Abstract
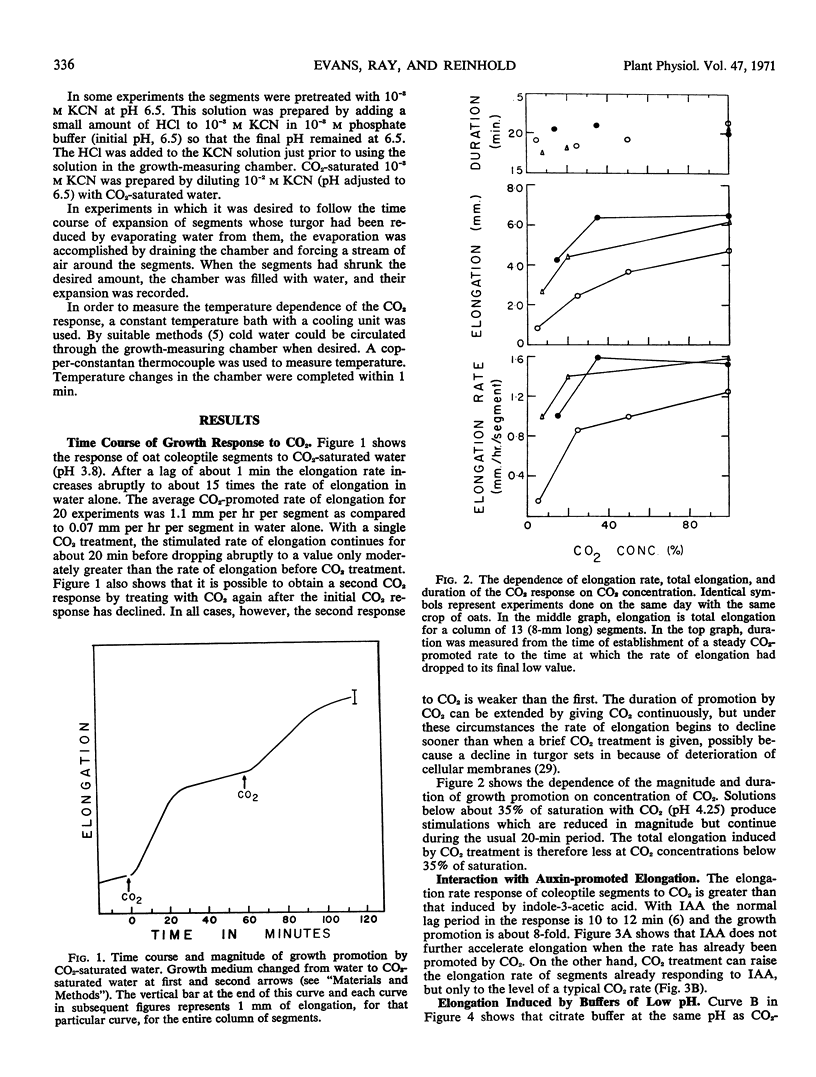
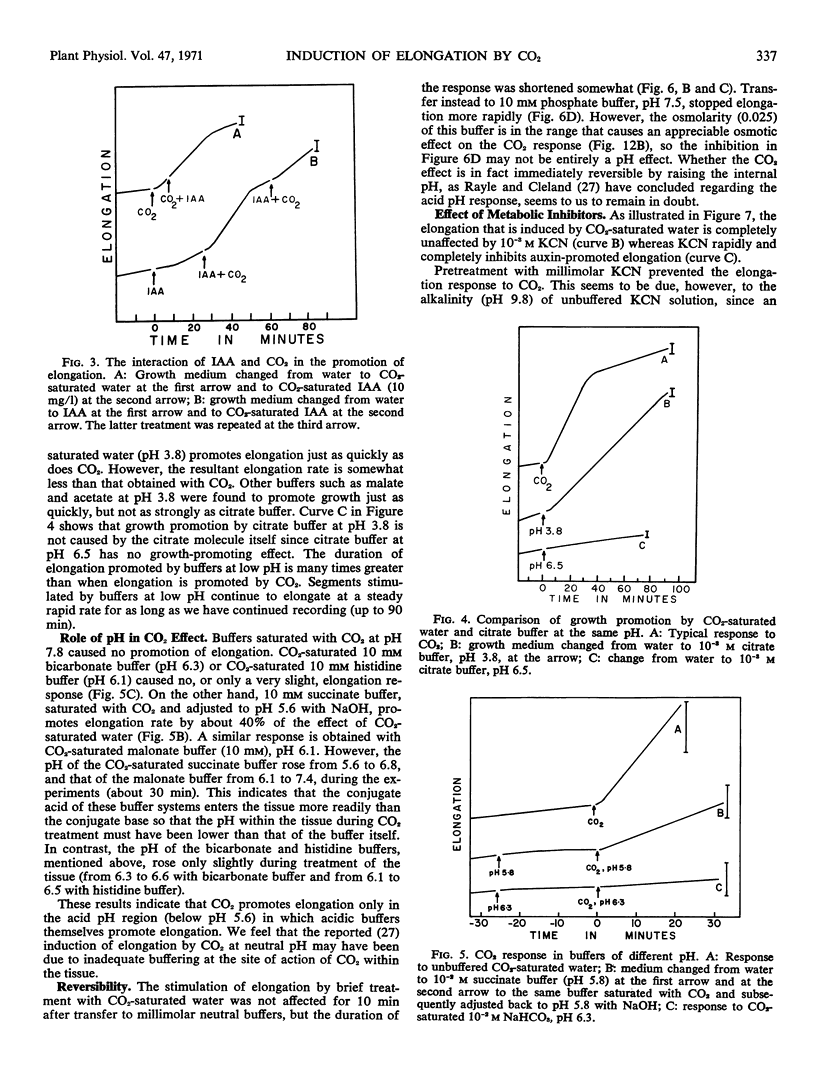
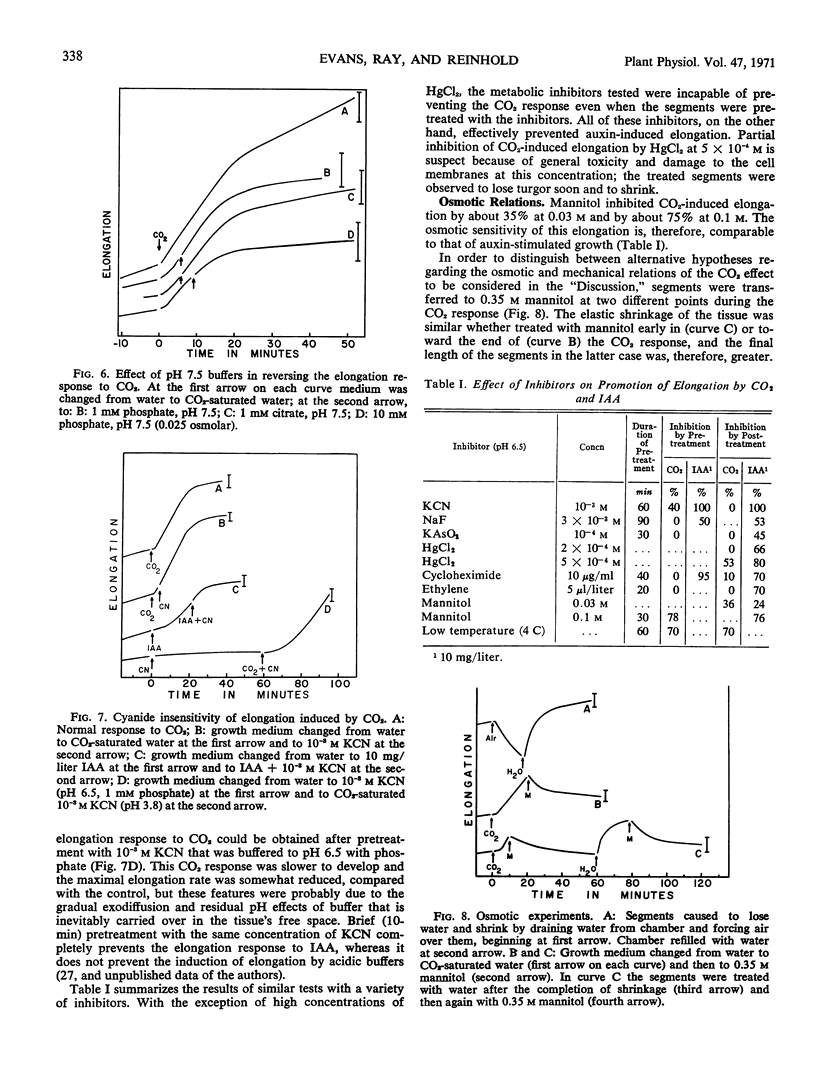
The ability of CO2 to induce elongation of Avena sativa coleoptile segments was examined with the use of a high resolution growth-recording device. CO2-saturated water causes an 8- to 16-fold promotion in the rate of elongation within 1 minute. This elongation is insensitive to a variety of metabolic inhibitors that suppress auxin-induced elongation, and the CO2 effect cannot be prevented by pretreatment with these inhibitors. Buffers of pH 3 to 4 also stimulate elongation quickly, and it seems that at least a major part of the action of CO2 depends upon its ability to reduce pH. The rate of elongation of auxin-promoted segments can be further enhanced by treatment with CO2 but not vice versa.
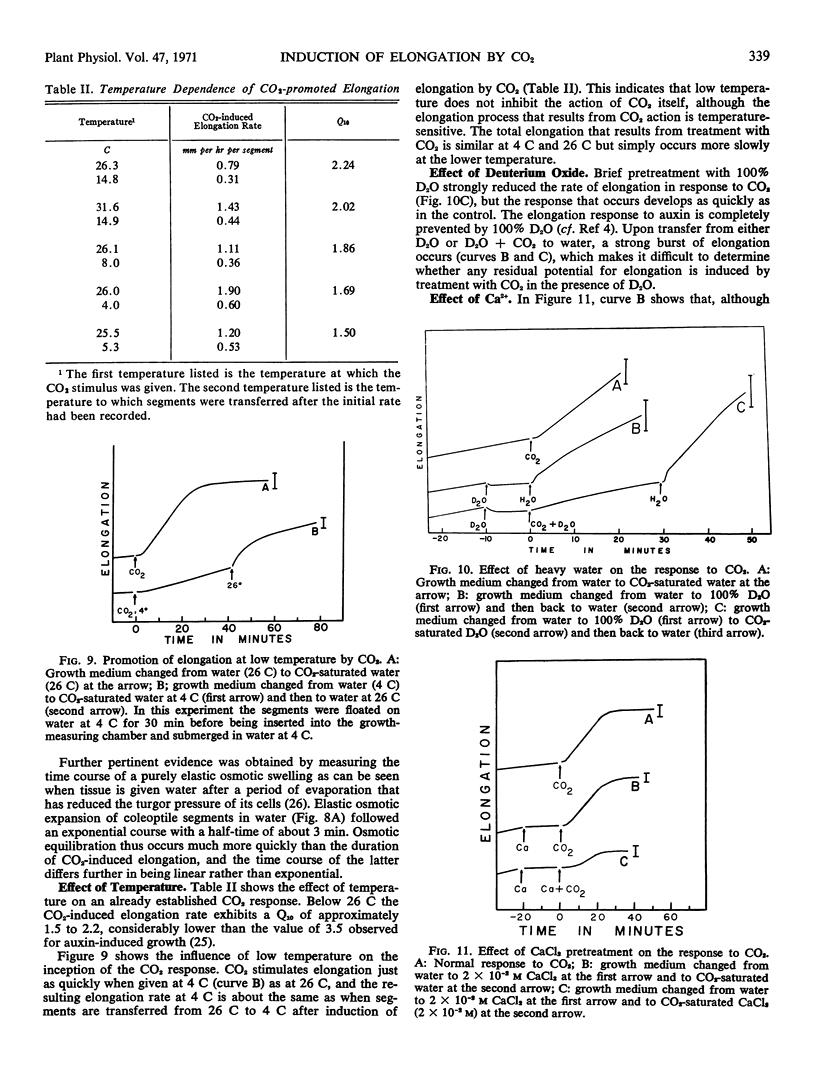
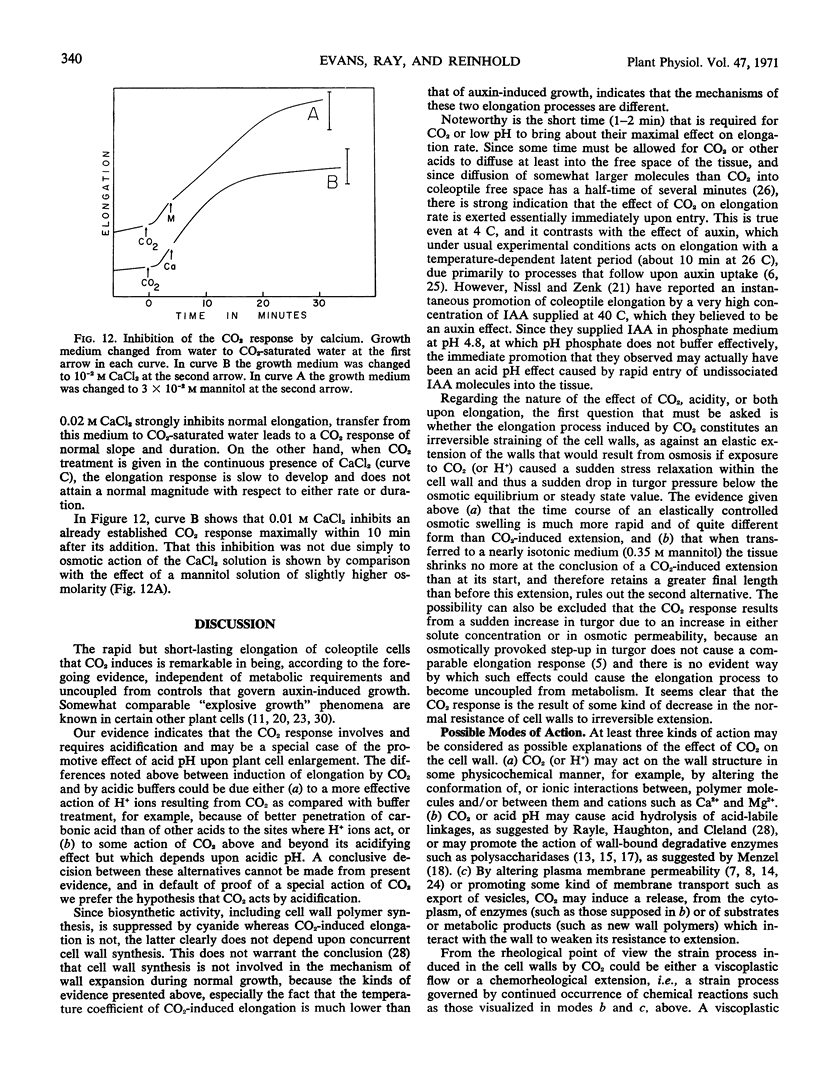
The response to CO2 can be inhibited by mannitol at osmotic concentrations that inhibit normal growth, by calcium, and by brief pretreatment with heavy water (D2O). The elongation rate that results from CO2 treatment is sensitive to temperature, but the induction by CO2 itself appears to be almost temperature-independent.
Elongation following treatment with CO2 may be a physical flow phenomenon, essentially independent of immediate biochemical participation, which occurs when wall polymer interactions that normally restrict strain in the cell wall are weakened or broken by CO2 in a manner that in effect substitutes for the role of metabolism in normal auxin-inducible cell enlargement.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans M. L., Ray P. M. Timing of the auxin response in coleoptiles and its implications regarding auxin action. J Gen Physiol. 1969 Jan;53(1):1–20. doi: 10.1085/jgp.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z., Reinhold L. Rapid Changes in Permeability of Cell Membranes to Water Brought About by Carbon Dioxide & Oxygen. Plant Physiol. 1962 Jul;37(4):481–486. doi: 10.1104/pp.37.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z., Reinhold L. Reversible Changes in the Hydraulic Permeability of Plant Cell Membranes. Plant Physiol. 1964 Nov;39(6):1043–1050. doi: 10.1104/pp.39.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Pramer D. Fungal Morphogenesis: Ring Formation and Closure by Arthrobotrys dactyloides. Science. 1967 Jan 20;155(3760):345–346. doi: 10.1126/science.155.3760.345. [DOI] [PubMed] [Google Scholar]
- Inoué S., Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967 Jul;50(6 Suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- Katz M., Ordin L. A cell wall polysaccharide-hydrolyzing enzyme system in Avena sativa L. coleoptiles. Biochim Biophys Acta. 1967 Jun 13;141(1):126–134. doi: 10.1016/0304-4165(67)90251-6. [DOI] [PubMed] [Google Scholar]
- Kitasato H. The influence of H+ on the membrane potential and ion fluxes of Nitella. J Gen Physiol. 1968 Jul;52(1):60–87. doi: 10.1085/jgp.52.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Kivilaan A., Bandurski R. S. In vitro autolysis of plant cell walls. Plant Physiol. 1967 Jul;42(7):968–972. doi: 10.1104/pp.42.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y., Yamamoto R. Effect of auxin on beta-1, 3-glucanase activity in Avena coleoptile. Dev Growth Differ. 1970 Mar;11(4):287–296. doi: 10.1111/j.1440-169x.1970.00287.x. [DOI] [PubMed] [Google Scholar]
- Nitsch J. P., Nitsch C. Studies on the Growth of Coleoptile and First Internode Sections. A New, Sensitive, Straight-Growth Test for Auxins. Plant Physiol. 1956 Mar;31(2):94–111. doi: 10.1104/pp.31.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Adaptation of barley roots to low oxygen supply and its relation to potassium and sodium uptake. Plant Physiol. 1969 Sep;44(9):1233–1240. doi: 10.1104/pp.44.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Ruesink A. W. Osmotic Behavior of Oat Coleoptile Tissue in Relation to Growth. J Gen Physiol. 1963 Sep 1;47(1):83–101. doi: 10.1085/jgp.47.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970 Aug;46(2):250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold L., Glinka Z. Reduction in Turgor Pressure as a Result of Extremely Brief Exposure to CO(2). Plant Physiol. 1966 Jan;41(1):39–44. doi: 10.1104/pp.41.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS D. F., EISENBERG R. M. A model representing a physiological role of CO2 at the cell membrane. J Gen Physiol. 1961 May;44:869–887. doi: 10.1085/jgp.44.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilescu V., Mărgineanu D. D20 effect on active transport through frog skin. Naturwissenschaften. 1970 Mar;57(3):126–127. doi: 10.1007/BF00600049. [DOI] [PubMed] [Google Scholar]
- Weinberg E. S., Voeller B. R. Induction of fern spore germination. Proc Natl Acad Sci U S A. 1969 Nov;64(3):835–842. doi: 10.1073/pnas.64.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin B. M., Henderson R. F., Henderson T. R. Effects of D2O on the association-dissociation equilibrium in subunit proteins. J Biol Chem. 1970 Aug 10;245(15):3733–3737. [PubMed] [Google Scholar]



