Abstract
Proximal row carpectomy (PRC) combined with distal radius hemiarthroplasty is a relatively novel procedure that rivals total wrist arthrodesis and offers a new surgical treatment option for select patients with painful, end-stage wrist disease. We present our early experience with this procedure. A retrospective chart review was conducted for nonrheumatoid patients diagnosed with wrist arthritis and subsequently treated with wrist hemiarthroplasty combined with PRC. The minimum follow-up duration was 12 months. Preoperative and postoperative flexion, extension, and grip strength were recorded. Postoperative radiographic findings were assessed. The Patient-Rated Wrist Evaluation (PRWE) questionnaire was administered to gauge postoperative pain and function. The records of 10 patients were reviewed. The mean age was 64 years and the mean postoperative follow-up duration was 19 months. Postoperative flexion, extension, and grip strength were all found to be less than the preoperative levels. The mean postoperative PRWE score for pain and function were 26 and 23, respectively. The complications were diverse and occurred at a relatively high rate. PRC combined with distal radius hemiarthroplasty is a novel procedure that offers a potential surgical option for the treatment of wrist arthritis in select patients. Our early experience has lead us to modify our technique with regard to the implant material, and at this stage, the surgical technique and the most appropriate implant may require further optimization. The level of evidence for this study is IV (therapeutic).
Keywords: wrist arthritis, hemiarthroplasty, proximal row carpectomy, wrist, motion preserving surgery
Several surgical options are available for the treatment of painful, end-stage wrist disease. These procedures can generally be classified into motion preserving or motion sacrificing. Proximal row carpectomy (PRC), scaphoid excision, and four-corner fusion and total wrist arthroplasty (TWA) are motion preserving procedures whereas total wrist arthrodesis is a motion sacrificing surgery. PRC and four-corner fusion have been used for more than two decades and long-term data indicate that these procedures can offer substantial pain relief and preserve some wrist motion for many years.1,2,3,4 However, for advanced disease that affects the lunate facet, and when PRC or four-corner fusion are not indicated, TWA or total wrist arthrodesis are often performed. TWA is usually reserved for low-demand patients and is generally avoided in younger and more active patient populations due to concerns related to implant loosening.5 Total wrist arthrodesis sacrifices all wrist motion but is a useful procedure because it provides predictable pain relief. Combined PRC and distal radius hemiarthroplasty represents a recently introduced motion preserving surgery and presents an alternative to TWA or total arthrodesis. The procedure was first described by Boyer and Adams in 2010 for the treatment of two patients with severe wrist arthritis.6 We began performing this surgery in April 2009, and by April 2012, we had performed 36 cases. In this article, we describe our surgical technique and our early experience with this relatively novel surgical technique for the treatment of nonrheumatoid patients.
Methods
Study Design
We performed a retrospective chart review of patients with multiple forms of wrist arthritis, treated by distal radius hemiarthroplasty combined with PRC. The study was conducted under a protocol approved by our local Institutional Review Board. The inclusion criteria were (1) patients diagnosed with wrist arthritis and treated with wrist hemiarthroplasty and PRC and (2) patients with at least 12 months duration of postoperative follow-up. Patients with inflammatory arthritis were excluded. Patients who did not have sufficient follow-up or unavailable medical records were also excluded. Preoperative and postoperative ranges of motion and grip strengths were included in this study. Wrist flexion, extension, and grip strength were recorded when available preoperatively and at the final follow-up visit. Postoperative ulnar and radial deviations were recorded only in the minority of cases, so their analysis was excluded. Postoperative radiographic findings were assessed. The Patient-Rated Wrist Evaluation (PRWE) questionnaire was administered by phone to gauge subjective, postoperative pain and function. Postoperative complications were also listed.
Indications for Surgery
Similar to alternative salvage procedures, debilitating wrist pain is the primary indication for this surgery. In the case of a spared lunate fossa, we utilize motion-sparing options (PRC or four-corner). Patients who desire more motion during physical activity but would otherwise undergo total wrist fusion or TWA may be appropriate candidates for this procedure, particularly when the lunate fossa shows signs consistent with arthritic changes. Arthritic changes of the lunate fossa are usually evident on preoperative radiographs, and intraoperative findings confirm arthrosis before the decision is made to resurface the distal radius. We have performed the procedure for posttraumatic arthritis, advanced radiocarpal, and midcarpal destruction in patients with stage III scapholunate advanced collapse (SLAC) (arthritis affecting the entire radioscaphoid joint and the capitolunate articulation), stage IV SLAC (arthritis affecting the radiocarpal and intercarpal joints), stage III scaphoid nonunion advanced collapse (SNAC) (involvement of the capitate), stage IV SNAC (pancarpal arthrosis), osteoarthritis and inflammatory arthritis, including both rheumatoid and psoriatic arthritis. However, the outcomes of this surgery remain in their infant stage and it is not yet clear which patient populations will benefit the most from this procedure.
The main absolute contraindication to this surgery is an active wrist infection, osteomyelitis or bacteremia. Relative contraindications include noncompliant patients, poor distal radius bone stock, and absent or insufficient extensor tendons.
Surgical Technique
Either regional axillary or general anesthesia is utilized on the basis of patient and anesthesiologist preferences. Preoperative prophylactic antibiotics are infused within 1 hour of the incision. The patient is positioned supine on the operating table with the effected arm abducted 90 degrees on a hand table. A tourniquet is placed on the arm and is used to limit blood flow and improve visualization of the field. The involved wrist is prepped and draped up to the level of the forearm.
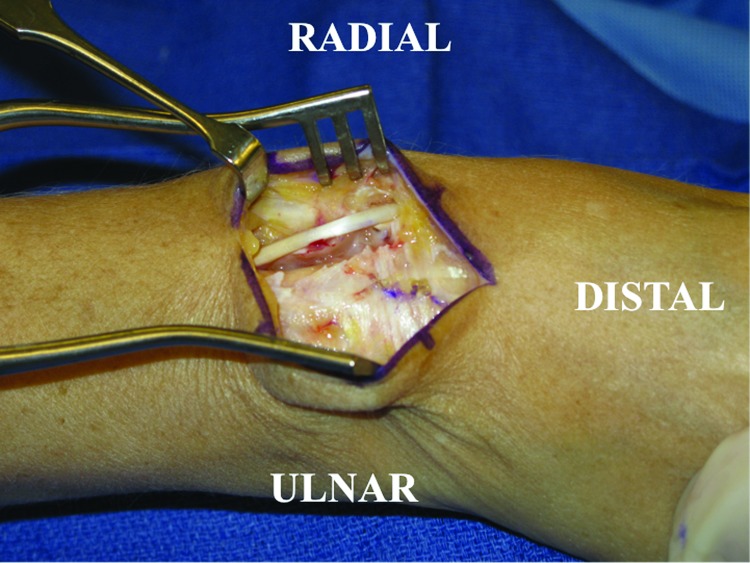
A dorsal longitudinal skin incision is made just ulnar to Lister tubercle and dissection is carried down to the level of the extensor retinaculum with care taken to preserve the sensory branches of the ulnar and radial sensory nerves. The third dorsal compartment containing the extensor pollicis longus (EPL) tendon should be visualized coursing obliquely just ulnar to Lister tubercle. The EPL is released and retracted in a radial direction while the extensor digitorum communis tendons contained in the fourth dorsal compartment are elevated subperiosteally and retracted ulnarward to visualize the dorsal wrist capsule (Fig. 1). The posterior interosseous nerve is visualized in the floor of the fourth compartment and is excised to provide postoperative pain relief.
Figure 1.
The extensor retinaculum is observed here prior to its release. The extensor pollicis longus is visible crossing the wrist obliquely. The extensor pollicis longus has been released from the third dorsal compartment and mobilized radially.
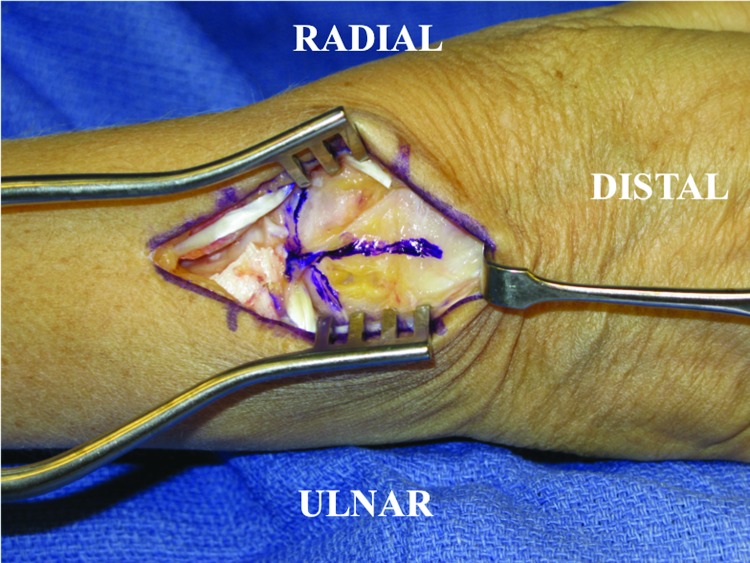
Next, an inverted T-incision is made in the dorsal wrist capsule, which allows visualization of the carpal bones and distal radius (Fig. 2). Inspection of the carpal bones is performed to determine the status and severity of the wrist pathology (Fig. 3). A PRC is then performed in piecemeal fashion with a combination of osteotomes, mallet, and rongeurs with care taken to protect the volar wrist extrinsic ligaments as well as the articular surface at the head of the capitate.
Figure 2.
Dorsal wrist exposure. Extensor digitorum communis tendons retracted ulnarward and the extensor pollicis longus radialized. The extensor retinaculum has been divided revealing the dorsal wrist capsule. An inverted T-incision is made over the dorsal wrist capsule as outlined by the marker.
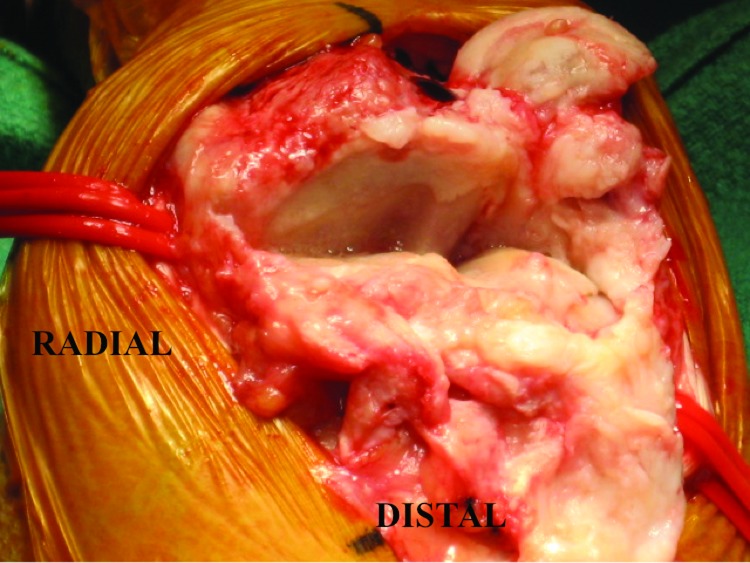
Figure 3.
The proximal row carpectomy component of the surgery has been completed. The distal radius shows an eburnated, sclerotic articular margin.
Following PRC, the preparation of the distal radius is the next step in the procedure. Initially, a cutting jig is used (Fig. 4) and standard guides and rasps specific to the implant model are used to create a path for the implant's stem (Fig. 5). Range-of-motion trials are performed in flexion, extension, and radial and ulnar deviation allowing for selection of the most appropriate implant size. The selected distal radius component is then placed in a press-fit fashion and, to date, we have only used bone cement for revision procedures. Between April 2009 and June 2010, we exclusively used the radial component of the Maestro Wrist Reconstructive System (BioMet, Warsaw, IN) and performed 17 cases using this implant. The radial stem of this implant is fabricated from titanium alloy, while the articulating surface that we used was composed of ultrahigh molecular weight polyethylene. Because of several complications (described in the Results section), from September 2010 and onward, we began using the distal radial component of the ReMotion Total Wrist Implant (Small Bone Innovations, Morrisville, PA). The articulating component of this implant is composed of cobalt chrome–molybdenum alloy. The intramedullary stem is coated with commercially pure titanium plasma coating. According to the manufacturer's guide, the distal surface is contoured to approximate the volar tilt and ulnar inclination angles of the distal radius. Once the implant is secured within the distal radius, its stability is tested in all ranges of motion by firmly rotating the hand and carpus on the bearing surface of the implant. The placement of the implant is fluoroscopically confirmed. At the conclusion of the procedure, the wound is copiously irrigated with antibiotic impregnated solution. The dorsal wrist capsule is closed with absorbable sutures. The third extensor compartment is left open with the EPL tendon radialized, while the extensor retinaculum overlying the fourth compartment is closed. The tourniquet is deflated, and final hemostasis achieved with bipolar cautery. Skin is closed according to surgeon preference. A bulky dressing with a protective volar splint is finally applied. Figs. 6A and 6B demonstrate preoperative and postoperative radiographs of the procedure.
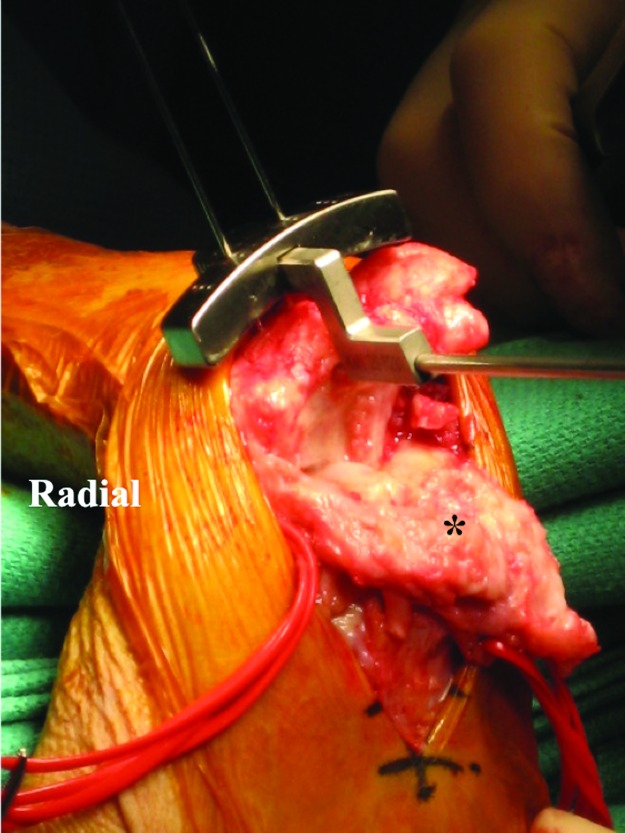
Figure 4.
Setting the jig to judge radial alignment for the radial implant in the neutral capsule. *Wrist capsule.
Figure 5.
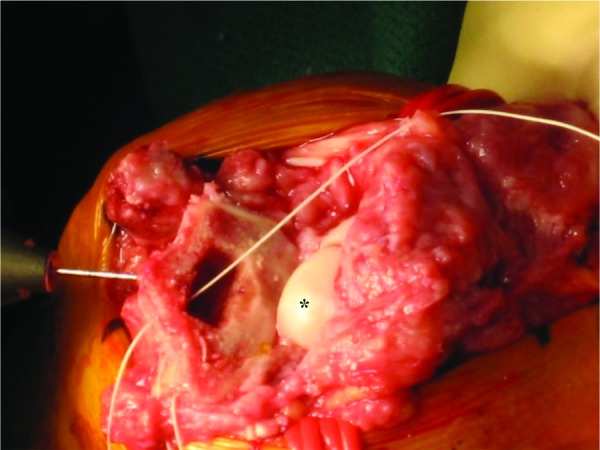
The radius has been broached in preparation for the distal radius component. *Capitate.
Figure 6.
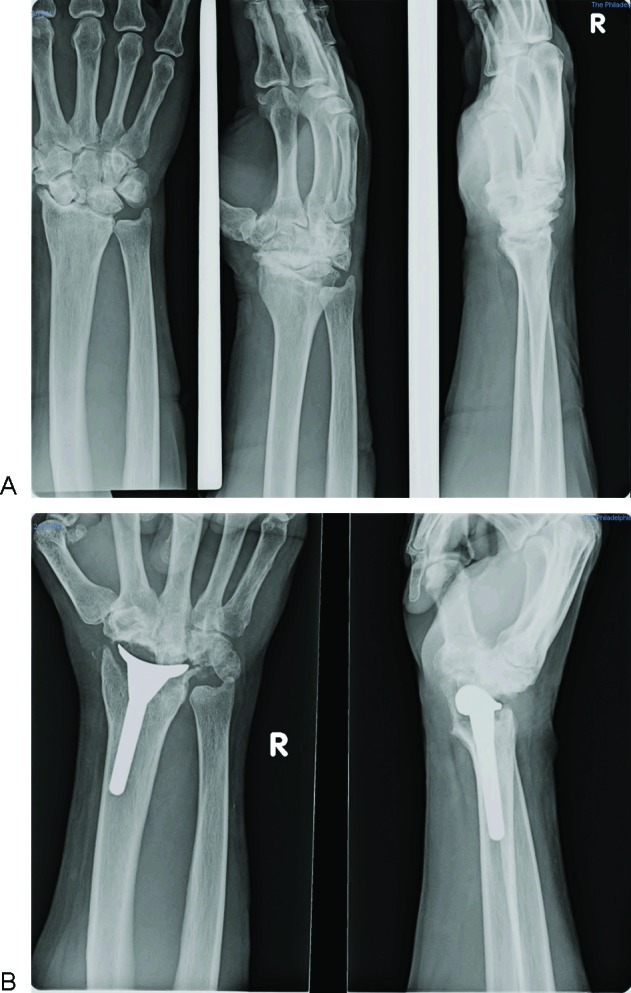
(A) Preoperative anteroposterior, oblique, and lateral radiographs of a patient diagnosed with stage IV scaphoid nonunion advanced collapse wrist. (B) Anteroposterior and lateral radiographs 14 months postoperatively illustrating an aligned and stable distal radius component as well as the removal of the proximal row.
Postoperative Management
Immediately postoperatively, emphasis is placed on edema control with ice, compression, and elevation. Early active range of motion of the metacarpophalangeal and interphalangeal joints is also encouraged. Patients are started on a formal therapy protocol 5 days postoperatively and this includes active range-of-motion exercises of the wrist and forearm. Two weeks postoperatively, gentle passive motion of the wrist is initiated. Strengthening exercises are incorporated into the rehabilitation protocol six weeks postoperatively.
Results
After applying the inclusion and exclusion criteria, 10 patient records were available for review. There were 6 men and 4 women, and the average age at surgery was 64 years (50 to 80 years). The mean clinical follow-up duration was 19 months (14 to 29 months). Mean postoperative flexion, extension, and grip strength were all less than the preoperative levels. Patient characteristics and objective outcomes are presented in Table 1. The outcomes of the PRWE questionnaire are presented in Table 2. The postoperative radiographic assessment is presented in Table 3, and the postoperative complications are listed in Table 4.
Table 1. Patients' Characteristics and Clinical Outcomes.
| Patient | Age, y | Diagnosis | Gender | Side | Implant | Flexion, Degrees | Extension, Degrees | Grip Strength, lbs | Follow-Up, mo | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop | Postop | Preop | Postop | Preop | Postop | |||||||
| 1 | 69 | SNAC IV wrist | Man | Right | Maestro | 30 | 30 | 20 | 35 | 60 | 38 | 15 |
| 2 | 50 | SLAC III wrist failed four-corner | Man | Left | ReMotion | 60 | 10 | 70 | 40 | 60 | 40 | 16 |
| 3 | 56 | SLAC III wrist | Man | Left | ReMotion | 20 | 30 | 30 | 30 | 30 | N/A | 14 |
| 4 | 69 | SLAC III wrist | Man | Right | Maestro | 50 | 30 | 50 | 20 | 50 | N/A | 29 |
| 5 | 80 | Osteoarthritis | Woman | Right | Maestro | 40 | N/A | 45 | N/A | 40 | N/A | 18 |
| 6 | 64 | SLAC III wrist | Woman | Right | Maestro | 60 | 30 | 70 | 45 | 55 | 40 | 20 |
| 7 | 58 | SLAC III wrist | Man | Left | Maestro | 45 | 20 | 45 | 30 | 30 | 38 | 21 |
| 8 | 56 | Posttraumatic arthritis | Woman | Right | Maestro | 20 | 20 | 35 | 20 | 10 | 20 | 15 |
| 9 | 65 | SLAC III wrist and posttraumatic arthritis | Woman | Left | Maestro | 30 | 30 | 15 | 35 | 35 | N/A | 29 |
| 10 | 74 | SLAC III wrist | Man | Right | Maestro | 30 | 20 | 30 | 40 | 40 | 25 | 17 |
| Mean | 64 | 39 | 24 | 41 | 33 | 41 | 34 | 19 | ||||
SLAC, scapholunate advanced collapse; SNAC, scaphoid non-union advanced collapse; N/A, not available.
Table 2. Patient-Rated Wrist Evaluation Outcomes.
| Patient | PRWE Pain (n = 10) | PRWE Function (n = 10) | PRWE Total (n = 10) | Follow-Up, mo (n = 10)a |
|---|---|---|---|---|
| 1 | 0 | 27.5 | 27.5 | 20 |
| 2 | 33 | 24.5 | 57.5 | 9 |
| 3 | 37 | 36.5 | 73.5 | 10 |
| 4 | 38 | 26.5 | 64.0 | 20 |
| 5 | 44 | 38.5 | 82.5 | 24 |
| 6 | 14 | 8.5 | 22.5 | 17 |
| 7 | 26 | 17.5 | 43.5 | 12 |
| 8 | 33 | 28.0 | 60.5 | 26 |
| 9 | – | – | – | – |
| 10 | 14 | 3.5 | 17.5 | 15 |
| Mean | 26.4 | 23.4 | 49.8 | 17 |
Note: Missing data indicated by (–).
Postoperative follow-up duration of telephone survey.
Table 3. Postoperative Radiographic Findings.
| Patient | X-ray Findings | Follow-Up, moa |
|---|---|---|
| 1 | Well-seated distal radius implant with no signs of lucency or subsidence. Capitate head remains seated in the lunate fossa. | 15 |
| 2 | Well-seated distal radius implant with no signs of lucency or subsidence. Capitate head and hamate remain seated in the lunate fossa. | 16 |
| 3 | Well-seated distal radius implant with no signs of lucency or subsidence. Capitate head remains seated in the lunate fossa. | 13 |
| 4 | Solidly fixed and well-aligned radial implant with osteolysis of distal carpal bones.b | 26 |
| 5 | Well-seated distal radius implant with no signs of lucency or subsidence. Capitate head and hamate remain seated in the lunate fossa. | 17 |
| 6 | Well-seated distal radius implant with no signs of lucency or subsidence. Capitate head and hamate remain seated in the lunate fossa. | 20 |
| 7 | Cyst formation in the hamate, capitate, and distal radius.b | 15 |
| Revised implant well-seated within bone cement. | 21 | |
| 8 | Well-seated distal radius implant with no signs of lucency or subsidence. Capitate head remains seated in the lunate fossa. | 14 |
| 9 | Well-seated distal radius implant with no signs of lucency or subsidence. Capitate head remains seated in the lunate fossa. | 29 |
| 10 | Osteolysis in the distal half of the radial prosthesis. Capitate head remains seated in the lunate fossa. | 14 |
Postoperative follow-up of radiographic study.
Underwent implant revision shortly thereafter.
Table 4. Postoperative Complications.
| Patient | Complications That Required Implant Revision | Treatment |
|---|---|---|
| 4 | Aseptic loosening secondary to “poly disease” | Revision distal radius hemiarthroplasty |
| 7 | Aseptic loosening secondary to “poly disease” | Revision distal radius hemiarthroplasty |
| Patient | Other Complications | Treatment |
| 2 | Left wrist contracture, bone spur development, and extensor tendon adhesion | Capsulectomy, bone spur removal and tenolysis respectively |
| 3 | Clicking during full flexion, pain accompanied by subchondral cysts, and degenerative changes in the capitate and hamate | Currently under observation |
| 5 | Ulnar tunnel syndrome | Ulnar nerve neurolysis and ulnar tunnel decompression |
| 6 | Right wrist contracture and extensor tendon adhesions | Capsulotomy and ECRL ECRB tenolysis |
| 8 | Wrist stiffness, pisotriquetral arthritis, ulnar tunnel syndrome, and extensor tenosynovitis of small finger | Capsulectomy, pisiformectomy, ulnar tunnel release, and steroid injection respectively |
| 9 | Flexor tenosynovitis | Flexor tenosynovectomy |
| 10 | Asymptomatic osteolysis | Nonea |
ECRB, extensor carpi radialis brevis; ECRL, extensor carpi radialis longus; EDC, extensor digitorum communis.
Patient deceased shortly thereafter.
Several of our patients experienced either aseptic loosening or reactive tenosynovitis, secondary to polyethylene wear that required further surgery (Fig. 7). Two cases consisted of implant revision. Other complications include two cases of ulnar tunnel syndrome that required decompression of Guyon canal, and one case of pisiform tenosynovitis that resolved with a corticosteroid injection (Table 4).
Figure 7.
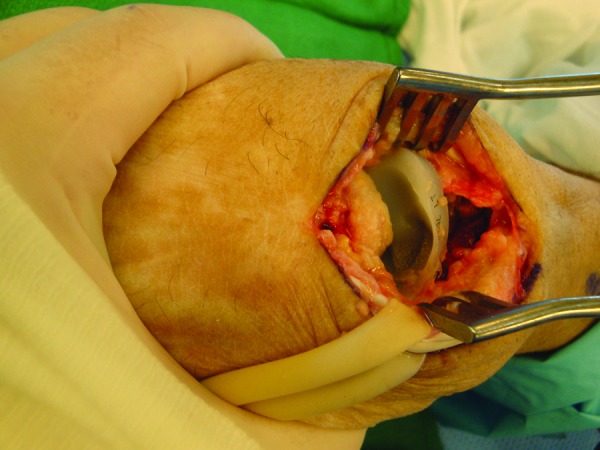
Revision hemiarthroplasty secondary to aseptic osteolysis. Note the erosion of the polyethylene bearing surface all the way to the metallic stem (Courtesy of Brian George, M.D., The Philadelphia Hand Center).
Discussion
Although the surgical treatment of end-stage wrist disease offers significant pain relief, new technology and thought continue to evolve regarding implant optimization and postsurgical recovery. Over the past few decades, PRC alone and scaphoid excision with four-corner fusion have proven to be useful and durable motion preserving procedures in terms of pain relief and residual motion for those patients with a preserved radiocarpal articulation.1,4 In the setting of advanced radiocarpal arthrosis and when PRC alone or four-corner fusion are not indicated, combined PRC and hemiarthroplasty may offer pain relief in addition to less restriction than traditional motion-sacrificing procedures. For end-stage wrist disease, we began using PRC and hemiarthroplasty 3 years ago (2009), primarily for pain relief and with the goal of minimizing postoperative restriction of wrist motion. The purpose of the study was to describe our surgical technique and to review the short-term outcomes of this procedure.
In our study, at a mean follow-up of 19 months, mean wrist flexion, wrist extension, and grip strength were all less than the preoperative levels. Since PRC with hemiarthroplasty is considered a salvage procedure, the fact that the postoperative flexion-extension arc of motion was less than preoperative levels was not unexpected. The relative maintenance of wrist extension provided the preservation of functional range of motion in the flexion-extension plane. Postoperative wrist flexion and extension were 22 and 30 degrees, respectively. Although there is no consensus regarding the functional range of wrist motion, Palmer et al determined the minimum range of motion to be 5 degrees of flexion, 30 degrees of extension, 10 degrees of radial deviation, and 15 degrees of ulnar deviation.7 However, Ryu et al reported that the minimum necessary range of motion to complete most tasks were 40 degrees of extension and flexion, 10 degrees of radial deviation, and 30 degrees of ulnar deviation.8
Postoperative pain and function were assessed using the PRWE. Our patients had a mean score of 26 of 50 for pain, 23 of 50 for function, and an overall score of 49 of 100 at a mean postoperative duration of 17 months (Table 2). TWA is often performed for severe wrist arthritis and has been shown to limit maximum disability at midterm follow-up, but it is not indicated for patients with high levels of activity or poor bone stock. Ferreres et al reported on 21 patients treated with the Universal 2 Total Wrist System (Integra LifeSciences, Plainsboro, NJ), 14 of whom were diagnosed with rheumatoid arthritis and 7 of whom were diagnosed with various other types of degenerative wrist arthritis, at a mean postoperative duration of 66 months. These patients had combined pain and function scores of 24 of 100 on the PRWE scale.9 Although these scores are superior to ours, the follow-up duration was considerably longer for the latter study and patients have a tendency to adapt to their limitations as time progresses. In addition, most of the patients in their study were rheumatoid patients. The pain relief afforded by total wrist arthrodesis has often been labeled as “predictable.” In a study that looked at the outcomes of total wrist fusion in a population of 22 patients with posttraumatic wrist arthritis, the authors found that after an average of 6 years, 14 patients reported persistent pain and 4 patients reported severe pain, with 1 patient considering the pain to be worse after the fusion.10 However, 15 patients did report that their pain had decreased after the fusion. In a separate study that looked at 20 patients with posttraumatic wrist arthritis, at an average of 2 years following total wrist arthrodesis, the authors found the mean pain score to be 1.6 ± 0.2 (1 to 4 scale; 1 being no pain and 4 being severe pain requiring regular analgesics).11 It is clear that many of our patients continued to experience pain postoperatively. However, because of the differences in patient populations, follow-up duration, and pain scales, we find it difficult to make any conclusive statements regarding how the pain levels experienced by our patients compare with either TWA or total wrist fusion.
The relatively high complication rates indicate that this novel procedure has not yet been optimized with regard to the surgical technique or the implant material. In our experience, postoperative wrist joint contracture leading to stiffness has been one of the most common complications. This was treated either by capsulotomy or capsulectomy. When we initially began performing this procedure, a distal radius component lined with a polyethylene articular surface was used (Maestro Wrist Reconstructive System). Soon thereafter, we began observing aseptic loosening and reactive synovitis secondary to “poly disease.” We postulate that the relatively small contact areas of the capitate on the soft polyethylene bearing surface lead to accelerated wear and subsequent tissue pathology (Fig. 7). As a consequence, we no longer utilize a polyethylene implant and have transitioned to a harder, all-metal component. After shifting to the all-metal implant, we have not seen any cases of reactive synovitis. The utility of the type of implant and material may become clearer as time progresses.
Our study has several limitations. First, the study falls victim to the intrinsic biases of a retrospective chart review. Second, we did not perform any statistical analysis mainly because of our small sample size. Our analysis of the range of motion was also restricted to flexion and extension whereas radial and ulnar deviations were excluded because these values were not prospectively collected. In our series of patients, standardized preoperative pain scores in addition to the postoperative scores would have been more informative. Despite these limitations, we believe that presenting our data is of value, given the novel concept of this surgical procedure, which has not yet gained a widespread audience.
To fully assess the efficacy of this procedure over others for severe wrist arthritis, further clinical refinement and research studies are required. Clinical studies should seek to follow a larger cohort of patients with a longer follow-up, whereas biomechanical studies may be able to shed light on contact areas, alignment, and force transmission. On the basis of the data we have presented, we believe that the surgical technique of combined PRC and hemiarthroplasty and the most appropriate type of implant should be optimized. We have attempted to improve on our initial findings by changing the type of implant that we use. We believe that in select patients, this novel technique represents a potential surgical option for the treatment of wrist arthritis.
Footnotes
Conflicts of Interests Dr. Culp is a consultant for SBi and for BioMet. Other authors have no conflict of interest to declare.
References
- 1.Cohen M S, Kozin S H. Degenerative arthritis of the wrist: proximal row carpectomy versus scaphoid excision and four-corner arthrodesis. J Hand Surg Am. 2001;26(1):94–104. doi: 10.1053/jhsu.2001.20160. [DOI] [PubMed] [Google Scholar]
- 2.Rettig M E, Raskin K B. Long-term assessment of proximal row carpectomy for chronic perilunate dislocations. J Hand Surg Am. 1999;24(6):1231–1236. doi: 10.1053/jhsu.1999.1231. [DOI] [PubMed] [Google Scholar]
- 3.Lumsden B C, Stone A, Engber W D. Treatment of advanced-stage Kienböck's disease with proximal row carpectomy: an average 15-year follow-up. J Hand Surg Am. 2008;33(4):493–502. doi: 10.1016/j.jhsa.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Bain G I, Watts A C. The outcome of scaphoid excision and four-corner arthrodesis for advanced carpal collapse at a minimum of ten years. J Hand Surg Am. 2010;35(5):719–2725. doi: 10.1016/j.jhsa.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Carlson J R, Simmons B P. Total wrist arthroplasty. J Am Acad Orthop Surg. 1998;6(5):308–315. doi: 10.5435/00124635-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Boyer J S, Adams B. Distal radius hemiarthroplasty combined with proximal row carpectomy: case report. Iowa Orthop J. 2010;30:168–173. [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer A K, Werner F W, Murphy D, Glisson R. Functional wrist motion: a biomechanical study. J Hand Surg Am. 1985;10(1):39–46. doi: 10.1016/s0363-5023(85)80246-x. [DOI] [PubMed] [Google Scholar]
- 8.Ryu J Y, Cooney W P III, Askew L J, An K N, Chao E Y. Functional ranges of motion of the wrist joint. J Hand Surg Am. 1991;16(3):409–419. doi: 10.1016/0363-5023(91)90006-w. [DOI] [PubMed] [Google Scholar]
- 9.Ferreres A, Lluch A, Del Valle M. Universal total wrist arthroplasty: midterm follow-up study. J Hand Surg Am. 2011;36(6):967–973. doi: 10.1016/j.jhsa.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Adey L, Ring D, Jupiter J B. Health status after total wrist arthrodesis for posttraumatic arthritis. J Hand Surg Am. 2005;30(5):932–936. doi: 10.1016/j.jhsa.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Field J, Herbert T J, Prosser R. Total wrist fusion. A functional assessment. J Hand Surg [Br] 1996;21(4):429–433. doi: 10.1016/s0266-7681(96)80039-8. [DOI] [PubMed] [Google Scholar]