Abstract
Autophagy, a highly conserved cellular mechanism wherein various cellular components are broken down and recycled through lysosomes, occurs constitutively in the heart and may serve as a cardioprotective mechanism in some situations. It has been implicated in the development of heart failure and is up-regulated following ischemia-reperfusion injury. Autophagic flux, a measure of autophagic vesicle formation and clearance, is an important measurement in evaluating the efficacy of the pathway, however, tools to measure flux in vivo have been limited. Here, we describe the use of monodansylcadaverine (MDC) and the lysosomotropic drug chloroquine to measure autophagic flux in in vivo model systems, specifically focusing on its use in the myocardium. This method allows determination of flux as a more precise measure of autophagic activity in vivo much in the same way that Bafilomycin A1 is used to measure flux in cell culture. MDC injected 1 h before sacrifice, colocalizes with mCherry-LC3 puncta, validating its use as a marker of autophagosomes. This chapter provides a method to measure autophagic flux in vivo in both transgenic and nontransgenic animals, using MDC and chloroquine, and in addition describes the mCherry-LC3 mouse and the advantages of this animal model in the study of cardiac autophagy. Additionally, we review several methods for inducing autophagy in the myocardium under pathological conditions such as myocardial infarction, ischemia/ reperfusion, pressure overloading, and nutrient starvation.
1. Introduction
Autophagy occurs constitutively in the normal myocardium and is up-regulated after ischemia reperfusion (Decker et al., 1980; Sybers et al., 1976). Since the early electron microscopy observations of autophagy more than 40 years ago, relatively little work has been done in the heart, largely due to the lack of suitable molecular reagents to facilitate mechanistic studies. However, a recent proteomic study reveals the up-regulation of autophagic proteins in chronically ischemic myocardium (Yan et al., 2005), and autophagy is up-regulated in the hearts of mice subjected to starvation (Pattingre et al., 2005), thus raising interest in the topic among cardiovascular investigators. Despite growing interest in the field, studies have been hampered by the lack of available molecular tools to investigate autophagy especially in vivo.
LC3, the mammalian homolog of yeast Atg8, is regularly used as a marker for autophagy both in Western blotting and as a fluorescently conjugated marker of autophagic vesicle (AV) formation. A transgenic mouse expressing GFP-LC3, created in Japan by Mizushima's group, has been used to demonstrate the occurrence of autophagy in the heart and provided great insight into the role of autophagy in vivo after starvation (Mizushima et al., 2005). Our publications and preliminary data with cardiac derived HL-1 cells subjected to simulated ischemia/reperfusion (sI/R) demonstrate that autophagy serves a protective role in ischemia/ reperfusion (Hamacher-Brady et al., 2006). We believe this is a salvage response, because suppression of autophagy increases cell death. Whereas autophagy plays an important role in facilitating replacement of damaged organelles and promoting survival during nutrient deprivation, excessive autophagy can result in caspase-independent cell death. Therefore in the context of the heart, low-level autophagy may be beneficial, whereas excessive autophagy may be deleterious. Moreover, the up-regulation of autophagy in the context of compensated cardiac hypertrophy may contribute to the transition to failure (Hein et al.,, 2003). To further investigate cardiac autophagy in vivo, we produced a line of transgenic mice expressing mCherry-tagged LC3 (mCherry-LC3) under the control of the cardiomyocyte-specific alpha-myosin heavy chain promoter. Here we describe the production of the mCherry-LC3 mouse line and its advantages in studying cardiac autophagy.
Despite the complex nature of cardiac autophagy, it is clear that data based on a snapshot of the cell at a given time are insufficient to accurately understand the behavior of autophagy. Static images of cells with numerous AVs could reflect increased autophagy but could also indicate reduced autophagic flux due to impaired fusion with lysosomes. A more thorough examination of the process requires researchers to measure autophagic flux directly. Autophagic flux is the measurement of the rate of autophagosome formation and clearance through the pathway (Fig. 16.1). Bafilomycin A1, a potent inhibitor of vacuolar H+-ATPase, is regularly used in the measurement of flux by preventing downstream clearance autophagosomes (Yamamoto et al., 1998). By inhibiting vacuolar acidification, Bafilomycin A1 results in the accumulation of autophagosomes by possibly preventing lysosome-autophagosome fusion, or by blocking intralysosomal degradation, which is dependent on lowered intralysosomal pH. A comparison of steady-state autophagosome levels with accumulated autophagosomes following Bafilomycin A1 treatment provides a good idea of the rate of production and clearance in the pathway. Differences between steady state and accumulated AV levels can be interpreted in several different ways as lower AV levels may indicate high turnover or decreased AV production. Alternatively, less significant differences in steady state and accumulated AV levels could denote that autophagy is unaffected, inhibited at both production and clearance stages or AV production is increased combined with a high rate of clearance.
Figure 16.1.
Conceptual representation of autophagic flux. Autophagic flux, the rate at which material is cleared by autophagy, is demonstrated here by the analogy of cars in a traffic jam. Lysosomal inhibitors such as Bafilomycin A1 and chloroquine act as blockades, resulting in the backup of vesicles as they are processed through the autophagic pathway. Right column: GFP-LC3-expressing cardiac-derived HL-1 cells subjected to 4-h serum starvation incubated with and without Bafilomycin A1.
Whereas Bafilomycin A1 provides a tool for studying autophagic flux in cell culture, it is costly and unsuitable for studying flux in animals. We present here a method using chloroquine to measure flux in vivo. Chloroquine is an anti-inflammatory drug that has been used in the treatment of malaria for more than 60 years (O'Neil et al., 1998). It is believed to work by raising lysosomal pH (Poole and Ohkuma, 1981; Kawai et al., 2007) and thereby inhibiting lysosomal activity (Ohkuma and Poole, 1978; Sewell et al., 1983). Because autophagosome-lysosome fusion is pH-dependent, the alkalinizing effects of chloroquine on lysosomes inhibit fusion and hydrolase activity, thus preventing AV clearance.
Additionally, we provide two methods for evaluating autophagy using monodansylcadaverine (MDC) and Alexa Fluor 488 Cadaverine (Invitrogen), which is known to label acidic endosomes, lysosomes and autophagosomes (Munafo and Colombo, 2001; Yan et al., 2007) (also see the chapter by Vázquez and Colombo in this volume). MDC labeling in vivo colocalizes with many mCherry-LC3 puncta and the number of labeled structures increases in parallel with induction of autophagy, validating its potential as a marker of autophagy in vivo (Iwai-Kanai et al., 2008). Our plate-based assay provides a quantitative measurement of autophagy using isolated autophagosomes from both fresh and frozen tissue samples.
We begin with an overview of common in vivo models of autophagy and methods to induce autophagy in the myocardium including nutrient starvation, ischemia/reperfusion, chronic ischemia, myocardial infarction and pressure overload by transverse aortic constriction (TAC). Specific precautions to studying autophagy in the heart are noted within each method.
2. In vivo Models of Autophagy in the Myocardium
2.1. Nutrient starvation
The heart is among the organs where rapid (within 30 min) and strong induction of autophagy is observed during the neonatal starvation period in mice (Kuma et al., 2004). In adult mice, autophagy is induced in the heart by food starvation (Mizushima et al., 2004; Pattingre et al., 2005). Induction of autophagic vacuoles is greater in the heart than in the liver, in the rat model of calorie restriction (Wohlgemuth et al., 2007). For induction of autophagy in the adult mouse heart, 48 h of food starvation has been commonly used (Mizushima et al., 2004; Pattingre et al., 2005). During starvation, mice have free access to water and their temperature and blood pressure should be checked periodically.
2.2. Ischemia/reperfusion
Both ischemia and ischemia followed by reperfusion (I/R) induce accumulation of autophagosomes in the heart. Decker et al report that 40-min ischemia causes up-regulation of autophagy and that subsequent reperfusion induces a drastic enhancement of autophagy in Langendorff perfused rabbit hearts (Decker and Wildenthal, 1980). In this model, increases in autophagy correlate with recovery of cardiac function and salvage of myocardium after I/R. Interestingly, 60-min ischemia causes lysosomal dysfunction during reperfusion, suggesting that extended ischemia may impair autophagy (Decker and Wildenthal, 1980). In another study, I/R was applied to the mouse heart by transiently occluding the coronary artery in situ. In this model, autophagy is induced by ischemia alone and it is further enhanced by reperfusion (Matsui et al., 2007). I/R experiments can be performed in two forms. One uses the ex vivo isolated perfused heart (Langendorff) preparation and the other the in situ coronary artery ligation. The effect of global I/R on autophagy can be studied using the Langendorff preparation without need for an extensive surgical setup (Decker and Wildenthal 1980; Hamacher-Brady et al., 2007). Changes in high energy phosphates and glycolysitic flux, as well as left ventricular (LV) cardiac function, can be monitored relatively easily during I/R (Luptak et al., 2007). On the other hand, in situ coronary artery ligation in experimental animals induces focal ischemia, which mimics human pathological conditions. Experiments can be conducted even in conscious animals after initial instrumentation, which allows one to obtain continuous monitoring of LV cardiac function during I/R. The technique of in situ I/R can be applied to genetically altered mouse models to monitor the extent of autophagy or to evaluate the functional significance of autophagy during I/R. In the following, methods to apply I/R to the mouse heart in situ are discussed.
2.2.1. Ischemia/Reperfusion
The extent of autophagosome accumulation in the myocardium after I/R is evaluated most conveniently using transgenic mice harboring GFP-LC3 (GFP-LC3 mice) made by Dr. N. Mizushima (C57BL/6J background) (Mizushima et al., 2004) or the mCherry-LC3 mouse as described later. In order to evaluate the effect of genetic interventions upon autophagy in the heart, genetically altered mice of interest can be crossed with the GFP-LC3 mice. It is important to use mice with a homogeneous genetic background. If cross breeding of mice with different genetic backgrounds is needed, sufficient generations of backcross should be conducted. Pathogen-free mice are housed in a temperature-controlled environment with a 12-h light/dark cycle where they receive food and water ad libitum.
Anesthetize mice by intraperitoneal injection of pentobarbital sodium (60 mg/kg). Use a rodent ventilator (model 683; Harvard Apparatus) with 65% oxygen during the surgical procedure.
Keep the mice warm using heat lamps and heating pads. Monitor the rectal temperature and maintain it between 36.8 °C and 37.2 °C, which is essential because the severity of ischemia depends in part on the rate of metabolism, which is temperature sensitive. The chest is opened by a horizontal incision through the muscle between the ribs at the third intercostal space.
Apply ischemia by ligating the anterior descending branch of the left coronary artery (LAD) using an 8–0 nylon suture, which is threaded through a silicon tubing (1-mm OD) propylene tube to form a snare on top of the LAD, 2 mm below the border between left atrium and LV. Confirm regional ischemia by ST-T changes in an electrocardiogram. The susceptibility of the mouse heart to ischemic injury is different from strain to strain. For example, the heart of FVB mice is more resistant to ischemia than that of C57BL/6 mice. Thus, longer ischemia is needed in FVB mice to achieve ischemic injury comparable to that in C57BL/6 mice. In order to observe induction of autophagy by ischemia alone, we use 30–45 min of ischemia for FVB mice and 20 min for C57BL/6. Longer ischemia may inhibit autophagosome formation since ATP-dependent steps are involved.
After transient occlusion of the coronary artery, remove silicon tubing to achieve reperfusion. An increase in autophagosome formation in the previously ischemic area is observed as early as 20 min after ischemia and it is further enhanced 2 h after reperfusion in C57BL/6 mice (Matsui et al., 2007).
- To observe LC3 dots representing autophagosomes, harvest the heart using the following method:
- After dissection of the heart, wash the heart several times with PBS.
- Cut the heart into several slices and fix them with 4% paraformaldehyde at 4 °C overnight.
- Replace 4% paraformaldehyde with 15% sucrose solution and keep the sample at 4 °C for at least 4–5 h (or overnight).
- Replace the 15% sucrose with a 30% sucrose solution and keep the sample at 4 °C overnight.
- Place the sample in the Tissue-Tek (Sakura Finetek USA, CA). Overlay the sample with Tissue-Tek O.C.T. Compound (Sakura Finetek USA, CA) and place it in ethanol-dry ice.
- Keep the sample at −80 °C.
To determine whether certain interventions increase or decrease the extent of myocardial damage, set the duration of ischemia to induce myocardial infarction with intermediate sizes, such as 20%–50% of the area at risk. It is difficult to see the protective effect of interventions when the infarction size is less than 10% without interventions. Conversely, it is also difficult to see the detrimental effect of interventions when the infraction size is greater than 60% without intervention. We have shown previously that heterozygous deletion of beclin 1 (C57BL/6 background) reduces the size of myocardial infarction after I/R from 45 to 20% (Matsui Y et al., 2007). In this case,weapplied 20 min of ischemia and the size of myocardial infarction was determined 24 h after reperfusion.
After I/R, reanaesthetize and intubate the animals, and open the chest. Arrest the heart at the diastolic phase by injecting 0.5 mL of 100 mM KCl. Canulate the ascending aorta and perfuse the heart with saline to wash out blood. Occlude the LAD again with the same suture, which has been left at the site of the ligation. To demarcate the ischemic area at risk (AAR), perfuse 1% Evans blue dye (Sigma Aldrich, 206334) into the aorta and coronary arteries. Excise the heart and slice the LV into 1-mm thick cross sections. Incubate the heart sections with a 1% triphenyltetrazolium chloride (Sigma Aldrich, T8877) solution at 37 °C for 10 min. Measure the infarct area (pale), the AAR (not blue), and the total LV area from both sides of each section using Adobe Photoshop (Adobe Systems), and average the values obtained. Multiply the percentage of area of infarction and AAR of each section by the weight of the section and then obtain the total from all sections. Express AAR/LV and infarct area/AAR area as a percentage.
2.3. Chronic ischemia
Although the myocardium under chronic hypoxia exhibits reduced contractility, the condition termed myocardial hibernation, it can show a significant recovery when hypoxia is eased. Chronic hypoxia in the myocardium not only up-regulates a series of cell survival mechanisms but induces autophagy in the myocardium (Yan et al., 2005; May et al., 2008). A previous study shows an inverse correlation between the occurrence of autophagy and apoptosis in the hibernating myocardium. Thus, autophagy in the myocardium caused by chronic hypoxia may contribute to survival of cardiac myocytes. Thus far, several animal models of myocardial hibernation have been reported. One is a large animal model, in which 6 episodes of repetitive reduction in coronary flow are applied to instrumented conscious pigs (Yan et al., 2005) The other is transgenic mice with conditional expression of a VEGF-sequestrating soluble receptor, which allows tetracycline-regulated VEGF blockade and fully reversible induction of the globally hypo-perfused heart with significant reduced contractility, mimicking the hibernating myocardium (May et al., 2008). Autophagy is induced by chronic ischemia in both pig and mouse models of hibernation. The pig model is useful for translational research because of the similarity in the anatomy of coronary arteries between pigs and humans. The mouse model is useful for mechanistic studies because they can be crossed with other genetically altered mouse models of autophagy.
2.4. Myocardial infarction
In humans, occlusion of the coronary artery by plaque rupture causes myocardial infarction, which alone exhibits a very high mortality. Even though patients manage to survive the acute event, the area of the infarction is replaced with a scar and the heart undergoes structural and functional remodeling, which eventually leads to cardiac dilation and heart failure. Myocardial infarction triggers inflammation and a tissue remodeling process through up-regulation of cytokines, proteases, and lysosomal enzymes. Autophagy is observed in the surviving myocardium after myocardial infarction.
To create myocardial infarction in mice, the same procedures as those for I/R can be used except that the coronary artery is permanently ligated. The size of the myocardial infarction can be determined by TTC staining at early stages (up to 3–4 days), whereas the proportion of the MI area/total left ventricle can be speculated by measuring the proportion of the circumference occupied by a thin scar visualized by Masson Trichrome staining (Odashima et al., 2007). Rupture of the LV wall due to vulnerability of the infarction area tends to occur at the acute phase, whereas LV dysfunction due to cardiac remodeling gradually develops after 2–4 weeks.
2.5. Pressure overload
Whether autophagy is stimulated in the heart under pathologically relevant stresses other than ischemia and, if so, whether autophagy is protective or detrimental in the failing heart are important issues. Transverse aortic constriction (TAC) is one of the most commonly used methods to induce pathological hypertrophy and heart failure by mimicking increased afterload caused by elevated blood pressure (Sadoshima et al., 2002). In one report, accumulation of autophagosomes is observed as early as 24 h after TAC and remains elevated at least 2 weeks (Zhu et al., 2007). In another report, autophagy is suppressed at 1 week after TAC, but up-regulated together with LV dysfunction at 4 weeks (Nakai et al., 2007).
2.5.1. TAC
Anesthetize mice at 3–6 months of age by intraperitoneal injection of pentobarbital sodium (65 μmg/kg). Intubate mice and ventilate them with a tidal volume of 0.2 ml and a respiratory rate of 110 breaths per minute using a rodent ventilator (model 683; Harvard Apparatus) with 65% oxygen during the surgical procedure.
Place mice in the supine position. Under a dissecting microscope, open the left side of the chest at the second intercostal space and expose the transverse aorta. Place a 7–0 prolene ligature around the aorta between the innominate and the left carotid artery. Place a 27-gauge needle on the aorta and remove after the ligature is tied. Use needles with a smaller diameter, such as a 28-gauge needle, to apply greater levels of pressure overload.
Close the chest in layers and maintain mechanical ventilation until the mice are able to breathe spontaneously. After extubation, keep the mice in an oxygenated warm chamber and monitor them until they recover from anesthesia.
Cardiac hypertrophy is developed within a week. If the purpose of the experiment is to induce heart failure, TAC is applied for more than 4 weeks in FVB mice, whereas 2 weeks are sufficient in C57BL/6 mice. To assess the severity of aortic constriction, measure the pressure gradient across the constriction, using two high-fidelity catheter tip transducers (1.4F; Millar Instruments, Houston). Insert one into the right carotid artery and the other into the right femoral artery. Advance them carefully to the ascending aorta and the abdominal aorta, respectively, where pressures are measured simultaneously. When the effect of the interventions upon cardiac responses, such as induction of autophagy, is compared, it is important to confirm that equal levels of pressure overload are applied to each animal. When some interventions facilitate the progression of heart failure, the pressure gradient measured after 2–4 weeks of TAC could be lower than that measured just after imposition of TAC due to the reduced contractility of the LV. In this case, the initial levels of pressure gradient could be assessed in a separate group of mice at earlier stages before the mice develop cardiac dysfunction.
2.6. Genetically altered mouse models
The functional significance of autophagy in the heart under various patho-physiological conditions can be studied in genetically modified mouse models where autophagy is impaired. These include beclin 1+/− mice (Qu et al., 2003), cardiac specific atg5−/− mice (Nakai et al., 2007), and atg7−/− mice (Komatsu et al., 2005). Beclin 1+/− mice have been used to examine the role of autophagy in mediating survival and death of cardiac myocytes in response to I/R (Matsui et al., 2007). Conditional deletion of atg5 has been used to examine the role of autophagy during pressure overload (Nakai et al., 2007).
2.7. Generation of mCherry-LC3 mouse line
2.7.1. Construction of plasmids
A 1.2-kbp DNA fragment containing rat LC3 cDNA fused to mCherry at the N-terminus (mCherry-LC3) was excised from mCherry-C1-LC3, originally cloned by replacement of EGFP with mCherry in pEGFP-C1-LC3 (gift from Tamotsu Yoshimori, Osaka, Japan) and inserted into the murine α-myosin heavy chain promoter expression vector C26-JM (Baines CP et al., 2005) to generate mCherry-LC3-mHC (Fig. 16.2).
Figure 16.2.
Map of mCherry-LC3 expression vector. mCherry-LC3 inserted into the cardiac-specific α-mHC promoter-driven expression vector proximal to the human growth hormone polyadenylation signal.
Cardiac-specific mCherry-LC3 transgenic mice were created in the FVB/NJ strain (Jackson Laboratories, Sacramento, CA) by pronuclear injection of murine alpha myosin heavy chain promoter-driven mCherry-LC3 transgene (mCherry-LC3-mHC) located proximal to the human growth hormone polyadenylation signal. Mice were screened for incorporation of the transgene by PCR using primers to the Human Growth Hormone poly A sequence (5′-GTCTGACTAGGTGTCCTTCT-3′ and 5′-CGTCCTCCTGCTGGTATAG-3′). The PCR is programmed for: 96 °C for 25 s, 56 ′C for 25 s, 72 ′C for 60 s, and repeats for a total of 30 cycles. The Platinum PCR Super-MIX (Invitrogen, Carlsbad, CA) was used according to manufacturer's instructions and positive DNA samples produce a 410-bp product.
Positive mice were crossed with wild-type FVB/NJ mice and maintained as heterozygotes for the mCherry-LC3 transgene. Production of the mCherry-LC3 line resulted in generation of three founder lines: J8138, J8139, J8295. One line, J8139, was chosen to maintain for studies due to its low expression level and reduced fluorescent protein aggregation (available upon request to Dr. Roberta Gottlieb, San Diego State University). mCherry-LC3 mice accurately reflect induction of autophagy under conditions commonly known to up-regulate the process (Fig. 16.3).
Figure 16.3.

mCherry-LC3 transgenic mouse heart sections. Representative images of cardiac tissue cryosections prepared from mCherry-LC3 overexpressing mice following 30-min ischemia and 2-h reperfusion (B) or no ischemic period (A). Increased fluorescent mCherry-LC3 puncta reflect induction of autophagy following IR. (Bar 50 μm)
2.7.2. Tissue processing and scoring in mCherry-LC3 mice
Excise the heart from pentobarbital anesthetized animals.
Rinse the heart in ice-cold 1× phosphate-buffered saline (PBS), pH 7.4. Rinse in fresh PBS until the heart is cleared of red blood cells.
Embed the heart in Tissue Tek OCT compound (Fisher Sci, Pittsburg, PA) and freeze in liquid nitrogen.
Store at −80 °C until ready to section.
Prepare tissue section slides on a cryostat using Fisherbrand Superfrost Plus glass slides (Fisher Sci).
Rinse sections in 1× PBS 5 min to remove the OCT compound.
Fix sections with 4% Formaldehyde (Ted Pella)/1× PBS for 15 min, covered at room temperature.
Immerse slide in 1× PBS to wash.
Dilute Hoechst 33342 (Invitrogen, H3570) 1:1000 in 1× PBS.
Cover tissue section with this solution using 100 μL (or as needed) and incubate covered for 30 min.
Rinse slide in 1× PBS and mount coverslip (Fisherbrand Premium cover glass, 22×50mm) with Aquapolymount (Polysciences, Warrington, PA) or other mounting media.
Image sections using 4× air objective lens on a fluorescence microscope (Nikon TE300, Nikon, Melville, NY) equipped with a 4× lens and cooled CCD camera (Orca-ER, Hamamatsu, Bridgewater, NJ) and automated excitation emission filter wheels controlled by a LAMBDA 10–2 (Sutter instruments) operated by MetaMorph Version 6.2r (Molecular Devices). Select the appropriate dichroic filters for visualization: DAPI (D360/40×) for Hoechst 33342 and MDC staining, and Texas Red (D560/40×) for mCherry-LC3. Quickly focus on the sample by eye to prevent bleaching of the fluorescent signal.
Capture images of both mCherry-LC3 and Hoechst staining of the identical optical fields. Fluorescent light is collected via a polychromic beam splitter (61002bs) and an emission filter for DAPI (D460/50m) or Texas Red (D630/60m). All filters are from Chroma Technology group (Rockingham, VT).
Score number of AVs per cell by counting total Hoechst-positive nuclei and mCherry-LC3-positive dots. The ratio of AVs/cell gives a measurement of autophagic activity.
Note: Alternatively to quantify the autophagic flux in vivo, the percentage of surface area covered by mCherry-LC3 fluorescence can be measured using ImageJ software (http://rsb.info.nih.gov/ij) (see Fig. 16.4 for example). However, users should be cautious of this method in instances where there are numerous fluorescent aggregates that may not represent true mCherry-LC3-positive autophagosomes but rather protein aggregates. In these cases, it is preferential to manually count AV/cell ratio as described.
Figure 16.4.
Flux in mCherry-LC3 mice treated with rapamycin. Cryosections of mCherry-LC3 mice injected with rapamycin +/− chloroquine (A). Quantification of punctate mCherry fluorescence in tissue sections (B). (Bars 500 μm and 100 μm, respectively) Reprinted from Iwai-Kanai et al. 2008, with permission from Landes Bioscience.
2.8. Chloroquine method
Weigh mice to determine dosage.
Prepare 100 μl chloroquine solution (EMD Bioscience, San Diego, CA) in sterile saline to administer at 10 μg/kg (★★2 ng/μl for average 20 g mouse).
Inject I.P. 100 μl solution using {1/2}-cc, 31-gauge BD Ultra fine II insulin syringe and needle (Becton Dickinson, Franklin Lakes, NJ). (★★This step can be done in conjunction with additional experimental treatments).
Wait a minimum of 2 h.
Sacrifice animals and harvest tissue as described previously.
Analyze tissue samples for autophagy (Fig. 16.4).
2.9. MDC method
Weigh animals to determine correct dosage.
Prepare 100 μl of MDC solution (Sigma Aldrich, St. Loius, MO, 30532) in sterile saline to administer at 1.5 mg/kg (★★0.3 μg/ml for average 20-g mouse).
Inject I.P. 100 μl solution using {1/2}-cc, 31-gauge BD Ultra fine II insulin syringe and needle (Becton Dickinson). (★★This step can be done in conjunction with additional experimental treatments).
Wait a minimum of 1 h.
Sacrifice animals and harvest tissue as described previously.
Analyze tissue samples for autophagy (Fig. 16.5). MDC excitation/emission is 365/525 nm respectively.
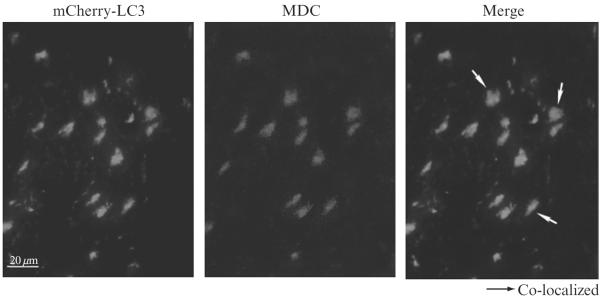
Figure 16.5.
Monodansylcadaverine (MDC) fluorescence in mCherry-LC3 mice. mCherry-LC3 transgenic heart tissue (after rapamycin stimulation and injection with monodansylcadaverine) showing colocalization. (Bar 20 μm.) Reprinted from Iwai-Kanai et al. 2008, with permission from Landes Bioscience.
Note: MDC is best visualized with a filter equipped for DAPI staining, however, its fluorescence diminishes quickly so great care must be taken to protect samples from light and to evaluate immediately after processing. Tissue fixation also reduces signal intensity so it is recommended not to process samples for fixation if possible.
2.10. Quantitative cadaverine plate reader assay
Mince 1- to 5-mm3 tissue sample in 1–2 mL of homogenization buffer in a 35-mm dish (adjust the volume depending on the starting tissue size).
Polytron at half speed, 5 s, on ice in a 15-mL round bottom polypropylene tube.
Spin out the nuclei and heavy membranes at 1000×g, 5 min, at 4 °C in a 15-mL Falcon tube.
Move the postnuclear supernatant fraction into a 1.5-mL microcentrifuge tube.
Add Alexa Fluor 488 Cadaverine (Invitrogen, A-30676) to a final concentration of 25 μM from a 5 mM stock.
Incubate on ice 10 min protected from light.
Centrifuge the sample at 20,000×g, 20 min, at 4 °C.
Aspirate the supernatant fraction and rinse the pellet fraction with 1 mL of cold resuspension buffer (see subsequently) twice.
Completely resuspend the pellet in 350 μL of resuspension buffer, pipetting well.
Add 100 μL per well in triplicate to a black 96-well plate (Corning, 3915).
Read on a fluorescence plate reader at excitation/emission 495/519 nm.
Subtract the readings from wells blanked with resuspension buffer alone.
Use the remaining sample to quantify the protein concentration with Coomassie Plus Better Bradford Reagent per the manufacturer's instructions (Thermo Fisher, 23238).
Calculate the results as relative fluorescent units (RFUs) per mg of protein.
- Homogenization Buffer:
- 1g of sucrose
- 2mL of 100 mM Na2EDTA
- 0.477 g of Hepes free acid
- Bring volume up to 200 mL with distilled H2O, pH 7.0
- Resuspension Buffer:
- 1.044 g of (140 mM) KCl
- 0.203 g of (10 mM) MgCl2
- 0.208 g of (10 mM) MOPS, pH 7.4
- 68 mg of (5 mM) KH2PO4
- 38 mg of (1 mM) EGTA
- Add fresh protease inhibitors to aliquot of each prior to use
3. Discussion
Microtubule-associated protein 1 light chain 3 (LC3), an 18-kDa mammalian homolog of autophagy-related protein 8 (Atg8) in yeast, is processed and conjugated to the nascent autophagosome membrane at the initiation of autophagy (Kabeya et al., 2000). A major obstacle to the study of autophagy in vivo is the difficulty of quantifying autophagosomes in tissue. This is greatly aided by the introduction of the transgenic mouse expressing GFP-LC3 in all tissues (Mizushima et al., 2004). However, due to the difficulty of obtaining this mouse from Japan, we created our own transgenic line, in which the red fluorescent protein, mCherry, is fused to LC3 and driven by the alpha-myosin heavy chain promoter for cardiac-restricted expression (Iwai-Kanai et al., 2008). For studies of cardiac autophagy, this eliminates the potentially confusing contribution of endothelial cells and fibroblasts present in numbers equal to cardiomyocytes. The use of mCherry-LC3 offers several advantages over GFP-LC3: it retains fluorescence even in the acidic environment of the lysosome, and there is very little red autofluorescence background. Additionally it will be possible to cross these mice with others expressing GFP-tagged proteins. Our characterization of the αMHC-targeted mCherry-LC3 mice indicates no apparent effects on cardiac function and the mCherry-LC3 reports autophagy as expected. Our comparisons of wild-type and mCherry-LC3 hearts thus far indicate that the transgene does not affect autophagy and functions as a reliable reporter of autophagosome formation (and accumulation).
Furthermore, this mouse model provides a rich source of fluorescently labeled autophagosomes that may be useful in future biochemical analysis requiring concentrated, pure AV preparations. For instance, we are currently developing protocols for the isolation and characterization of autophagosomes sorted by flow cytometry (unpublished data). This technique enables us to characterize associated proteins under various conditions such as starvation and organelle-targeted insults (e.g., rotenone) in a pure preparation of isolated AVs and most importantly provides quantitative data on particle numbers.
As discussed, increased numbers of autophagosomes do not necessarily mean increased autophagic flux, as autophagosomes may accumulate if they are not cleared through lysosomal degradation. To address this, we have established a method to assess flux in vivo using chloroquine in place of Bafilomycin A1 as presented here. Studies in cell culture indicated that Bafilomycin A1 and chloroquine were equally effective and that adding inhibitors of lysosomal proteases was not necessary (data not shown). Accordingly, we injected chloroquine i.p. into mice to assess effects on autophagy in vivo. In transgenic mice expressing mCherry-LC3, rapamycin administration caused an increase in the abundance of mCherry-LC3-labeled AVs in the myocardium (Fig. 16.4). This was further enhanced by the co-administration of chloroquine, indicating that the increase in AVs after rapamycin administration was due to increased flux, not diminished clearance. These findings clearly indicate that chloroquine is useful in evaluation of autophagic flux in vivo. Moreover, these studies show that chloroquine can be used to suppress the late phase of autophagy, which may be of therapeutic value in certain conditions.
Whereas both the GFP-LC3 mice and our mCherry-LC3 mice provide a great advantage in the study of autophagy, we also wanted to develop a method to measure autophagy in nontransgenic mice that can be more widely used and does not require maintenance of an additional colony. Monodansylcadaverine has been used to detect autophagosomes, although it has been criticized for being nonspecific. We assessed colocalization of MDC with mCherry-LC3 in mouse hearts, and found excellent colocalization, and that MDC labeling increased in parallel with mCherry-LC3 puncta (Fig. 16.5). Under our conditions, MDC represents a valid marker of autophagosomes. Whereas GFP-LC3 fluorescence is lost in the acidic environment of the lysosome, mCherry fluorescence is stable at an acidic pH allowing detection of both early and late autophagosomes (Kimura et al., 2007). Thus it is conceivable that the colocalization of MDC with many (but not all) LC3-mCherry puncta is an indication of the number of autolysosomes. If true, MDC would represent an accurate indicator of flux, as it would only label autophagosomes that have fused with lysosomes. However, MDC is also suggested to incorporate into membranes based on lipid characteristics independent of pH (Neimann et al., 2000). If the incorporation is related to the double-membrane structure of the autophagosome, then MDC incorporation would be expected even when chloroquine is used to block autophagosome-lysosome fusion, as we have observed. In further validation studies, we have found that this method is suitable for nontransgenic animals and potentially other tissue types and therefore will advance the study of autophagy. In addition, we have investigated the efficacy of alternative fluorescently labeled cadaverine compounds such as Alexa Fluor 488 Cadaverine and Cadaverine Texas Red and provide here a completely novel quantitative assay for examining autophagy using isolated autophagosomes (manuscript in preparation). These innovative methods provide necessary tools to advance the study and understanding of cardiac autophagy in animal models.
ACKNOWLEDGMENTS
This work was funded by San Diego State Research Foundation and grants from NIH/NHLBI P01 HL08557701-02, NIH/NHLBI F31 HL091723-01 and NIH/NIA R01 AG33283. Mice were treated in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the Animal Care Committee of the San Diego State University in San Diego, CA.
REFERENCES
- Decker RS, Poole AR, Crie JS, et al. Lysosomal alterations in hypoxic and reoxygenated hearts. II. Immunohistochemical and biochemical changes in cathepsin D. Am. J. Pathol. 1980;98(2):445–456. [PMC free article] [PubMed] [Google Scholar]
- Sybers HD, Ingwall J, DeLuca M. Autophagy in cardiac myocytes. Recent Adv. Stud. Cardiac Struct. Metab. 1976;12:453–463. [PubMed] [Google Scholar]
- Yan L, Vatner DE, Kim SJ, et al. Autophagy in chronically ischemic myocardium. Proc. Natl. Acad. Sci. USA. 2005;102(39):13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, et al. Bcl-2 anti-apoptotic proteins inhibit beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, et al. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophago-some marker. Mol. Biol. Cell. 2004;15(3):1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J. Biol. Chem. 2006;281(40):29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- Hein S, Arnon E, Kostin S, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: Structural deterioration and compensatory mechanisms. Circulation. 2003;107(7):984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, et al. Bafilomycin A1 prevents maturation of autophagic vescicles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 1998;23(1):33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- O'neil PM, Bray PG, Hawley SR, Ward SA, Park BK. 4-Amnoquino-lines-past, present and future: A chemical perspective. Pharmacol. Ther. 1998;77:29–58. doi: 10.1016/s0163-7258(97)00084-3. [DOI] [PubMed] [Google Scholar]
- Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J. Cell Biol. 1981;90:665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A, Uchiyama H, Takano S, Nakamura N, Ohkuma S. Autophago-some-lysosome fusion depends on the pH in acidic compartments in CHO cells. Autophagy. 2007;3:154–157. doi: 10.4161/auto.3634. [DOI] [PubMed] [Google Scholar]
- Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell RB, Barham SS, LaRusso NF. Effect of chloroquine on the form and function of hepatocyte lysosomes: Morphologic modifications and physiologic alternations related to the biliary excretion of lipids and proteins. Gastroenterology. 1983;85:1146. [PubMed] [Google Scholar]
- Munafo DB, Colombo MI. A novel assay to study autophagy: Regulation of autophagosome vacuole size by amino acid deprivation. J. Cell Sci. 2001;114:3619–3629. doi: 10.1242/jcs.114.20.3619. [DOI] [PubMed] [Google Scholar]
- Yan CH, Yang YP, Qin ZH, Gu ZL, Reid P, Liang ZQ. Autophagy is involved in cytotoxic effects of crotoxin in human breast cancer cell line MCF-7 cells. Acta Pharmacol. Sin. 2007;28:540–548. doi: 10.1111/j.1745-7254.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- Iwai-Kanai E, Yuan H, Huang C, et al. A Method to Measure Cardiac Autophagic Flux in vivo. Autophagy. 2008;4(3):322–329. doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth SE, Julian D, Akin DE, et al. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion. Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR, Logue SE, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- Luptak I, Yan J, Cui L, et al. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007;116:901–909. doi: 10.1161/CIRCULATIONAHA.107.691253. [DOI] [PubMed] [Google Scholar]
- May D, Gilon D, Djonov V, et al. Transgenic system for conditional induction and rescue of chronic myocardial hibernation provides insights into genomic programs of hibernation. Proc. Natl. Acad. Sci. USA. 2008;105:282–287. doi: 10.1073/pnas.0707778105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odashima M, Usui S, Takagi H, et al. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ. Res. 2007;100:1344–1352. doi: 10.1161/01.RES.0000265846.23485.7a. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Montagne O, Wang QM, et al. The MEKK1-JNK pathway plays a protective role in pressure overload, but does not mediate cardiac hypertrophy. J. Clin. Invest. 2002;110:271–279. doi: 10.1172/JCI14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Tannous P, Johnstone JL, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J. Clin. Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007;13(5):539–541. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized on autophagosome membranes after processing. EMBO J. 2001;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3(5):452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- Niemann A, Takatsuki A, Elsasser HP. The lysosomotropic agent monodansylcadaverine also acts as a solvent polarity probe. J. Histochem. Cytochem. 2000;48(2):251–258. doi: 10.1177/002215540004800210. [DOI] [PubMed] [Google Scholar]