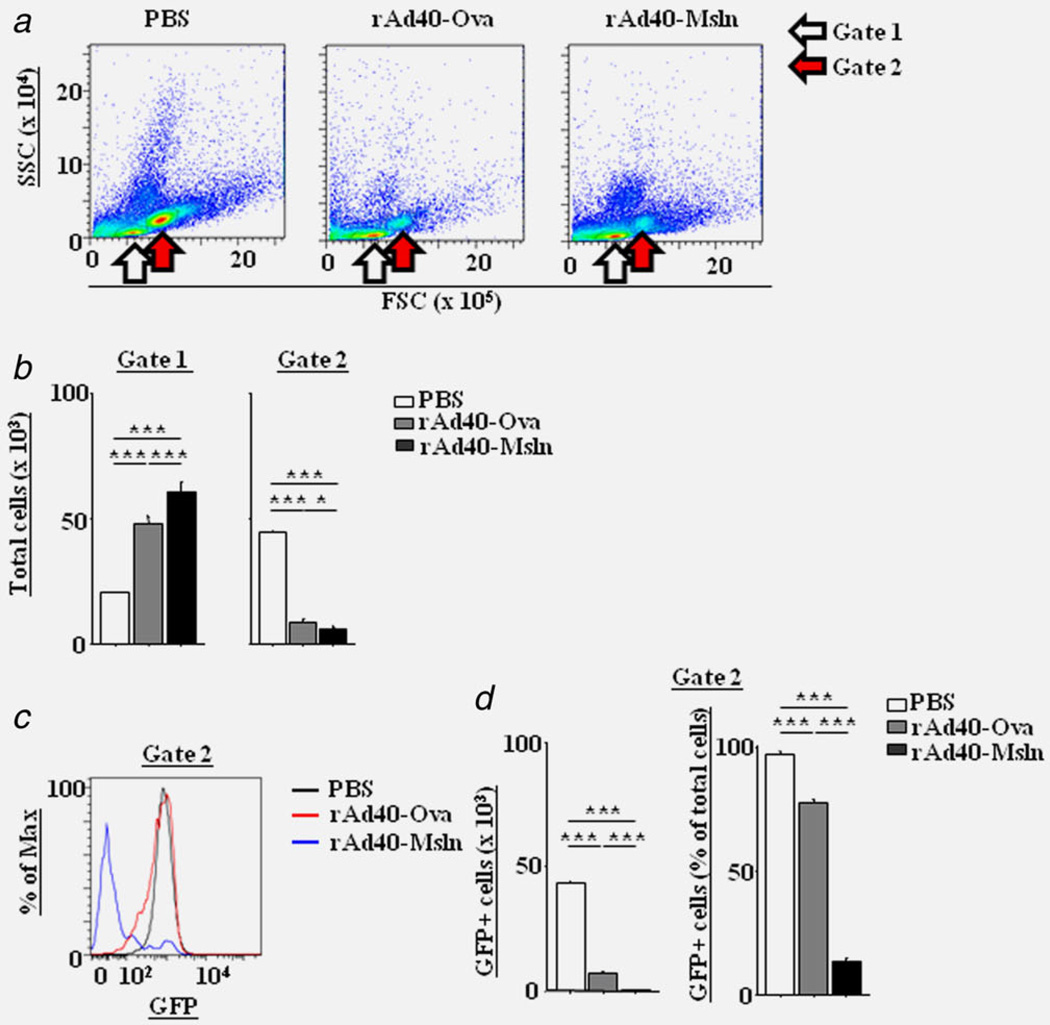
The effect of rAd40-Msln on the metastasis was compared to rAd40-Ova in an orthotopic pancreatic cancer model with Pan02-LuciGFP. By in vivo bioluminescense, untreated (PBS-pretreated), orthotopically transplanted Pan02-LuciGFP tumors progressed to extensive loco-regional and disseminated disease as described above. In PBS-pretreated mice, in vivo luciferase gene expression of the tumor cells was detected in spleen, and periportal and intestinal LNs (Fig. 4a). Autopsy (29 days post tumor inoculation) revealed that all PBS- and rAd40-Ova-pretreated mice showed metastatic diseases in the spleen, periportal and intestinal LNs, and gastrointestinal tract. Additionally, metastatic diseases in the liver and peritoneum were observed only in PBS- and rAd40-Ova-pretreated mice. On the other hand, tumor cells of rAd40-Msln-pretreated mice were found only in the pancreas. Also, the levels of abdominal bioluminescence were significantly lower in rAd40-Msln-pretreated mice compared to PBS- and rAd40-Ova-pretreated mice at day 21 and 28 after tumor inoculation (Fig. 4b). Only rAd40-Msln could suppress the organ distribution of metastases in orthotopic Pan02-LuciGFP tumor models. To assess the responses of inflammatory markers in orthotopic models, plasma IL-6 levels were measured (Fig. 4c). Plasma IL-6 was upregulated only in PBS-pretreated mice after orthotopically Pan02-LuciGFP inoculation. Tumors were confirmed histologically by H & E staining (Supporting Information Fig. 8 and Table 1). Also, GFP expression in all tumors was evaluated by IF. By FSC/ SSC dot-plots of FCM, the cells harvested from spleens of PBS-pretreated mice yielded higher number of cells in the specific gate (gate 2) for GFP-positive cells compared to the lymphocyte-specific gate (gate 1, Figs. 5a and 5b). In the gate 2, the higher numbers of GFP-expressing cells were detected in PBS-pretreated mice compared to rAd40-pretreated mice (Figs. 5c and 5d). A definite reduction in carcinomatosis and metastases to the spleen was shown after rAd40-Msln pre-treatment. Additionally, pretreatment with rAd40-Msln reduced the frequency of metastases to LNs, gastrointestinal tract, liver, and peritoneum compared to PBS and rAd40-Ova, coinciding with less Msln protein expression in the pancreas and spleen. rAd40-Msln pretreatment appeared to have a dramatic effect on the inhibition of pancreatic cancer invasion and metastasis.
Figure 4.
Reduction of tumor growth achieved by a single intravenous administration with rAd40 expressing mouse mesothelin in orthotopic pancreatic cancer mouse models. (a) The growth of Pan02-LuciGFP tumors was monitored weekly by live animal imaging using bioluminescence imaging. (b) Quantitation of luminescence signals generated by the Pan02-LuciGFP tumors in (a). Regions of interest (ROIs) are marked with red circles. White arrows indicate metastatic lesions. (c) Plasma IL-6 levels at 29 days after tumor inoculation of Pan02-LuciGFP into the pancreas of PBS-, rAd40-Ova- or rAd40-Msln-pretreated mice.
Table 1.
Organ distribution and growth of experimental metastases at orthotopic models
| Target organ | H&E | GFP | Msln |
|---|---|---|---|
| Pancreas | 9/91, 9/9, 9/9 | 9/9, 9/9, 9/9 | 4/9, 0/9, 0/9 |
| Spleen | 9/9, 9/9, 0/9 | 9/9, 9/9, 0/9 | 9/9, 9/9, 0/9 |
| Lymph nodes | 9/9, 9/9, 0/9 | 9/9, 9/9, ND | 9/9, 9/9, ND |
| Gastrointestinal tract | 9/9, 9/9, 0/9 | 9/9, 9/9, ND | 9/9, 9/9, ND |
| Liver | 3/9, 3/9, 0/9 | 3/3, 3/3, ND | 3/3, 3/3, ND |
| Peritoneum | 3/9, 6/9, 0/9 | 3/3, 6/6, ND | 3/3, 6/6, ND |
Three different samples from three different sites in PBS-, rAd40-Ova, or rAd40-Msln are shown. H & E, Hematoxylin and eosin staining; Msln, mouse mesothelin protein expression evaluated by IF; ND, not detected.
Figure 5.
Suppression of splenic tumor metastasis induced by a single intravenous administration with rAd40 expressing mouse mesothelin in orthotopic pancreatic cancer mouse models. Increased number of cells in the specific gate for GFP-positive cells at 29 days after tumor inoculation of Pan02-LuciGFP into the pancreas of PBS-, rAd40-Ova- or rAd40-Msln-pretreated mice. Cells harvested from the spleen were prepared for FCM and shown at FSC/SSC dot-plots (a). White and red arrows indicate the lymphocyte gate (gate 1) and the specific gate for GFP-positive cells in control mice (gate 2), respectively. Shown are representative quantitative evaluation on the numbers of cells in gate 1 and 2 (b). Preventive splenic Pan02-LuciGFP growth induced by rAd40-Msln i.v. Shown are representative histogram (c) and quantitative evaluation (d).
Discussion
This study showed that pretreatment with a one-time i.v. administration of rAd40 prevented pancreatic cancer growth in an antigen-specific manner and the number of metastases in mouse models. In vivo transgene delivery and expression after rAd40 i.v. were different from a conventional rAd5 i.v., and this difference is supposed to be the reason why Ad40-derived vector outperformed Ad5-derived one. Intravenously administered rAd40 did not cause harmful inflammatory responses and liver toxicity and stimulated systemic antigen-specific immune responses.
Our study elucidated the capability of rAd40 i.v. to overcome current issues with rAd systemic applications for prophylactic cancer vaccine. Among viral vectors as vaccines, rAd5 is historically the most widely used vector due to a high level of transfection efficiency, engineering capabilities, and production scalabilities. However, rAd5 i.v. induced high sequestration and transgene expression in the liver. We also observed inflammatory responses and liver toxicity after rAd5 i.v. In contrast, rAd40 i.v. should be safer than rAd5 i.v. thanks to reduced liver transduction, as well as less inflammatory responses and liver toxicity. The mechanism of this difference can be partly explained by the fact that Ad40 lacks the RGD motif in the penton base,25 which is the main player in sinusoid endothelial cell-binding in the liver.26 Liver antigen-presenting cells regulate liver tolerance and the induction of peripheral tolerance.27 Our data suggest that transgenes expressed by rAd5 can stimulate the immune reaction in the liver as previously reported.28 As a result, rAd5-Ova i.v. lacked the induction of functional Pan02-Ova-specific CTLs and Pan02-Ova tumor suppression in vivo, supporting the hyposis that rAd5 i.v. induces liver tolerance as previously described.29,30 On the other hand, rAd40-Ova i.v. induced functional Pan02-Ova-specific systemic immunity along CTL and Th1-cell dependent pathways. Also, antitumor immune responses against Pan02-Ova tumor were observed only by rAd40-Ova i.v. Our data show that rAd40 i.v. can induce a strong enough immune reaction to prevent tumor growth in antigen-specific manner.
It is reported that Msln vaccination can enhance antitumor effects against Msln-expressing tumors through Msln peptide-specific CD8+ T cell-mediated immunity.31–33 Consistent with this, pretreatment with rAd40-Msln i.v. prevented the primary tumor growth and the number of metastases in mouse pancreatic models. Msln protein expression was not detected in subcutaneous tumors derived from Pan02-Ova and Pan02-LuciGFP, which did not express Msln protein in vitro nor develop any metastatic diseases in vivo. Oppositely, orthotopically transplanted Pan02-LuciGFP tumors expressed Msln protein in the some population of primary pancreatic tumor cells and all metastatic tumors. Msln protein expression may regulate invasion and metastasis of mouse pancreatic models. The high correlation of Msln protein expression in metastatic tissues of our pancreatic cancer models indicated a possible mechanism that Msln protein expression in pancreatic cancer cells acts as a metastasis inducer. Further, Msln protein expression disappeared after ex vivo culture of Msln protein-positive tumor cells obtained from orthotopic tumors. Our findings make Msln an ideal target for intervention of metastatic pancreatic cancer. In fact, Msln-specific cytotoxicity against human pancreatic cancer has been found in multiple studies.34–36
We have also established that Msln protein expression is important for pancreatic cancer metastases in mice. While obvious Msln protein expression was found only in the limited sites of metastatic lesions, pretreatment with rAd40-Msln i.v. completely prevented the development of metastases in an orthotopic pancreatic mouse model. In the tumor micro-environment, Msln protein-expressing cells may be using abundant inflammatory cytokines (e.g., IL-6) to support tumor survival in vivo.37 In line with this hypothesis, we could detect the up-regulation of plasma IL-6 only in control mice of orthotopic pancreatic models. Interestingly, rAd40-Msln stimulated CTLs specific to Msln-directed antigens and also other tumor-antigens. Unexpectedly, a negative control rAd40 (i.e., rAd40-Ova) induced tumor antigen-specific IL-10-secreting helper T cells in orthotopic pancreatic models, suggesting that rAd40-Ova i.v. promoted tumor-specific T-cell tolerance. Our results indicate that rAd40 i.v. has the possibility to be an attractive method to stimulate both transgene- and tumor-specific immunity.
Our present study revealed that rAd40 i.v. showed the induction of stable immune responses and effective antitumor activity in mice. Additionally, rAd40 i.v. had wider biodistribution, while reducing inflammatory responses and liver toxicity compared to a conventional rAd5 i.v. Thus, our methods may improve the efficacy and safety of prophylactic vaccines for metastatic pancreatic cancer after surgical resection of the primary tumor. The same approach may also work for other cancers and infectious diseases.
Supplementary Material
What’s new?
Mesothelin is a cell-surface glycoprotein that is overexpressed in some cancers, including pancreatic cancers. In this study, the authors explored the possibility that a vaccine based on mesothelin could prevent growth and metastasis of these cancers. They designed a novel adenovirus vector that expresses mesothelin, and tested it in a mouse model of pancreatic cancer. The vaccine not only produced an anti-tumor immune response, but also suppressed metastasis. Prophylactic vaccines based on this formulation may provide a new strategy for treating metastatic pancreatic cancer, as well as other cancers and infectious diseases.
Acknowledgements
The authors thank Ashok K. Saluja (University of Minnesota) for helpful discussions, Toshio Kitamura (University of Tokyo, Tokyo, Japan) for the gift of pMX-puro, Matthew F. Mescher (University of Minneota) for the gift of E.G7 cell lines, and Stephen R. Nelson for manuscript assistance.
Grant sponsor: NIH; Grant number: P50CA101955
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 3.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 4.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M, Bharadwaj U, Zhang R, et al. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7:286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000;20:2902–2906. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 8.Maitra A, Adsay NV, Argani P, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–912. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- 9.Hassan R, Laszik ZG, Lerner M, et al. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–845. [PubMed] [Google Scholar]
- 10.Uehara N, Matsuoka Y, Tsubura A. Mesothelin promotes anchorage-independent growth and prevents anoikis via extracellular signal-regulated kinase signaling pathway in human breast cancer cells. Mol Cancer Res. 2008;6:186–193. doi: 10.1158/1541-7786.MCR-07-0254. [DOI] [PubMed] [Google Scholar]
- 11.Bharadwaj U, Li M, Chen C, et al. Mesothelin-induced pancreatic cancer cell proliferation involves alteration of cyclin E via activation of signal transducer and activator of transcription protein 3. Mol Cancer Res. 2008;6:1755–1765. doi: 10.1158/1541-7786.MCR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat Biotechnol. 2009;27:129–139. doi: 10.1038/nbt0209-129. [DOI] [PubMed] [Google Scholar]
- 13.Liu MA. Immunologic basis of vaccine vectors. Immunity. 2010;33:504–515. doi: 10.1016/j.immuni.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hangalapura BN, Oosterhoff D, Gupta T, et al. Delivery route, MyD88 signaling and cross-priming events determine the anti-tumor efficacy of an adenovirus based melanoma vaccine. Vaccine. 2011;29:2313–2321. doi: 10.1016/j.vaccine.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukens JR, Dolina JS, Kim TS, et al. Liver is able to activate naïve CD8+ T cells with dysfunctional anti-viral activity in the murine system. PLoS One. 2009;4:e7619. doi: 10.1371/journal.pone.0007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidd AH, Chroboczek J, Cusack S, et al. Adenovirus type 40 virions contain two distinct fibers. Virology. 1993;192:73–84. doi: 10.1006/viro.1993.1009. [DOI] [PubMed] [Google Scholar]
- 18.de Jong JC, Wigand R, Kidd AH, et al. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11:215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]
- 19.Tiemessen CT, Kidd AH. Adenovirus type 40 and 41 growth in vitro: host range diversity reflected by differences in patterns of DNA replication. J Virol. 1994;68:1239–1244. doi: 10.1128/jvi.68.2.1239-1244.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamasaki S, Miura Y, Brown E, et al. Development of a method for an effective amplification of human adenovirus 40. Arch Virol. 2010;155:1059–1068. doi: 10.1007/s00705-010-0683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corbett TH, Roberts BJ, Leopold WR, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717–726. [PubMed] [Google Scholar]
- 22.Miura Y, Yoshida K, Nishimoto T, et al. Direct selection of targeted adenovirus vectors by random peptide display on the fiber knob. Gene Ther. 2007;14:1448–1460. doi: 10.1038/sj.gt.3303007. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Deng ZL, Luo X, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 24.Kim MP, Evans DB, Wang H, et al. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4:1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiemessen CT, Kidd AH. The subgroup F adenoviruses. J Gen Virol. 1995;76:481–497. doi: 10.1099/0022-1317-76-3-481. [DOI] [PubMed] [Google Scholar]
- 26.Di Paolo NC, van Rooijen N, Shayakhmetov DM. Redundant and synergistic mechanisms control the sequestration of blood-born adenovirus in the liver. Mol Ther. 2009;17:675–684. doi: 10.1038/mt.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Zajac AJ, McPherson SA, et al. Primary adenovirus-specific cytotoxic T lymphocyte response occurs after viral clearance and liver enzyme elevation. Gene Ther. 2005;12:1079–1088. doi: 10.1038/sj.gt.3302494. [DOI] [PubMed] [Google Scholar]
- 29.Flatz L, Hegazy AN, Bergthaler A, et al. Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8+ T cell immunity. Nat Med. 2010;16:339–345. doi: 10.1038/nm.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schweichel D, Steitz J, Tormo D, et al. Evaluation of DNA vaccination with recombinant adenoviruses using bioluminescence imaging of antigen expression: impact of application routes and delivery with dendritic cells. J Gene Med. 2006;8:1243–1250. doi: 10.1002/jgm.952. [DOI] [PubMed] [Google Scholar]
- 31.Hung CF, Tsai YC, He L, et al. Control of mesothelin-expressing ovarian cancer using adoptive transfer of mesothelin peptide-specific CD8+ T cells. Gene Ther. 2007;14:921–929. doi: 10.1038/sj.gt.3302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly RJ, Sharon E, Pastan I, et al. Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther. 2012;11:517–525. doi: 10.1158/1535-7163.MCT-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CL, Wu TC, Hung CF. Control of human mesothelin-expressing tumors by DNA vaccines. Gene Ther. 2007;14:1189–1198. doi: 10.1038/sj.gt.3302974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokokawa J, Palena C, Arlen P, et al. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res. 2005;11:6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyazawa M, Iwahashi M, Ojima T, et al. Dendritic cells adenovirally-transduced with full-length mesothelin cDNA elicit mesothelin-specific cytotoxicity against pancreatic cancer cell lines in vitro. Cancer Lett. 2011;305:32–39. doi: 10.1016/j.canlet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Bharadwaj U, Marin-Muller C, Li M, et al. Mesothelin promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis. 2011;32:1013–1024. doi: 10.1093/carcin/bgr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.