Abstract
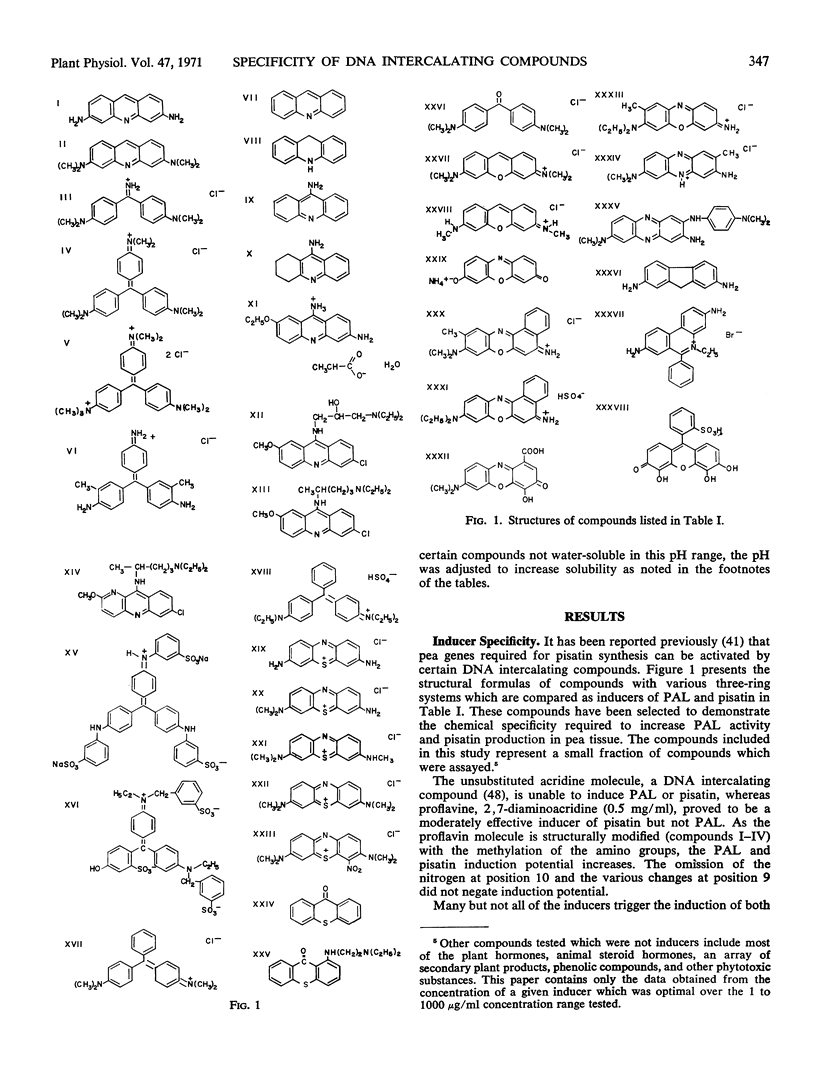
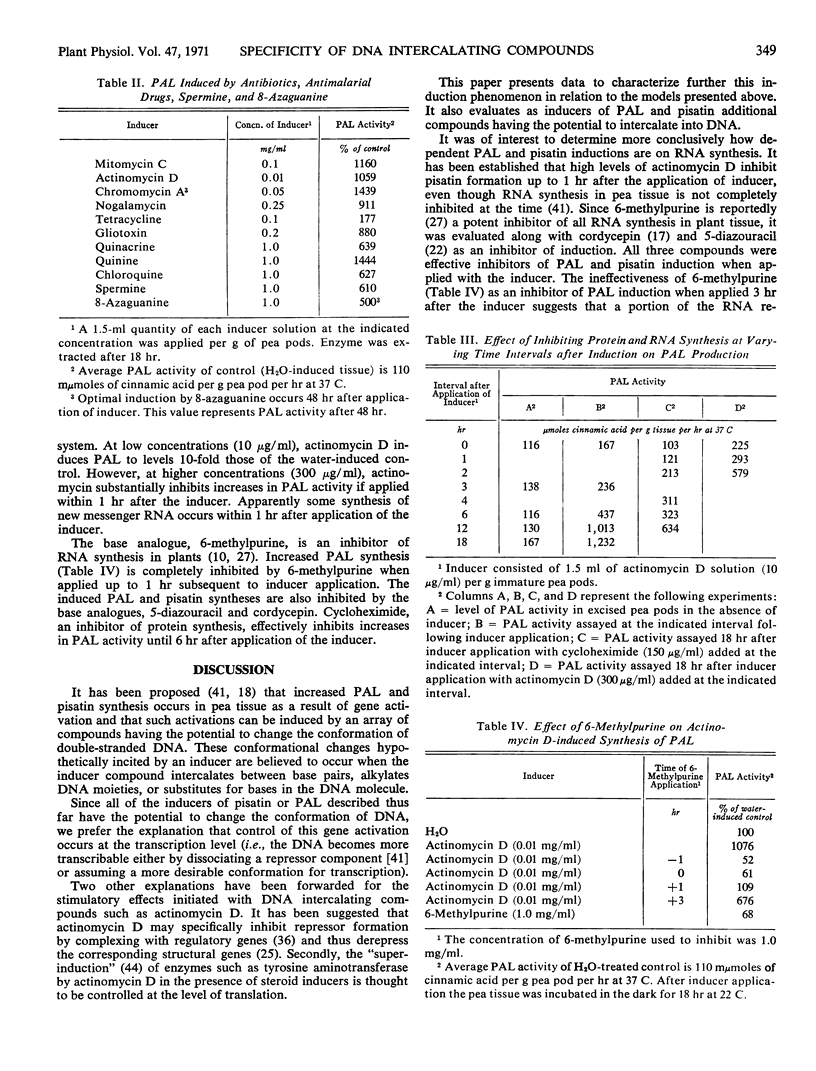
Compounds with planar triple ring systems such as acridine orange, 9-amino acridine, 9-amino-1,2,3,4-tetrahydroacridine (tacrine), 6,9-diamino-2-ethoxyacridine lactate monohydrate (DE-acridine), 6-chloro-9-(3′-diethylamino-2′-hydroxypropylamino) -2-methoxyacridine·2 HCl (CDM-acridine), quinacrine, 6-chloro-9-(4′-diethylamino-1′-methylbutylamino) -2-methoxy-1,10-diazaanthracene (CDM 1,10-diazaanthracene), thionine, azure A, methylene blue, and pyronine Y when applied to excised pea pods were potent inducers of phenylalanine ammonia lyase or of pisatin, or of both. Compounds with an array of structural variation around the planar three-ring system were tested for their ability to induce these responses in pea tissue. In general, dimethylamino, diethylamino, or amino substitutions at position 2 and 6 or an amino (with or without an aliphatic side chain) substitution at position 9 of the three-ring system augmented induction potential. Methyl green, methylene blue, 2,7-diaminofluorene, nile blue, neutral red, pyrogallol red, ethidium bromide, nogalamycin, quinine, chloroquine, spermine, 8-azaguanine, gliotoxin, chromomycin A3, actinomycin D, and mitomycin C were also potent inducers. The inhibition of phenylalanine ammonia lyase induction by the application of actinomycin D (300 micrograms per milliliter) or 6-methylpurine (1 milligram per milliliter) within 1 hour after inducer application indicated that newly synthesized RNA is necessary for induction. Phenylalanine ammonia lyase induction was also inhibited by cycloheximide (150 micrograms per milliliter).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. L., O'Brien R. L., Hahn F. E. DNA: reaction with chloroquine. Science. 1965 Sep 3;149(3688):1111–1113. doi: 10.1126/science.149.3688.1111. [DOI] [PubMed] [Google Scholar]
- Ambrose C. T. Regulation of the secondary antibody response in vitro. Enhancement by actinomycin D and inhibition by a macromolecular product of stimulated lymph node cultures. J Exp Med. 1969 Nov 1;130(5):1003–1029. doi: 10.1084/jem.130.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansfield F. J., Korbitz B. C., Davis H. L., Jr, Ramirez G. Triple drug therapy in testicular tumors. Cancer. 1969 Sep;24(3):442–446. doi: 10.1002/1097-0142(196909)24:3<442::aid-cncr2820240304>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Bhuyan B. K., Smith C. G. Differential interaction of nogalamycin with DNA of varying base composition. Proc Natl Acad Sci U S A. 1965 Aug;54(2):566–572. doi: 10.1073/pnas.54.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman R. Studies of the binding of ethidium bromide to transfer ribonucleic acid: absorption, fluorescence, ultracentrifugation and kinetic investigations. J Mol Biol. 1969 Dec 14;46(2):251–268. doi: 10.1016/0022-2836(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Burlington R. F., Maher J. T., Angelakos E. T. Effect of temperature on contractility of isolated atria from a hibernator and a non-hibernator. Life Sci. 1968 May 1;7(9):449–452. doi: 10.1016/0024-3205(68)90046-5. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., BARNETT L., BRENNER S., WATTS-TOBIN R. J. General nature of the genetic code for proteins. Nature. 1961 Dec 30;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Zech L., Modest E. J., Foley G. E., Wagh U., Simonsson E. Chemical differentiation with fluorescent alkylating agents in Vicia faba metaphase chromosomes. Exp Cell Res. 1969 Nov;58(1):128–140. doi: 10.1016/0014-4827(69)90123-2. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Zech L., Modest E. J., Foley G. E., Wagh U., Simonsson E. DNA-binding fluorochromes for the study of the organization of the metaphase nucleus. Exp Cell Res. 1969 Nov;58(1):141–152. doi: 10.1016/0014-4827(69)90124-4. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Hormonal control of enzyme synthesis: on the mode of action of gibberellic Acid and abscisin in aleurone layers of barley. Plant Physiol. 1967 Jul;42(7):1008–1016. doi: 10.1104/pp.42.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciak J., Hahn F. E. Quinacrine (atebrin): mode of action. Science. 1967 May 5;156(3775):655–656. doi: 10.1126/science.156.3775.655. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Waring M. J. Supercoiling of polyoma virus DNA measured by its interaction with ethidium bromide. J Mol Biol. 1967 Apr 14;25(1):23–30. doi: 10.1016/0022-2836(67)90276-8. [DOI] [PubMed] [Google Scholar]
- Fox C. L., Jr, Rappole B. W., Stanford W. Control of pseudomonas infection in burns by silver sulfadiazine. Surg Gynecol Obstet. 1969 May;128(5):1021–1026. [PubMed] [Google Scholar]
- Goldberg I. H. Mode of action of antibiotics. II. Drugs affecting nucleic acid and protein synthesis. Am J Med. 1965 Nov;39(5):722–752. doi: 10.1016/0002-9343(65)90094-x. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., FURTH J. J., MALAMY M., ALEXANDER M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1222–1230. doi: 10.1073/pnas.48.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger L. A., Schwochau M. E. Induction of phenylalanine ammonia lyase and pisatin in pea pods by poly-lysine, spermidine or histone fractions. Biochem Biophys Res Commun. 1970 Feb 20;38(4):683–691. doi: 10.1016/0006-291x(70)90635-2. [DOI] [PubMed] [Google Scholar]
- Hahn F. E., O'Brien R. L., Ciak J., Allison J. L., Olenick J. G. Studies on modes of action of chloroquine, quinacrine, and quinine and on chloroquine resistance. Mil Med. 1966 Sep;131(9 Suppl):1071–1089. [PubMed] [Google Scholar]
- Hunt D. E., Pittillo R. F. Antimicrobial evaluation of 5-diazouracil. Appl Microbiol. 1968 Nov;16(11):1792–1793. doi: 10.1128/am.16.11.1792-1793.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Key J. L., Shannon J. C. Enhancement by Auxin of Ribonucleic Acid Synthesis in Excised Soybean Hypocotyl Tissue. Plant Physiol. 1964 May;39(3):360–364. doi: 10.1104/pp.39.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey A. K., Hahn F. E. Berberine: complex with DNA. Science. 1969 Nov 7;166(3906):755–757. doi: 10.1126/science.166.3906.755. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Olenick J. G., Hahn F. E. Reactions of quinine, chloroquine, and quinacrine with DNA and their effects on the DNA and RNA polymerase reactions. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1511–1517. doi: 10.1073/pnas.55.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Terzaghi E., Streisinger G., Emrich J., Inouye M., Tsugita A. A frame-shift mutation involving the addition of two base pairs in the lysozyme gene of phage t4. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1692–1698. doi: 10.1073/pnas.56.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. THE DIFFERENTIAL EFFECT OF ACTINOMYCIN D ON THE BIOSYNTHESIS OF ENZYMES IN BACILLUS SUBTILIS AND BACILLUS CEREUS. Biochim Biophys Acta. 1963 Sep 17;76:80–93. [PubMed] [Google Scholar]
- Sarabhai A., Lamfrom H. Mechanism of proflavin mutagenesis. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1196–1197. doi: 10.1073/pnas.63.4.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L. H. Chemotherapy of the drug-resistant malarias. Annu Rev Microbiol. 1969;23:427–454. doi: 10.1146/annurev.mi.23.100169.002235. [DOI] [PubMed] [Google Scholar]
- Schwochau M. E., Hadwiger L. A. Regulation of gene expression by actinomycin D and other compounds which change the conformation of DNA. Arch Biochem Biophys. 1969 Oct;134(1):34–41. doi: 10.1016/0003-9861(69)90247-1. [DOI] [PubMed] [Google Scholar]
- Schwochau M. E., Hadwiger L. A. Stimulation of pisatin production in Pisum sativum by actinomycin D and other compounds. Arch Biochem Biophys. 1968 Aug;126(2):731–733. doi: 10.1016/0003-9861(68)90463-3. [DOI] [PubMed] [Google Scholar]
- Shapiro J. T., Leng M., Felsenfeld G. Deoxyribonucleic acid-polylysine complexes. Structure and nucleotide specificity. Biochemistry. 1969 Aug;8(8):3219–3232. doi: 10.1021/bi00836a014. [DOI] [PubMed] [Google Scholar]
- TABOR H. The protective effect of spermine and other polyamines against heat denaturation of deoxyribonucleic acid. Biochemistry. 1962 May 25;1:496–501. doi: 10.1021/bi00909a021. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Van DYDE K., Lantz C., Szustkiewicz C. Quinacrine: mechanisms of antimalarial action. Science. 1970 Jul 31;169(3944):492–493. [PubMed] [Google Scholar]
- Van Duuren B. L., Sivak A., Katz C., Melchionne S. Inhibition of tumor induction in two-stage carcinogenesis on mouse skin. Cancer Res. 1969 Apr;29(4):947–952. [PubMed] [Google Scholar]
- Ward D. C., Reich E., Goldberg I. H. Base specificity in the interaction of polynucleotides with antibiotic drugs. Science. 1965 Sep 10;149(3689):1259–1263. doi: 10.1126/science.149.3689.1259. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Drugs which affect the structure and function of DNA. Nature. 1968 Sep 28;219(5161):1320–1325. doi: 10.1038/2191320a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D. Actinomycin Binding to DNA: Inability of a DNA Containing Guanine To Bind Actinomycin D. Science. 1969 Jul 4;165(3888):75–76. doi: 10.1126/science.165.3888.75. [DOI] [PubMed] [Google Scholar]



