Abstract
The members of the Aeromonas genus are ubiquitous, water-borne bacteria. They have been isolated from marine waters, rivers, lakes, swamps, sediments, chlorine water, water distribution systems, drinking water and residual waters; different types of food, such as meat, fish, seafood, vegetables, and processed foods. Aeromonas strains are predominantly pathogenic to poikilothermic animals, and the mesophilic strains are emerging as important pathogens in humans, causing a variety of extraintestinal and systemic infections as well as gastrointestinal infections. The most commonly described disease caused by Aeromonas is the gastroenteritis; however, no adequate animal model is available to reproduce this illness caused by Aeromonas. The main pathogenic factors associated with Aeromonas are: surface polysaccharides (capsule, lipopolysaccharide, and glucan), S-layers, iron-binding systems, exotoxins and extracellular enzymes, secretion systems, fimbriae and other nonfilamentous adhesins, motility and flagella.
1. Introduction
Ever since the first reference of an organism that could be considered a motile aeromonad in 1891 the taxonomy of the genus Aeromonas, initiated in 1943, is complex and continuously changing. Although historically the genus Aeromonas was included in the family Vibrionaceae, together with the genera Vibrio, Photobacterium, and Plesiomonas, phylogenetic investigations indicated that they should form their own family: Aeromonadaceae [1]. The family Aeromonadaceae consists of Gram-negative, facultative anaerobic, chemoorganotroph bacteria with an optimal growing temperature of about 22°C to 28°C. Generally they are motile by polar flagellation, able to reduce nitrates to nitrites and able to catabolize glucose and several carbohydrates while producing acids and often gases as well. Initially, in Bergey's Manual of Systematic Bacteriology this family only included the genus Aeromonas and was divided into two principal subgroups: the nonmotile and psycnrophilic species (A. salmonicida) and the motile and mesophilic species (A. hydrophila, A. caviae, and A. sobria) [2]. The current edition, list three genera in this family: Aeromonas, Oceanimonas, and Tolumonas [3].
The first classifications within the Aeromonas genus have been determined phenotypically (phenospecies), based on growth characteristics and biochemical tests. Nevertheless, there is a great difficulty in identifying the different Aeromonas strains on a species level by these characteristics, due to the phenotypical heterogeneity and growing number of known species [4]. One of the biggest steps forward in the taxonomic process was the introduction and continuous use of genotypical methods (genospecies). DNA-hybridisation groups (HG) have been established, associated with the already described phenotypical species. Though some genospecies stay without an associated phenospecies, some phenospecies without associated genospecies and major problems occurred due to differences between the phenotypical and genotypical groups.
A number of molecular chronometers have been used to evaluate phylogenetic relationships and relatedness among Aeromonas species. The ribosomal gene 16S (small subunit) was very useful to classify the Aeromonads [5], and a fast and low-cost method based on Restriction Fragment Length Polymorphisms (RFLP) of the 16S rDNA amplified by polymerase-chain-reaction (PCR) was developed [6, 7]. To better estimate the nucleotide substitution of the 16S-rDNA gene, recent sequence analysis of housekeeping genes, like gyrB (B subunit DNA-gyrase) and rpoD (σ 70 factor), has been proposed [8, 9].
According to the latest edition of Bergey's Manual of Systematic Bacteriology [3], 17 species have been officially accepted within the genus Aeromonas (DNA-hybridisation groups are indicated in parenthesis): A. hydrophila (HG1), A. bestiarum (HG2), A. salmonicida (HG3), A. caviae (HG4), A. media (HG5), A. eucrenophila (HG6), A. sobria (HG7), A. veronii [(bv. sobria (HG8) and bv. veronii (HG10)], A. jandaei (HG9), A. schubertii (HG12), A. trota (HG14), A. allosaccharophila (HG15) [6], A. encheleia (HG16) [10], and A. popoffii (HG17) [11]; recently three new species have been described: A. culicicola [12], A. simiae [13], and A. molluscorum [14]. Two DNA-hybridisation groups, Aeromonas sp. (HG11) and Aeromonas Group 501 (HG13; previously enteric group 501), remain without association with an actual species [15].
The members of the Aeromonas genus are ubiquitous, water-borne bacteria. They have been isolated from marine waters, rivers, lakes, swamps, sediments, chlorine water, water distribution systems, drinking water, and residual waters, especially during hot months in greater numbers [16]. The number of isolates from drinking water is generally low compared to its numbers found in food. Aeromonas strains have been found in different types of food, such as meat, fish, seafood, vegetables and processed foods. Potentially they could represent a serious problem in food, as many strains are able to grow at temperatures of a common refrigerator, at a pH of 4–10 and in presence of higher concentrations of salts [17]. Furthermore it has been shown that they are able to produce exotoxins at low temperatures [18].
Aeromonas strains are predominantly pathogenic to poikilothermic animals including amphibians, fish and reptiles, whereas they also can be found associated with infections of birds and mammals. In fish, they cause hemorrhagic septicemia that often leads to an elevated mortality and major economic losses in aquaculture. The psicrophilic A. salmonicida is considered an important pathogen among a variety of fishes, provoking systemic furunculosis in Salmonidae [19]. Mesophilic species (A. hydrophila and A. veronii) cause a similar assortment of diseases in fishes as carp, tilapia, perch, catfish, and salmon [20], and A. hydrophila and A. jandaei provoke aeromoniasis in eels [21]. On the other hand, A. hydrophila has been linked to major die-offs and fish kills around the globe over the past decade.
The mesophilic Aeromonads are emerging as important pathogens in humans, causing a variety of extraintestinal and systemic infections, as well as gastrointestinal infections. Approximately 85% of the clinical isolates of the genus Aeromonas consist of two species and a single biotype of a third species: A. hydrophila, A. caviae, and A. veronii sv. sobria [19]. Caused extraintestinal infections include: septicemia, provoked by the dissemination of the organism, from the intestinal tract to systems of circulation, and in the majority observed in immunocompromised patients; wound infections, mostly superficial cutaneous infections, but also infections of tendons, muscles, and bones; infections of the respiratory tract, from epiglottitis to pneumonia; and, less frequent, meningitis, peritonitis, eye infections and hemolytic uremic syndrome [22]. The most commonly described disease caused by Aeromonas is the gastroenteritis that can appear in the form of a selflimiting liquid diarrhea to a more severe and invasive diarrhea, which specially is a problem for young children and infants. In the last couple of years also cases of travelling diarrhea caused by Aeromonas have been documented [23]. Also, an increased isolation rate of Aeromonas species was reported in the floodwater samples following Hurricane Katrina in New Orleans [24], suggesting that this microbe could pose potential public health threats during natural disasters. Despite the demonstration of the enterotoxic potential of some Aeromonas strains, there is still a debate on its consideration as an etiological agent, as there were no big epidemical outbreaks described and no adequate animal model is available to reproduce the gastroenteritis caused by Aeromonas.
Microbiological infections implicate interaction of host and pathogen. Microbes use their own strategies for survival and multiplication, fighting the defense mechanisms of the host's immune system. The observed clinical manifestations of Aeromonas infections suggest that there could be a complex network of pathogenic mechanisms forming of a multifactorial process. Recent studies seem to strengthen this hypothesis as the virulence of this genus depends on the bacterial strain, the infection route, and the animal used as model organism [25].
Over the last years there has been a big increase in the number of sequenced genomes of different bacteria in the databases. This information permits a better understanding of the bacteria's potential, though always within limits. To date five complete genomes of genus Aeromonas have been sequenced entirely, three of them made available to the public in publications: the strain A449 of Aeromonas salmonicida, subspecies salmonicida [26], the strain ATCC 7966T of A. hydrophila [27], and the strain B565 of A. veronii [28]. This information is of great value, although there is a great diversity within the genus and some virulence factors will probably not be present in these strains or these strains show different mechanisms to infect the host than others.
The main pathogenic factors associated with Aeromonas are surface polysaccharides (capsule, lipopolysaccharide, and glucan), S-layers, iron-binding systems, exotoxins and extracellular enzymes, secretion systems, fimbriae and other nonfilamentous adhesins, motility and flagella.
2. Surface Polysaccharides
2.1. Capsule
The capsule (CPS) is a structure composed of polysaccharides that usually covers the outer membrane of the bacterial cell. It is highly hydrated (approximately 95% is water) and made up by repetitions of monosaccharides that are linked within each other by glycosidic bonds that can cause homo- or hetero-polymers. The variety of capsule-forming monosaccharides, different linkage and possible modifications contribute to an additional elevated diversity and structural complexity [29]. This structure frequently forms the most outer layer of the bacterial cell and therefore participates in the bacteria interactions with the environment. In consequence, capsules have been described as a major virulence factor of many pathogens, as they prevent phagocytosis, favor interactions to other bacteria and host tissue, and act as a barrier against hydrophobic toxins [30].
A. salmonicida, in vivo and in TSA medium (Tryptic Soy Agar), is able to form a capsular polysaccharide which is not detectable when growing in liquid TSB medium (Tryptic Soy Broth) [31]. The reported O-chain polysaccharide of A. salmonicida produced in TSB and consisting of L-rhamnose, D-mannosamine and D-glucose [32] which was also detected in the bacterial inoculums TSB culture used to prepare the in vivo growth chambers. It could be established that the structure of the CPS and lipopolysaccharide (LPS) O-chain polysaccharide of A. salmonicida strain 80204-1 produced under in vitro growth conditions on TSA. Both polysaccharides were shown by composition, methylation analysis, NMR and MS methods to be composed of linear trisaccharide repeating units containing 3-linked2-acetamido-2-deoxy-D-quinovose, 4-linked 3-[(N-acetyl-L-alanyl)amido]-3-deoxy-D-quinovose and 2-acetamido-2-deoxy-D-galacturonic acid. It has been confirmed by direct different chemical analysis that both CPS and O-chain polysaccharide were also present in the in vivo-grown cells of A. salmonicida strain 80204-1 harvested at 72 h after implant surgery. These polysaccharides were not detected in the in vitro-grown bacterial inoculum TSB culture used for the implants. The role of this structure as a virulence factor was demonstrated, as it reduces opsonization by hindering phagocytosis [33–35] and contributes to the invasion in cell lines of fish [36]. Formation of capsular polysaccharide (CPS) covering the A-layer has been reported to be produced during the in vivo culture of A. salmonicida in surgically implanted intraperitoneal culture chambers [34]. Moreover, Merino et al. [36] have reported that when grown under conditions promoting capsule formation, strains of A. salmonicida exhibited significantly higher ability to invade fish cell lines. It suggests that, as with the A-layer and LPS, CPS is an important virulence factor, essential for host cell invasion and bacterial survival.
Mesophilic Aeromonas spp., A. hydrophila AH-3 (serogroup O: 34), and A. veronii bv. sobria (serogroup O: 11) are also able to produce a capsule when grown in glucose-rich media [37]. The strains PPD134/91 and JCM3980 of A. hydrophila (serogroup O: 18) also produce capsular polysaccharides, and it was the strain PPD134/91 where genes for biosynthesis and export of the capsule have been described for the first time within the genus. The genetic organization in three regions is similar to the group II of capsular polysaccharides in other bacteria like Escherichia coli [38, 39].
2.2. Lipopolysaccharide (LPS)
The cell envelope of some Gram-negative bacteria display a form of subcellular differentiation in which peptidoglycan and outer membrane proteins at the cell poles remain stable for generations while material in the lateral walls is diluted by growth and turnover. The outer membrane has a defined in vivo organization in which a subfraction of proteins and lipopolysaccharide (LPS) are embedded in stable domains at the poles and along one or more helical ribbons that span the length of the Gram-negative rod [40].
LPS is a surface glycoconjugate unique to Gram-negative bacteria and a key elicitor of innate immune responses, ranging from local inflammation to disseminated sepsis. Gram-negative bacteria have two membrane layers separated by a periplasmic space: an inner or plasma membrane and the outer membrane. LPS is a major component of the outer leaflet of the outer membrane [41] and consists of lipid A, core oligosaccharide (OS), and O-specific polysaccharide or O antigen [41, 42]. The O antigen, which is the most surface-exposed LPS moiety, mediates pathogenicity by protecting infecting bacteria from serum complement killing and phagocytosis [42–45]. O antigens are polymers of OS repeating units. The chemical composition, structure, and antigenicity of the O antigens vary widely among Gram-negative bacteria, giving rise to a large number of O-serogroups [6]. LPS biosynthesis involves a large number of enzymes and assembly proteins encoded by more than 40 genes, recently reviewed in references [46–48]. It begins at the cytosolic or inner membrane, followed by the transit of the molecule to the outer leaflet of the outer membrane where it becomes surface-exposed. The O antigen is synthesized as a lipid-linked glycan intermediate by a process that is remarkably similar to the biogenesis of lipid linked OSs for protein N-glycosylation [49]. The lipid carrier in bacteria is undecaprenyl phosphate (Und-P), while eukaryotic cells and Archaea utilize dolichyl phosphate (Dol-P).
The A. hydrophila AH-3 WecP represents a new class of UDP-HexNAc: polyprenol-P HexNAc-1-P transferases (ref WecP). These transferase-catalyzed reactions involve a membrane-associated polyprenol phosphate acceptor and a cytoplasmic UDP-D-N-acetylhexosamine sugar nucleotide as the donor substrate. Four subgroups of bacterial enzymes have been identified based on their specific substrate preference [50]. A. hydrophila AH-3 WecP transfers GalNAc to Und-P and is unable to transfer GlcNAc to the same enzyme substrate [51]. Furthermore, the WecP enzyme (UDP-GalNAc: polyprenol-P GalNAc-1-P transferase) differs from WecA (UDP-GlcNAc: polyprenol-P GlcNAc-1-P transferase) in membrane topology (ref WecP). The differences in substrate specificity and membrane topology between WecP and WecA indicate a different phylogenetical branch [51].
The lipid A is a highly conserved structure and covalently linked to the polysaccharide complex. It is the lipid component of LPS and contains the hydrophobic, membrane-anchoring region of LPS. Lipid A consists of a phosphorylated N-acetylglucosamine (NAG) dimer with 6 or 7 saturated fatty acids (FAs) attached. Some FAs are attached directly to the NAG dimer and others are esterified to the 3-hydroxy fatty acids that are characteristically present. Its biological activity appears to depend on a peculiar conformation that is determined by the glucosamine disaccharide, the PO4 groups, the acyl chains, and also the 3-deoxy-D-manno-octulosonic acid (Kdo) containing inner core.
The most prominent activity of LPS is its immunostimulatory potency leading to the complex clinical syndrome of Gram-negative sepsis when the initial host response to an infection becomes deregulated. The clinical manifestation of sepsis is characterized by fever, hypotension, respiratory and renal failure, and intravascular disseminated coagulation [52]. These effects are not the result of LPS toxicity, but are rather a consequence of cell activation by LPS and a subsequent deregulation of the inflammatory host response. The biological activity of LPS is harbored in the lipid anchor of the molecule, termed lipid A or “the endotoxic principle” of LPS [53]. The endotoxic LPS properties derive from the release of lipid A of lysed bacteria which can provoke a major systemic inflammation known as septic or endotoxic shock.
The lipid A components of Aeromonas salmonicida subsp. salmonicida contained three major lipid A molecules differing in acylation patterns corresponding to tetra-, penta- and hexa-acylated lipid A species and comprising 4′-monophosphorylated β-2-amino-2-deoxy-D-glucopyranose-(1→6)-2-amino-2-deoxy-D-glucopyranose disaccharide, where the reducing end 2-amino-2-deoxy-D-glucose was present primarily in the α-pyranose form [54]. The tetra-acylated lipid A structure containing 3-(dodecanoyloxy)tetradecanoic acid at N-2′,3-hydroxytetradecanoic acid at N-2 and 3-hydroxytetradecanoic acid at O-3, respectively, was found. The penta-acylated lipid A molecule had a similar fatty acid distribution pattern and, additionally, carried 3-hydroxytetradecanoic acid at O-3′. In the hexaacylated lipid A structure, 3-hydroxytetradecanoic acid at O-3′ was esterified with a secondary 9-hexadecenoic acid. Interestingly, lipid A of the in vivo rough isolate contained predominantly tetra- and pentaacylated lipid A species suggesting that the presence of the hexa-acylated lipid A was associated with the smooth-form LPS [54].
The core can be subdivided into two regions, the inner and the outer core, based on their sugar composition. The inner core is attached to the lipid A at the 6′ position of one NAG, and all known inner cores contain Kdo or a derivative residue (3-glycero-D-talo-octulosonic acid). In addition, the base structure of the inner core typically contains L-glycero-D-mannoheptose (L,D-Hep), but some bacteria contain D-glycero-D-mannoheptose (D,D-Hep) alone or in combination with L-D-Hep, while other lack heptoses entirely, like Rhizobium. Within a genus or family, the structure of the inner core tends to be highly conserved. In contrast, the outer core provides an attachment site to the O polysaccharide and shows more structural diversity, although variation within a given species, or even a genus, is still limited. The complete lipid A-core OS unit is translocated to the periplasmic face of the inner membrane by the MsbA transporter, which is a member of the glyco ATP-binding cassette (ABC) transporters superfamily requiring ATP hydrolysis [55].
The chemical structure of the LPS core of A. hydrophila O: 34 [56] and A. salmonicida [57]. The complete genomics and proteomics of the LPS core biosynthesis in A. hydrophila AH-3 (serogroup O34) were achieved by the identification and characterization of three genomic regions [58]. Combining these data together with the structure elucidation of the LPS core in mutants in each gene from the three gene clusters enabled a presumptive assignment of all LPS core biosynthesis gene functions.
The comparison between the LPS core structures of A. salmonicida subsp. salmonicida A450 and A. hydrophila AH-3 renders a great similarity in the inner and part of the outer LPS core, but some differences in the distal part of the outer LPS core. The three genomic regions encoding LPS core biosynthetic genes in A. salmonicida A450 were fully sequenced, being regions 2 and 3 with identical genes to A. hydrophila AH-3. A. salmonicida A450 region 1 showed seven genes, three of them identical to A. hydrophila AH-3, three of them similar but not identical to A. hydrophila AH-3 and one of them without any homology to any well characterized gene. Combining the gene sequence and complementation test data with the structural data and phenotypic characterization of mutants, the complete genomics and proteomics of A. salmonicida were established [59]. By hybridization studies with internal probes of the A. salmonicida specific genes using different A. salmonicida strains (besides their subspecies or being atypical), was shown a unique or prevalent LPS core type.
The O polysaccharide (O antigen) is usually attached to a terminal residue of the outer core and consists of repeating oligosaccharide subunits made up of 1 to 6 sugars. The individual chains vary in length ranging up to 40 repeat units, which constitute the hydrophilic domain of the LPS molecule, as well as a major antigenic determinant of the Gram-negative cell wall. The structural diversity of O-polysaccharides repeated units with more than 60 monosaccharides, different position and stereochemistry of the O-glycosidic linkage and presence or absence of different noncarbohydrate substituents least to great variability between species and even strains of Gram-negative bacteria [60]. The variability of O-polysaccharides repeated units, particularly the terminal sugar, confer immunological specificity of the O-antigen. The first useful scheme for the serogroup of Aeromonas strains included 44 serogroups based on O antigens for a total of 307 A. hydrophila and A. caviae strains [61]. Afterwards it was extended to 97 O serogroups [62]. More than 60% of the septicemia cases are related to four of these serogroups: (O: 11; O: 16; O: 18; O: 34) [22]. Serogroup O: 11 is associated with severe infections in humans, like septicemia, meningitis and peritonitis while serogroup O: 34, the most common in mesophilic Aeromonas, is associated with wound infections in humans and outbreaks of septicemia in fishes [63]. Furthermore, the LPS of serogroups O: 13, O: 33, O: 34 and O: 44 shows thermoadaptation. Thus, high growth temperatures (37°C) increase the levels of hydroxylated and saturated fatty acids in the lipid A of serogroup O: 34 [64] and in serogroups O: 13, O: 33, O: 34, and O: 44, the S forms of LPS predominate in growth conditions of 20°C or 37°C at higher osmolarity, while R forms predominate at 37°C at lower osmolarity [65, 66].
The chemical structure of A. hydrophila O: 34 [67] and A. salmonicida subsp. salmonicida [68] has been characterized, also the chemical structure of the O: 11 antigen of A. hydrophila LL1 with S-layer [69] and Aeromonas caviae ATCC15468 [70]. Furthermore, the O antigen biosynthesis genes in the A. hydrophila strain PPD134/91 (serogroup O: 18) and AH-3 (serogroup O: 34) have been described [38, 71]. Like in other polysaccharides biosynthesis clusters, three classes of genes have been found: genes involved in the biosynthesis of activated sugars, genes that encode glycosyltransferases, and genes whose products are necessary for the O antigen translocation and polymerization.
The interaction of LPS with cells of the innate immune system leads to the formation and release of endogenous mediators initiating inflammatory and immune responses essential for an antibacterial defense [72]. This primarily protective mechanism may become overshadowed by an acute pathophysiological response with the typical clinical symptoms of septic shock that frequently follows the release of inflammatory mediators, such as tumor necrosis factor (TNF)-a during infection [73]. LPS induces no degranulation in macrophages, but like allergens, it stimulates the de novo synthesis and release of cytokines in these cells. Activation of cells by LPS is mediated by the Toll-like receptor 4 (TLR4), a member of the highly conserved protein family of TLR, which is specialized in the recognition of microbial components. In mice, defects in TLR4 result in LPS unresponsiveness [72]. For functional interaction with LPS, TLR4 requires association with myeloid differentiation protein 2 (MD-2) [74]. According to current consensus activation of TLR4 is preceded by the transfer of LPS to membrane-bound or soluble CD14 by LPS-binding protein (LBP) [75]. This mechanism is believed to be true for LPS signaling generally. However, in a recent study showed that R-form LPS and lipid A, but not S-form LPS, are capable of inducing TNF-α responses also in the absence of CD14 [76].
LPSs from Aeromonas are mainly high heterogeneous mixtures of S-form LPS molecules containing 1 to over 50 repeating oligosaccharide units and contain ubiquitously a varying proportion of R-form molecules lacking the O-specific chain. Many clinically relevant Gram-negative bacteria synthesize this type of LPS. LPSs are amphipathic molecules whose hydrophobicity decreases with increasing length of the sugar part [77]. Based upon these differences, S- and R-form LPS show marked differences in the kinetics of their blood clearance and cellular uptake as well as in the ability to induce oxidative burst in human granulocytes [78] and to activate the host complement system [79]. In relation to the Aeromonas ssp biological activities, like in other Gram-negative bacteria, the lipid A induce the B cell polyclonal activation and the response to immunoglobulin M, both by a T mitogen-independent mechanism. Furthermore, different effects were observed after injection into animals: pyrogenicity, leucopenia followed by leucocytosis, septic shock, hemorrhagic necrosis of tumors, diarrhoea, and also death [80, 81]. On the other hand, the S form of LPS protects the bacteria from the bactericide effects of the nonimmune serum, since the complement component C3b binds to the long O antigen chains being far away from the membrane and unable to form the complement attack complex, and therefore avoids cell lysis [82]. The long O: 34 antigen chains increase hemolytic activity, virulence in fishes and mice [64], and adherence to human epithelial cells [65] and can be considered an important in vivo colonization factor [66].
2.3. Surface α-Glucan
Bacteria, such as Escherichia coli, could show up to six distinct saccharide polymers simultaneously present within the glycocalyx. At present, the known components of the saccharide matrix include LPS O-antigens [46], enterobacterial common antigen (ECA) [83], capsular polysaccharides (K-antigen) [84], colanic acid (CA or M-antigen) [85], poly β-1,6-N-acetyl-D-glucosamine (PNAG) [86], and the β-1,4-glucan bacterial cellulose [87]. The A. hydrophila AH-3 α-glucan (D-Glucose linked α1-4 and sometimes branched in a α1-6) is a surface polysaccharide exported via the WecP which is also used by the O34-antigen LPS, and ligated to the surface through the O34-antigen polysaccharide ligase (WaaL) [88]. Nevertheless, it is an independent polysaccharide versus the O34-antigen LPS despite the common use of the export and ligation system, because we could find mutants devoid of either polysaccharide or both. The surface glucan is common to the mesophilic Aeromonas strains tested. Aeromonas surface glucan production may not have a significant role in cell adhesion but clearly has a role in biofilm formation [88]. Some E. coli exopolysaccharides (in particular CA and PNAG [89]) are integral components of biofilms, acting as the “cement,” which holds together the various protein, lipid, and polysaccharide components [90]. A similar role seems to be played Aeromonas surface α-glucan polysaccharide.
Several published reports indicate that the use of β-glucans enhances Aeromonas disease resistance in fish by potentiating innate immunity [91, 92]. The β-glucans used are from yeast representing a heterogeneous group of glucose polymers, consisting of a backbone of β-(1→3)-linked β-d-glucopyranosyl units with β-(1→6)-linked side chains of varying length and distribution. However, in no case the authors were able to show the scientific reason for this Aeromonas resistance. The fact that Aeromonas produces a surface α-glucan may explain these results and also suggests that the use of α-glucans instead of β-glucans could be more helpful to enhance the fish resistance to Aeromonas disease [88].
2.4. S-layers
The S-layer is a surface protein layer of paracrystalline nature that is produced by a broad range of bacteria to form the outermost cell envelope. Chemical analysis showed that it is composed of a single protein or glycoprotein (40-200 kDa) and exhibits either oblique, square or hexagonal lattice symmetry with unit cell dimensions in the range of 3 to 30 nm. S-layers are generally 5 to 10 nm thick and show pores of identical size (diameter, 2–8 nm) and morphology. S-layers have been associated with a number of possible functions that relate to pathogenicity. Due to its exposition on the cell surface, it plays a mayor role in diverse biological functions: adhesion, protection against complement and attack by phagocytes, antigenic properties, anchoring site for hydrolytic exoenzymes, bacteriophage receptor, and others [93].
In 1981, Kay and coworkers identified a layer associated with virulence, initially called A-layer, outside the cell wall of A. salmonicida [94]. Later, the constituting protein and the sequence of the encoding gene, vapA, were identified [95], and it was observed that this layer got lost after growth at temperatures above 25°C, due to a deletion of the genetic material [96]. In parallel, the S-layers of mesophilic Aeromonas belonging to serogroup O: 11, and the encoding gene ahsA were identified [97]. Although these layers are similar to the one identified in A. salmonicida at a morphological level, they differ on the genetic and functional level and could therefore carry out a different role in pathogenicity [98]. More recently the presence of an S-layer was also described in pathogenic isolates of A. hydrophila belonging to serogroups O.14 and O: 81 [99]. The S-layer of Aeromonas is composed by autoassembling subunits of a single protein that form a tetragonal complex that covers the entire bacterial cell and constitutes the predominant surface antigen [100]. The secretion of Aeromonas S-layer subunits involves the cleavage of a signal peptide to be translocated across the plasma membrane, as well as different specific proteins, homologous to components of the type II secretion system (T2SS), to be transferred from the periplasm to the exterior.
The S-layer of A. salmonicida promotes association with extracellular matrix proteins and macrophages, binding to porphyrins [101] and immunoglobulins [102], and protection against proteases [100] and oxidative killing [34]. Its presence in the mesophilic Aeromonas spp. of serogroup O: 11 increases their capacity of adherence which contributes to the colonization of intestinal mucosa as well as generating a major resistance to opsonophagocytosis which could facilitate systemic dissemination after invasion through the gastrointestinal mucosa [94].
Since the early days of S-layer glycoprotein research, it was evident that these cell surface components occur on Archaea as well as on bacteria [103]. S-layer glycoproteins have been known for their occurrence among the major lineages of Archaea. Among bacteria, for a long time only Gram-positive members of the Bacillaceae family have been known to possess S-layer glycoproteins [104]. Only very recently there were the first reports on the occurrence of glycosylated S-layer proteins in the Gram-negative species. Evidence was obtained from biochemical analyses and so far nothing is known about either glycan structure or linkage of the glycans to the S-layer protein portion. However, in contrast to the known S-layer glycoproteins from Bacillaceae investigated these glycosylated S-layer proteins originate from potential pathogens and, therefore, might be of medical relevance [105]. Until today all S-layer Aeromonas strains have one thing in common: a lipopolysaccharide (LPS) that contains O-antigen polysaccharides of homogeneous chain length. This fact leads to the speculation of the possible implication of the LPS in linking the S-layer to the bacterial cell surface [100, 106], or to participate in S-layer glycosylation.
3. Iron-Binding Systems
Numerous environments contain less than 1 μM of iron, which is considered the optimum for microbial growth. The low availability of free iron makes bacterial growth and pathogenicity more difficult, but not impossible. Microorganisms developed a series of mechanisms to sequester iron from their hosts or from insoluble polymers of the environment, including reduction of ferric to ferrous iron, occupation of intracellular niches, utilization of host iron compounds, and production of siderophores. While direct evidence that high-affinity mechanisms for iron acquisition function as bacterial virulence determinants has been provided in only a small number of cases, it is likely that many if not all such systems play a central role in the pathogenesis of infection [107]. The competition for iron between the host and the bacterial invader shows the advance of an invasion. Due to the presence of iron-binding proteins in the host, such as hemoglobin, transferrin, lactoferrin, or ferritin, the iron is little accessible in vivo. Iron concentrations in the serum are far from the required minimum for growth during the infections of many bacteria. This capacity to deprive an essential nutrient of a microorganism is known as nutritional immunity [108].
Two high affinity mechanisms to acquire iron are known in Aeromonas strains: siderophore-dependent and siderophore-independent mechanisms [109]. Siderophores are low-molecular-weight peptides which present functional groups with elevated affinity and specificity towards iron ions. These peptides need specific cell membrane bound receptors as well as a cell-associated apparatus to incorporate the metal into the bacterial metabolism. Mesophilic Aeromonas synthesize, enterobactin or amonabactin siderophores, but never both of them. The enterobactin is found in different Gram-negative bacteria, while the amonabactin is only known in Aeromonas ssp. Both siderophores are catecholates (phenolates), as they have 2,3-dihydroxybenzoic-acid (2,3-DHB) conjugated with aminoacids [110]. Therefore, their biosynthesis in Aeromonas spp. is encoded by two distinct gene groups: the amo genes, in strains that produce amonabactin, and the aeb genes (aeromonad enterobactin biosynthesis), in enterobactin hydroxypyroridone producing strains [111]. Furthermore, the amonabactin receptor of A. hydrophila shows low specificity, permitting the transport of an extraordinary ample range of siderophores, with various chelating groups like catecholate, hidroxamate and hydroxypyroridone [108]. Recently, the A. salmonicida gene cluster involved in the catechol-type siderophore biosynthesis has been described [112]. Using a proteomic approach, a recent study demonstrated that under iron-limited conditions A. salmonicida expresses three iron-regulated outer membrane receptors, and one of these receptors was proposed to be a putative heme receptor based on sequence homology [113]. Heme uptake in Gram negative bacteria usually involve outer membrane receptors as well as a TonB-dependent internalization process with two accessory proteins ExbB and ExbD, and this system is believed to transduce the energy of the proton motive force of the cytoplasmic membrane into transport energy required by the receptor. Subsequently, transport of heme across the cytoplasmic membrane is driven by ATP hydrolysis, and an ATP-binding cassette (ABC) transporter is involved in this transport. A. salmonicida siderophore-independent mechanisms consist of bacterial outer membrane proteins able to bind specific host iron- or heme-binding proteins without the intervention of siderophores [108, 114].
The acquisition of iron is recognized as one of the key steps in the survival of bacterial pathogens within their hosts, and contributes significantly to virulence [115]. The expression of genes involved in iron acquisition is tightly regulated by the ferric uptake regulator protein Fur, which acts as an iron-responsive DNA binding repressor [116]. A genetic screening known as the Fur titration assay on this bacterium identify Fur-regulated genes for siderophore biosynthesis and for ferrisiderophore transport previously described. A screening of gene distribution demonstrated that all the analyzed strains shared genes for siderophore biosynthesis and transport and for heme utilization, indicating that these two systems of iron acquisition are a conserved trait [112]. Iron-regulated A. salmonicida proteins have demonstrated to be protective antigens for fish and are good candidates for the improvement of vaccines [117].
4. Exotoxins and Other Extracellular Enzymes
4.1. Exotoxins
It has been described that the genus Aeromonas produces a wide range of exotoxins. However, all toxins described are not produced by all strains, although strains may possess their genes. Furthermore, some strains only express toxin genes in certain growth conditions. Two main types of enterotoxins have been described in Aeromonas spp. cytotoxic and cytotonics.
Cytotoxic enterotoxins, also known as cytolytic enterotoxins, provoke degeneration of crypts and villi of the small intestine and their producing strains are generally isolated from patients suffering diarrhea. These toxins can lead to produce hemolysis, cytotoxicity and enterotoxicity [118, 119]. They are synthesized as a preprotein containing a signal peptide which separates after crossing the inner membrane. The secreted protein is inactive and can be activated by proteolytic nicking near the C-terminus. The active toxin binds to a glycoprotein on the surface of the target cell and oligomerizes forming pores in the host's cell membrane that cause cell death. The cytotoxic enterotoxin Act, from A. hydrophila SSU [120], plays an important role in Aeromonas infections [121], since it induces early cell signaling in eukaryotic cells, which leads to the production of inflammation mediators in macrophages and in human epithelial cells. Furthermore, it also contributes to apoptosis [122]. Detailed structural-functional studies of the Act enterotoxin and two aerolysins from A. trota and A. bestiarum [123] showed that these proteins are closely related. However, some heterogeneity at the aminoacid level in some regions could lead to possible differences in folding of these molecules, resulting in differential neutralization of these toxins by specific monoclonal antibodies [124].
Act is an aerolysin-related pore-forming toxin that is responsible for the hemolytic, cytotoxic, and enterotoxic activities of A. hydrophila, being its main virulence factor. Hemolysis involves pore formation in the membrane of the target cell and water entry from the external media, resulting in swelling of the cells and subsequent lysis. The toxin interacts with the membranes of erythrocytes, inserts into the lipid bilayer as oligomers, and creates pores in the range of 1.14 to 2.8 nm. Cholesterol serves as the receptor for Act, and the 3′-OH group of this membrane constituent is important for the interaction. Once Act has interacted with cholesterol on the cell membranes, the toxin is activated with subsequent oligomerization and pore formation [125, 126]. The toxin activity also includes tissue damage and high fluid secretion in intestinal epithelial cells, resulting from the induction of a proinflammatory response in the target cells. Act upregulates the production of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β) and IL-6 in macrophages. TNF-α and IL-1β stimulate the production of the inducible nitric oxide synthase (iNOS) that, through nitric oxide (NO) production, is an essential element of antimicrobial immunity and host-induced tissue damage. Simultaneously, Act has the ability to activate arachidonic acid (AA) metabolism in macrophages that leads to the production of eicosanoids (e.g., prostaglandin E2 [PGE2]) coupled to cyclooxygenase-2 (COX-2) pathway. AA is a substrate for PGE2 production, but is present at limited concentrations in cells [127]. Act increases the amount of AA from phospholipids by inducing group V secretory phospholipase A2 (sPLA2), which acts in the membrane of eukaryotic cells. Act increases cyclic AMP (cAMP) production in macrophages by indirect activation of adenylate cyclase by PGE2. The A. hydrophyla toxin also induces the production of antiapoptotic protein Bcl-2 in macrophages, preventing the occurrence of massive apoptosis resulting from the induction of the inflammatory response, which would be undesirable for the bacteria. Act also promotes an increased translocation of the nuclear factor kB (NF-kB) and cAMP-responsive element binding protein (CREB) to the nucleus [127]. Transcription factor NF-kB is important in a number of inflammation-related pathways. The enhancer/promoter regions of some immunoregulatory cytokine genes, including the TNF-α, IL-1β, and IL-6, present binding elements for NF-kB and CREB [128]. These transcription factors have also important regulatory functions in the transcription of cox-2 and are implicated in the induction of Act cytotoxic activities. The mature protein is 52 kDa and contains 493 aminoacids. It is secreted as an inactive precursor and undergoes processing at both the N- and C-terminal ends to demonstrate biological activity. It has a leader sequence of 23 amino acids that allows the protein to transverse the inner membrane. This leader peptide is removed when the toxin enters the periplasmic space [129].
Cytotonic enterotoxins do not produce degeneration of the epithelium and have mechanisms of action similar to those of the choleric toxin, since they increase the cyclic adenosine monophosphate (cAMP) levels and prostaglandins in intestinal epithelial cells. Aeromonas species produces cytotonic enterotoxins that show different molecular weights and variable reactivity to the choleric antitoxin [130, 131]. These enterotoxins have been divided into two groups: heat-labile (56°C for 10 min.), without cross-reactivity with the choleric anti-toxin, and heat-stable (100°C for 30 min.) that react with the choleric antitoxin Chopra and colleagues purified a heat-labile cytotoxic enterotoxin, Alt, from A. hydrophila SSU [119] that increased the cAMP levels and prostaglandins in rats intestinal mucosa. The protein sequence shows similarity to the C-terminus of A. hydrophila phospholipase C (PLC). They also detected a heat-stable (56°C for 20 min.) cytotonic enterotoxin, Ast, that provokes fluid secretion in rats small intestine and increases the cAMP levels in mucosal cells [119].
In addition to cytotoxic hemolytic enterotoxins, Aeromonas spp. strains produce at least two other classes of hemolysins without enterotoxic properties: α-hemolysins and β-hemolysins. The α-hemolysins are synthesized in the stationary growth phase and lead to reversible cytotoxic effects and incomplete erythrocytes lysis [132]. The β-hemolysins, on the other hand, are usually synthesized in the exponential growth phase. They are thermostable (5 min. at 56°C) and pore forming toxins which lead to osmotic lysis and complete destruction of erythrocytes [17, 132].
4.2. Other Extracellular Enzymes
Aeromonas spp. secretes a wide range of extracellular enzymes, including proteases, lipases, amylases, chitinases, nucleases, and gelatinases. Although in many cases their role in pathogenicity is still to be determined, they represent a big potential to adapt to environmental changes. Extracellular proteases contribute to the metabolic versatility that allows Aeromonas to persist in different habitats and that facilitate ecological interactions with other organism. In general, proteases can contribute to the pathogenicity promoting invasion by direct damage of host tissue or by proteolytic activation of toxins [17]. Furthermore, they can also contribute to the establishment of infection overcoming the initial host defenses, for example, inactivating the complement system, or to providing nutrients for cell proliferation [133]. In Aeromonas spp. three different types of proteases have been identified: a temperature-labile serine-protease and two metalloproteases, both temperature-stable but EDTA (ethylenediaminetetraacetic acid)-sensitive and -insensitive, respectively. Additionally, aminopeptidases with a number of specific activities have been described: catabolism of exogenously supplied peptides, extracellular activation of aerolysine, or cleavage of amino-terminal methionine from newly synthesized peptide chains (methionin aminopeptidase) [134].
Lipases or triacylglycerol hydrolases are produced by a wide range of bacteria. They may provide nutrients and constitute virulence factors by interacting with human leukocytes or by affecting several immune systems functions through free fatty acids generated by lipolytic activity. In A. hydrophila different lipases, such as the Ah65 lipase/acyltransferase, H3, Apl1 and Lip, have been described [134, 135], with the Apl1 lipase showing phospholipase C activity. In Aeromonas spp.serogroup O: 34 two lipases have been described: phospholipase A1 and C. The phospholipase C shows lecithinase and cytotoxic activities and its role as a virulence factor has been demonstrated [136]. Furthermore, glycerophospholipid-cholesterol acyltransferases (GCAT), which digest erythrocytes membranes and leads their lysis, have been isolated from A. hydrophila and A. salmonicida [134].
5. Secretion Systems
Gram-negative bacteria have a cytoplasmic membrane, a thin peptidoglycan layer, and an outer membrane containing lipopolysaccharide. There is a space between the cytoplasmic membrane and the outer membrane called the periplasmic space. In order to transport proteins to the cell surface or the extracellular space Gram-negative bacteria had developed different secretion systems: secretion systems type I, II, III, IV, V, and VI [137]. This classification is based on proteins transport across the outer membrane and refers to the molecular nature of the transport machinery and the catalyzed reactions. The mechanisms involved in protein transport across the cytoplasmic membrane, in all the above mentioned secretion systems, can be divided into two main groups: Sec-dependent and Sec-independent [138]. Proteins secreted via the Sec-dependent pathway contain an N-terminal signal peptide and utilize the Sec translocase for transport across the cytoplasmic membrane. The Sec-dependent pathway includes the secretion system type II and V. Sec-independent pathways allow the export from the cytoplasm to the extracellular environment in one step and do not involve periplasmic intermediates. These pathways include secretion systems type I, III, IV, and VI, although type IV can also employ the Sec-dependent pathway. An alternative Sec-independent pathway know as twin arginin translocation system (Tat-system), which recognize proteins containing two “twin”-arginine residues in the signal sequence, is employed to transport already folded proteins across the inner membrane [139].
Type III and VI secretion systems (T3SS and T6SS, resp.) have been documented to play a critical role in the virulence of many Gram-negative bacteria, are often activated upon contact with target cells and deliver their toxin proteins, the so-called effectors, directly into the host cells cytosol.
5.1. Type III Secretion System
The T3SS was first identified in pathogenic strains of Yersinia spp. [140], and consist of a complex multicomponent system which transports bacterial proteins, frequently involved in pathogenicity, directly from the bacterial cytoplasm across the inner and outer membrane of the bacterial envelope to either the external medium or directly into the eukaryotic cells. The T3SS contains three different types of proteins: (a) structural components that form needle-like structures, so called injectisomes; (b) secretion substrates, so called effectors; (c) chaperones that assist and protect structural and effector proteins during transport. The injectisome consists of approximately 20 different proteins that assemble to form a needle-like structure with thin and rigid hollow needles that extend from the cell surface and are anchored to the envelope by basal structures resembling flagella basal bodies. This structure is usually induced upon contact with the host cells and allows the translocation of the effectors into the eukaryotic cytosol [141].
The T3SS is independent of the Sec system; however the assembly of the secretion apparatus probably requires the Sec machinery, since several components have the characteristic N-terminal signal sequences [142]. The signal that allows effectors recognition and secretion or translocation into the host cells is unknown, although various theories have been suggested [143]. Regardless the differences of these theories it seems that the region that codes the first 20 aminoacids, either in RNA or peptide form, is essential for the effectors recognition and secretion [142].
Until today the presence of a functioning T3SS has been described in A. salmonicida [144] and in A. hydrophila strains AH-1, AH-3 and SSU [145–147]. The Aeromonas T3SS is similar to the Yersinia T3SS [146]. Furthermore, four T3SS-effector proteins have been identified in A. salmonicida, AexT, AopP, AopO and AopH [148–150], and one, AexT and AexT-like (or AexU), in the A. hydrophila strains AH-3 and SSU, respectively [151, 152]. AexT is a bifunctional toxin, homologous to the also bifunctional effectors ExoT/ExoS of P. aeruginosa, showing ADP-ribosyltransferase and GAP (GTPase acting protein) activities. AopP belongs to the YopJ family, a group of T3SS effectors that interferes with signaling pathways of mitogen-activated protein kinases (MAPK) and/or the nuclear factor kappaB (NF-κB). The biological functions of AopO and AopH are unknown; nevertheless they are homologues of the Y. enterocolitica effectors AopO and YopH, respectively.
5.2. Type VI Secretion System
The T6SS was described by Pukatzki and coworkers and seems to constitute a phage-tail-spike-like injectisome, which again serves the purpose of translocations effectors into host cells [153, 154]. It appears to be highly conserved and can be found in one or more copies in diverse Gram-negative species, such as V. cholerae, P. aeruginosa, Y. pestis, E. coli, S. enterica, Agrobacterium tumefaciens, Rhizobium leguminosarum, Francisella tularensis, Burkholderia spp, and Edwardsiella spp. However, the macromolecular structure of this system has not yet been resolved yet, and it is not known how T6SS machines assemble or deliver effectors. A hallmark of all T6SSs is the presence of Hcp (hemolysin coregulated protein) and VgrG (valine-glycine repeat protein G) proteins in culture supernatants [155]. Neither of these proteins is made with a signal peptide, and they are not proteolytically processed. Furthermore, they show structural similarities to components of viral injection machineries indicating that they do not act as classical secreted effectors but are rather surface exposed structural components that might be released in culture supernatants or into eukaryotic cells. Although necessary for E. tarda or Francisella pathogenesis, T6SS are required for processes as different as resisting predation in V. cholerae, symbiosis in Rhizobium leguminosarum, biofilm formation in enteroaggregative E. coli, killing of niche competitors in P. aeruginosa, Burkholderia thailandensis, and V. cholerae, and stress sensing in V. anguillarum for a recent review [156].
Several regulatory mechanisms controlling T6SS gene cluster expression have been identified in recent years: they are regulated at the transcriptional level by alternate sigma factors, two-component systems, or transcriptional factors. Several cases of regulation by quorum sensing have also been reported. Because T6SS gene clusters are often found in pathogenicity islands or have been acquired by horizontal gene transfer, their GC content is sometimes different from the GC content of the core genome, and they are silenced by histone-like proteins. T6SS subunit production is also regulated at the translational level through the action of small regulatory RNA, and several T6SS need to be activated by posttranslational mechanisms [157].
Recently, a functional T6SS has been described in A. hydrophila strain SSU [158] and its involvement in virulence has been demonstrated. T6SSs gene clusters are also present in the genome of A. hydrophila AH-3 and ATCC7966, but their implication in virulence has not been proven. By bioinformatics approach we identified several Aeromonas strains with clusters which possess typical −24/−12 sequences, recognized by the alternate sigma factor 54, which directs the RNA polymerase to these promoters which requires the action of a bacterial enhancer binding protein (bEBP), which binds to cis-acting upstream activating sequences. Putative bEBPs are encoded within the T6SS gene clusters possessing σ 54 boxes.
The importance of vasH (σ 54 activator) and vasK of A. hydrophila SSU in the expression of the T6SS gene cluster and the secretion and translocation of T6SS associated effectors proteins and their crucial roles in evoking mouse lethality. A vasH isogenic mutant was unable to express and produce known T6SS proteins, such as Hcp and VgrG2/3, and vasK isogenic mutant was able to express and translocate Hcp into the host eukaryotic cell but unable to secrete it into the extracellular milieu [158]. The proteomics analysis indicated the existence of VgrG1, with its gene localized outside the T6SS gene cluster. This protein has a COOH-terminal extension containing a vegetative insecticidal protein-2 (VIP-2) domain, known for its actin ADP-ribosylating activity (18). VgrG1 is an important virulence factor of A. hydrophila that is secreted and also translocated by the T6SS with actin ADP-ribosylating activity [159].
6. Adhesins
The bacterial capacity to adhere and colonize the hosts' mucosa is a critical step in the infection process. Two classes of adhesins which allow bacteria to bind to specific receptors on the eukaryotic cell surface have been described in Aeromonas: those associated with filamentous structures and those associated with proteins of the outer membrane [160] or other structures.
6.1. Filamentous Adhesins: Fimbriae/Pili
Fimbriae/pili are filamentous structures on the bacterial surface, formed by subunits known as pilin. Although pili are often described as adhesive organelles, they have been implicated in other functions, such as phage binding, DNA transfer, biofilm formation, cell aggregation, host cell invasion, and twitching motility. The pili of Gram-negative bacteria have been placed into four groups based on their assembly pathway: (a) pili assembled by the chaperone-usher pathway; (b) the type IV pili; (c) pili assembled by the nucleation/precipitation pathway; (d) pili assembled by the alternative chaperon-usher pathway (CS1 pili family) [161].
In clinical and environmental isolates of mesophilic Aeromonas, two distinct types of fimbriae have been found based on their morphology: short, rigid fimbriae (S/R) that can be found in high numbers on the bacterial cell and long, wavy fimbriae (L/W) that can be found in smaller numbers. The S/R fimbriae have a length of 0,6 to 2 μm and are common epitopes in different analyzed species. They are widely distributed (more than 95% of strains) and able to cause autoaggregation, but not hemagglutination or binding to intestinal cells. Furthermore, they are the predominant type in Aeromonads with elevated pili number [162] and in some clinical strains they can be induced under determined environmental conditions (<22°C, in liquid media). The L/W fimbriae are large, fine (4–7 nm), flexible, and considered hemagglutinins. They are also the predominant type in strains isolated from fish which present a small number of pili (<10 per cell). Aminoacid sequence analysis indicates that they correspond to type IV pili [163], known as important structures for adhesion to epithelial cells and involved in biofilm formation and twitching motility. Two different type IV pili have been described in gastroenteritis-associated Aeromonas species: the bundle-forming pili (Bfp) and the type IV pili (Tap) [164]. Bfp pili are involved in adhesion to intestinal cells [165] and exhibit N-terminal sequence homology with the mannose-sensitive hemagglutinin pilus of V. cholerae. Tap pili differ from Bfp pili in their N-terminal sequences and molecular weights and exhibit highest homology with the type IV pili of Pseudomonas and pathogenic Neisseria species [164]. Furthermore, one of the Tap-family proteins (TapD) is essential for secretion of aerolysin and proteases, contributing to type II secretion [163]. Recently, the role of Bfp in A. veronii bv. sobria adherence, by the study of a 22-kb locus encoding the bundle-forming that contained 17 pilus-related genes similar to the mannose-sensitive hemagglutinin (MSHA) of V. cholerae. The bundle-forming pilus is required for A. veronii adherence and biofilm formation, and both the major and minor pilin proteins are essential for this process [166].
A. salmonicida subsp. salmonicida, a bacterial pathogen of Atlantic salmon, has no visible pili, yet its genome contains genes for three type IV pilus systems. One system, Tap, is similar to the P. aeruginosa Pil system, and a second, Flp, resembles the Actinobacillus actinomycetemcomitans Flp pilus [167]. The Tap pili appeared to be polar, while the Flp pili appeared to be peritrichous. The Tap pilus made a moderate contribution to virulence, while the Flp pilus made little or no contribution. Unlike the pili of other piliated bacterial pathogens, A. salmonicida subsp. salmonicida type IV pili are not absolutely required for virulence in Atlantic salmon.
6.2. Nonfilamentous Adhesins
On the Aeromonas spp. surface there are also other macromolecules considered adhesins, like the S-layer monomers, the lipopolysaccharide and different outer membrane proteins. Among the outer membrane proteins, the porins have been specially described to act like a lectin-type adhesins, binding the bacteria to carbohydrate-rich surfaces like erythrocytes and probably intestinal human cells [168].
7. Motility and Flagella
7.1. Motility
Depending on the environmental conditions, bacteria are able to move in a free individual manner or remain in the same place to form colony groups and colonize surfaces. Bacteria living in surface colonies have several advantages over single cells. As a group, bacteria can optimize growth and survival by the presence of different cell types that are able to perform specialized functions; can have better access to nutrients; better defense mechanisms for protection against unfavorable environmental conditions such as desiccation. Furthermore, bacteria in colonies secrete polysaccharides to form biofilms which enhance adhesion, survival, and movement.
Different types of bacterial movement have been described: swimming, swarming, gliding, twitching, and sliding among others. All of them are associated to movement over surfaces; except for swimming, that is also used for motility in liquid media. It has been shown that the twitching requires type IV pili, like several forms of gliding, whereas other forms of gliding remain to be explained. The sliding represents a form of passive translocation. Only swimming and swarming are correlated with the presence of flagella. However, whereas swimming is an individual endeavour, swarming is the movement of a group of bacteria. Swimming in liquid media alternates between straight and tumbling movements. In bacteria with peritrichous flagella, such as E. coli, the counter-clockwise (CCW) flagella rotation results in the formation of a helical bundle that propels the cell forward in one direction in a smooth-swimming motion, a so-called run. By contrast, the clockwise (CW) rotation causes unbundling of the helical bundle, allowing the bacterium to randomly reorient its direction, so-called tumbling. In bacteria with a single polar flagellum, such as Aeromonas, CCW rotation propels the cell forward in a run, whereas CW rotation propels the cell backward with a concomitant random reorientation. About 60% of mesophilic Aeromonas strains showed swarming motility [169].
7.2. Flagella
7.2.1. Structure
Within the Gram-negative bacteria, the most studied model of flagellum has been that of E. coli and S. enterica sv. Typhimurium [170]. The prokaryotic flagellum has been structurally divided into an external part, constituted by the filament and the hook, and an internal part embedded in the bacterial surface, the so-called basal body. E. coli and Salmonella express approximately 20.000 copies of a single flagellin (FliC). However, V. parahaemolyticus is able to synthesize 6 different polar flagellins [171], A. caviae presents two polar and two lateral flagellins, and A. hydrophila presents two polar and only one lateral flagellin [172–174].
The flagellar motor is divided into two substructures: the rotor and the stator. The rotor, composed of the FliM, FliN, and FliG proteins that form the C ring structure at the base of the flagella basal body, and the stator, consisting of membrane-embedded proteins surrounding the MS-ring, that constitute proton or sodium ion channels and couple the flow of ions to flagella rotation [175]. In the proton-driven motor of E. coli and S. enterica serovar Typhimurium, the stator is composed of two integral membrane proteins, MotA and MotB [175] polar flagella stator of Vibrio species, such as V. alginolyticus and V. parahaemolyticus, require four proteins: PomA, PomB, MotX, and MotY [171]. MotP/PomA and MotS/PomB proteins are homologous to the proton-driven MotA and MotB, respectively. MotX and MotY do not have paralogous proteins in E. coli and are components of the T-ring [176], which is located beneath the P-ring of the polar flagella basal body in Vibrio species.
Mesophilic Aeromonas also showed two additional polar stator genes named pomA 2 and pomB 2. A. hydrophila PomA2 and PomB2 are highly homologous to other sodium-conducting polar flagella stator motors. Aeromonas PomA-B and PomA2-B2 are redundant sets of proteins, as neither set on their own is essential for polar flagella motility either in aqueous or high-viscosity environments. Both PomA-B and PomA2-B2 are sodium-coupled stator complexes although PomA2-B2 is more sensitive to low sodium concentrations than PomA-B [177]. Furthermore, the stator of the mesophilic Aeromonas proton-driven lateral flagella motor (MotAL [LafT] and MotBL [LafU]) is additional to the polar flagella stators [169, 177].
7.2.2. Genetics
In bacteria with polar flagella as Vibrio spp., mesophilic Aeromonas and Pseudomonas aeruginosa, genes are distributed in at least five chromosomal regions, but the majority is located in two of them (Figure 1) [171, 174].
Figure 1.
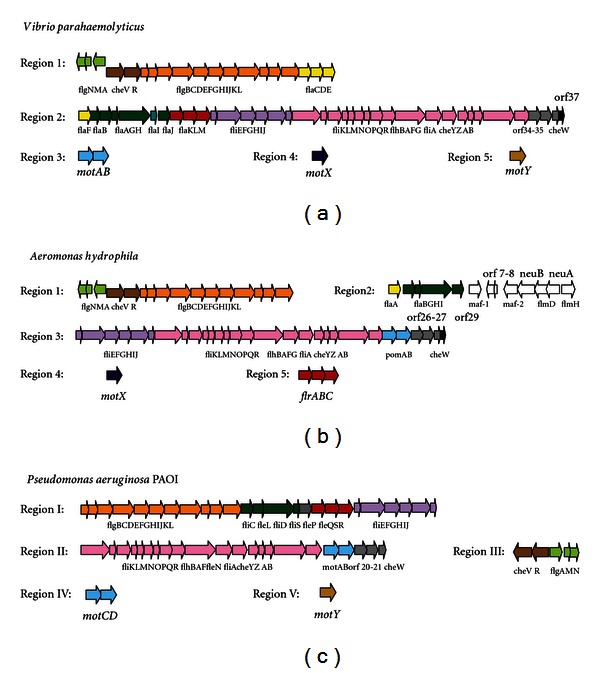
Genetic organization of polar flagellar genes in V. parahaemolyticus, A. hydrophila and P. aeruginosa.
Region 1 is similar in Vibrio and Aeromonas, though Vibrio possesses three more flagellin gene (flaCDE). Organization of the fla genes in region 2 is also similar in Vibrio and Areomonas, with the difference that the fli genes of region 2 in Vibrio are found in Aeromonas' region 3. However, Vibrio also possesses one more flagellin gene (flaF) and a gene encoding a putative chaperone (flaI) between flaH and flaJ. Furthermore, downstream of the Aeromonas flaJ lies a gene which encoded a homolog of the Maf proteins reported in H. pylori, Clostridium acetobutylicum, and Campylobacter jejuni [178]. Aeromonas polar flagella region 3 shows similar organization to the genes downstream of flaM in Vibrio region 2 with the absence of the motor genes. No master regulatory genes encoding homologues of Vibrio parahaemolyticus FlaK, FlaL and FlaM or P. aeruginosa FleQ, FleS, and FleR, were found upstream of A. hydrophila fliE. In contrast, Aeromonas have two genes (pomA and pomB) which encode orthologues of the MotA and MotB, motor proteins of Pseudomonas. Region 4 of Vibrio and Aeromonas includes a gene that encodes the sodium-driven motor protein MotX which is involved with MotA, MotB and MotY in torque generation of polar flagellum; however in Pseudomonas this region contains genes that encode the motor proteins MotCD. Region 5 of Vibrio and Pseudomonas includes a gene that encodes the motor protein MotY, however in Aeromonas it contains the master regulatory genes (flrABC). Recently, the Aeromonas MotY has been found in a different region with different behavior than the Vibrio MotY [179].
The complete set of genes involved in the formation of a functional and inducible lateral flagella system was described in two bacterial species: V. parahameolyticus and A. hydrophila. These lateral flagella are encoded by 38 genes distributed in two discontinuous regions (region 1 and 2) on chromosome II in V. parahameolyticus [180] and in one unique chromosomal region in A. hydrophila [181] (Figure 2). In these two bacterial species, the polar and lateral flagella systems do not share either structural or regulatory genes. A partial set of genes was described in other bacteria with functional dual flagella, such as A. brasilense, A. caviae, R centenum, and B. japonicum [169].
Figure 2.
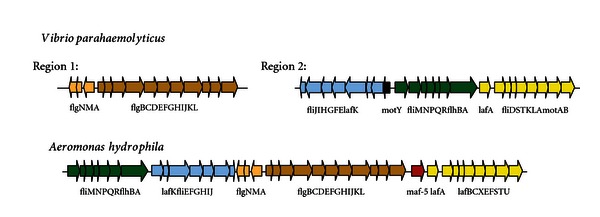
Genetic organization of lateral flagella genes in V. parahaemolyticus and A. hydrophila [180, 181].
V. parahaemolyticus region 1 genes are divided into two divergent transcribed sets of genes: flgAMN L and flgBCDEFGHIJKL L. Homologous A. hydrophila genes exhibit the same distribution and direction of transcription. V. parahaemolyticus region 2 genes are arranged in four clusters: fliMNPQRLflhBA L, lafA and fliDSTKLA L motAB L transcribed in the same direction, and motYLlafKfliEFGHIJ L transcribed in the opposite direction. Homologous A. hydrophila genes are transcribed in the same direction and do not contain any homologous gene to V. parahaemolyticus motYL. Furthermore, the A. hydrophila lateral flagella region contains, between flgL L and lafA, a modification accessory factor gene [178], maf5, which is independently transcribed.
The expression of flagellar genes is highly regulated, due to the energetic cost of biosynthesis and use of the flagellum for the bacteria. Flagellar systems are regulated by numerous environmental factors and global regulators of the bacteria. To ensure the maximum efficiency and precision of the biosynthesis, the bacteria use hierarchic regulatory networks that include transcriptional and posttranslational mechanisms to control expression of the flagellar components. In E. coli and S. enterica sv. Typhimurium models for peritrichous flagellation a hierarchy of three transcriptional classes were established. The early genes that correspond to the operon flhDC are controlled by class I promoters (independent of σ 70) and code for the transcriptional activator, FlhD2C2, for the next genes in the hierarchy. The class II genes (also independent of σ 70) code components of the exportation complex, the basal body, the hook, the flagellum specific sigma-factor σ 28 (FliA) and the anti-σ 28 factor (FlgM). FlgM is a negative regulator that binds to σ 28 and therefore inhibits its function. During flagellum biosynthesis, when the structure of the basal body is completed, FlgM is secreted out of the cell by the flagellum exportation complex, resulting in FlgM-depletion in the cytoplasm and release of σ 28 to activate the late genes [182]. The late genes (class III) are activated by σ 28 and include the system of chemotaxis, the motor, HAPs, and the flagellins.
There have also been described regulatory cascades with four transcriptional classes of bacteria with polar flagellation, like V. cholerae and P. aeruginosa. The early genes express the protein FlrA in V. cholerae and FleQ in P. aeruginosa, which binds to the σ 54 factor and thus activates the class II genes. Class II genes include two-component-regulators, V. cholerae flrBC or P. aeruginosa fleSR, and fliA (σ 28). FlrC and FleR are regulators that also associated with the σ 54 factor and activate class III promoters, whereas σ 28 factor activates class IV genes (figure D) [183]. In V. parahaemolyticus possible regulatory cascades for its two flagellar systems have been described. The V. cholerae and P. aeruginosa model was proposed for regulating the polar flagellum with the regulators flaK, an early gene, and flaLM, class II genes [180]. On the other hand, the lateral flagellar system is regulated by LafK. This transcriptional regulator, that shows similarity to FlrA and FleQ, associates with the σ 54 factor to activate the expression of class II genes. Within these genes we find the gene that codes the σ 28 factor, which activates the late genes [180]. In this species it was also observed that the loss of the FlaK-regulator of polar flagellation can be compensated by LafK, the lateral regulator [184].
A. hydrophila polar flagellum class I gene transcription is σ 70-dependent, which is consistent with the fact that A. hydrophila polar flagellum is constitutively expressed [185]. In contrast to other bacteria with dual flagella systems such as V. parahaemolyticus, the A. hydrophila LafK protein does not compensate for the lack of the polar flagella regulator FlrA (V. parahaemolyticus FlaK homologue). This is consistent with the fact that the A. hydrophila FlrA mutation abolishes polar flagella formation in liquid and on solid surfaces but does not affect inducible lateral flagella formation. The results highlight that the polar and lateral flagella inter-connections and control networks are specific and that there are differences between the dual flagellar systems in A. hydrophila and V. parahaemolyticus. Furthermore, the A. hydrophila polar flagellum transcriptional hierarchy (also in class II, III and IV genes) shares not only some similarities, but also many important differences with those of V. cholerae and P. aeruginosa [185].
Comparative proposed A. hydrophila, V. cholerae [186], and P. aeruginosa [183] polar flagellum gene transcription hierarchies are shown in Figure 3.
Figure 3.
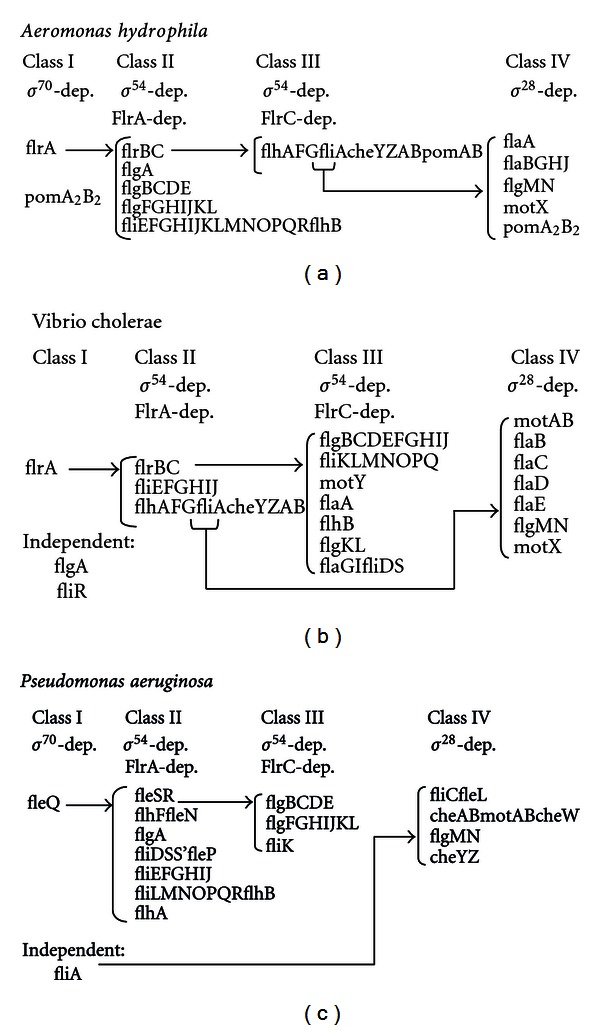
Polar flagellum gene transcription hierarchies.
7.2.3. Virulence Factor
The flagella of pathogenic bacteria promote the colonization and invasion of the host's mucosa. Once the bacteria reach the mucosa the flagellum structure becomes necessary for motility, adhesion and invasion. This motility, coupled with chemotaxis, permits the pathogens to reach the target-tissue of the mucosa. In Helicobacter pylori and P. aeruginosa motility is crucial for the infection of stomach and lungs, respectively. V. cholerae motility is necessary to colonize the intestinal mucosa. In P. mirabilis the swarming is associated with provoking important urinary tract infections. Motility is associated with the invasion of epithelial cells in Y. enterocolitica. Furthermore the flagella are reported to act like adhesins, most probably via its flagellin dominions D2 and D3 [187]. The flagellin of P. aeruginosa is able to bind to the lungs mucine [188]. Some enteropathogenic strains of E. coli adhere to the intestinal mucosa via a flagellum-dependent mechanism [189]. Motility and presence of flagella is also associated with biofilm formation, which generally goes along with persistent infections.
The colonization of the mucosa provokes a proinflamatory response or inducible innate immune response, mainly stimulated by specific cells of the mucosa. In mammals TLR5 (Toll-like receptor 5) is implicated in flagellin recognition by its dominion D1 [190]. TLR5 stimulate the transcription of the NF-κB (nuclear factor-kappaB) and MAPK (mitogen-activated protein kinase) dependent proinflamatory genes. Systemic injection of flagellins in mice induces the production of proinflammatory cytokines like TNF-α (Tumor Necrosis Factor α), IL-6 (Interleukine 6) and nitric oxide. In epithelial cells flagellins of E. coli, S. enterica sv. Typhimurium and P. aeruginosa stimulate the secretion of IL-8, the essential chemokine to attract neutrophiles and macrophages to the infection site [187]. Additionally, glycosylation of the flagellin protein was shown to play a role in the proinflammatory action of P. aeruginosa flagellin. IL-8 release from A549 cells stimulated with nonglycosylated flagellin was significantly reduced when compared to wildtype flagellin [191]. Similar pathogenic mechanisms have been observed for Aeromonas polar and lateral flagella ([174], [181], and unpublished results).
7.2.4. Glycosylation
Glycosylation is the most abundant polypeptide chain modification in nature. Glycans can be covalently attached to the amide nitrogen of Asn residues (N-glycosylation), to the hydroxyl oxygen of, typically, Ser or Thr residues (O-glycosylation), and, in rare cases, to the indole C2 carbon of Trp. Protein glycosylation was first demonstrated in the late 1930s and was long thought to exist only in eukaryotes. As more than two-thirds of eukaryotic proteins are predicted to be glycosylated [192] and these modifications are essential for a multitude of cellular functions [193], it is not surprising that countless publications have been dedicated to this topic. It took 40 years until the first bacterial and archaeal glycoproteins were discovered on the surface layers (S-layers) of the archaeon Halobacterium salinarum [194] and on the S-layers of two hyperthermophilic Clostridium species [195, 196].
O-linked protein glycosylation occurs in all three domains of life, and the eukaryotic and bacterial pathways are well characterized. In this section, we focus on the O-glycan pathways that modify bacterial flagella and pili. Our understanding of bacterial flagellin- and pilin-specific O-glycosylation systems has also been growing, and general O-glycosylation pathways have been identified. A distinguishing feature is that the O-linkages can be formed with Ser, Thr or Tyr residues [197].
More than 20 years later, the first bacterial N-linked protein glycosylation (Pgl) pathway was described in the bacterium C. jejuni [198].Examples of surface-associated glycoproteins in Gram-negative bacteria are the pilins of P. aeruginosa and Neisseria spp., the adhesins TibA and AIDA-1 of E. coliand HMW1of Haemophilus influenzae, and the flagellins of P. aeruginosa, H. pylori, Clostridium botulinum, and C. jejuni/C. coli [199]. These species solely possess a polar flagellar system and the filaments are constituted by the two flagellins FlaA and FlaB. The structural characterization of the flagellins in this species led to identify the presence of pseudaminic acid (Pse5Ac7Ac) decorated in O-linkage to serine (Ser) or threonine (Thr) residues in the central region of the primary sequence, which is predicted to be surface exposed in the assembled filament [200]. Pseudaminic acid (5,7-diacetamido-3,5,7,9-tetradeoxy-L-glycero-L-manno-nonulosonate acid) is a sugar of 9 carbon-atoms, similar to N-acetylneuroaminic acid or sialic acid (Neu5Ac). In C. jejuni also other residues that modify flagellins have been found, all of them deriving from pseudaminic acid [201], whereas in C. coli flagellin modification with pseudaminic acid and two derivates of the related nonulosonate sugar legionaminic acid (5,7-diacetamido-3,5,7,9-tetradeoxy-D-glycero-D-galacto-nonulosonic acid, Leg5Ac7Ac) was reported. Up to 19 glycosylation sites were found in both Campylobacter flagellins and these modifications represent about 10% of the total protein mass In H. pylori, 7 glycosylation sites in FlaA and 10 in FlaB were found. The glycosylation sites do not seem to be related to a certain conserved peptide sequence, though a hydrophobic region next to the Ser/Thr residues was often described [202].
At the genetic level, glycosylation islands (GI) have been identified in P. aeruginosa and P. syringae. The GI of Campylobacter appears to be one of the most variable loci in the genome, containing in between 25 and 50 genes, depending on the strain, that is situated close to those of the flagellins [203]. A number of genes of this locus encode proteins with homology to carbohydrate biosynthetic enzymes, including some (neu-locus) with homology to sialic acid biosynthesis enzymes [204]. Extensive mutational analyses, in addition to novel metabolomic analyses and functional studies on recombinant enzymes, have defined the precise function of a number of GI genes in the Pse5Ac7Ac biosynthetic pathway and provided preliminary evidence for the role of the ptm genes from this locus in the legionaminic acid biosynthetic pathway [201]. In addition to carbohydrate biosynthetic genes, the Campylobacter GI contains multiple copies of hypothetical genes encoding proteins which belong to the motility accessory factor (MAF, Cj1318 family) of proteins. Insertional inactivation of individual copies of these genes has provided evidence for a role in motility or glycosylation although the precise function of each MAF gene remains to be determined [205].
The functionality of glycosylated flagellins and the precise nature of glycosylation in pathogenic bacteria are still to be determined. In some species, like C. jejuni and H. pylori, glycosylation is necessary for filament assembly [205]; whereas the absence of glycosylation does not affect assembly nor motility in P. aeruginosa. Flagellar glycosylation reportedly plays an important role in intestine colonization of C. jejuni [203] and the proinflammatory action of P. aeruginosa [191]. In P. syringae the flagellum-glycosylating residues determine the specificity to the host plant and also play a role in stabilization of the filament structure [206].
In A. caviae, the polar flagellins, FlaA and FlaB, were shown to be glycosylated, at six or seven sites, respectively, with a novel nonulosonate sugar derivate of 373 Da mass, and glycosylation was required for flagellar assembly [207, 208]. Though bacterial adherence to Hep-2 cells requires polar flagella, it is not yet known if the novel glycan plays a role in this process [209]. A. caviae Sch3N possesses a small genomic island that is involved in both flagellin glycosylation and LPS O-antigen biosynthesis. This island appears to have been laterally acquired as it is flanked by insertion element-like sequences and has a much lower G-C content than the average aeromonad G-C content. Most of the gene products encoded by the island are orthologues of proteins that have been shown to be involved in pseudaminic acid biosynthesis and flagellin glycosylation. Two of the genes, lst and lsg, are LPS specific as mutation of them results in the loss of only a band for the LPS O-antigen. The proteins encoded by flmA, flmB, neuA, flmD, and neuB are thought to make up a pseudaminic acid biosynthetic pathway, and mutation of any of these genes resulted in the loss of motility, flagellar expression, and a band for the LPS O-antigen [210]. Studies on lateral flagella from A. hydrophila have indicated that their flagellins are also glycosylated, although the structure of the glycan has yet to be determined [173]. Of significance is the identification of a homologue (maf5) of the Campylobacter MAF family of genes, in the lateral flagella structural locus of A. hydrophila [181]. The product of this gene is required for lateral flagella production and provides the first preliminary evidence that glycosylation may also be required for lateral flagella assembly in this species.
A. hydrophila strain AH-3 in-frame deletion mutants of pseudaminic acid biosynthetic genes pseB and pseF homologues resulted in abolition of polar and lateral flagella formation by posttranscriptional regulation of the flagellins, which was restored by complementation with wildtype pseB or F homologues or Campylobacter pseB and F [211]. Polar and lateral flagellin proteins from A. hydrophila strain AH-3 (serogroup O34) were found to be glycosylated with different carbohydrate moieties. The lateral flagellin was modified at three sites in O-linkage, with a single monosaccharide of 376 Da, which we show to be a pseudaminic acid derivative. The polar flagellin was modified with a heterogeneous glycan, comprised of a heptasaccharide, linked through the same 376 Da sugar to the protein backbone, also in O-linkage [212].
Acknowledgments
This work was supported by Plan Nacional de I+D+i (Ministerio de Educación, Ciencia y Deporte and Ministerio de Sanidad, Spain) and from Generalitat de Catalunya (Centre de Referència en Biotecnologia). The author also thanks Dr. Susana Merino for her support.
References
- 1.Colwell RR, MacDonell MT, De Ley J. Proposal to recognize the family Aeromonadaceae fam. nov. International Journal of Systematic Bacteriology. 1986;36(3):473–477. [Google Scholar]
- 2.Popoff M. Genus III. Aeromonas. Kluyver and Van Niel 1936, 398 AL. In: Krieg NR, Holt JJ, editors. Bergey's Manual of Systematic Bacteriology, Vol. 1. 9th edition. section 5. London: Williams and Wilkins; 1984. pp. 545–547. [Google Scholar]
- 3.Martin-Carnahan A, Joseph SW. Order XII. Aeromonadales. In: Brenner DJ, Krieg NR, Staley JT, editors. Bergey's Manual of Systematic Bacteriology, Vol. 2 Part B. 2nd edition. New York, NY, USA: Springer; 2005. pp. 556–578. [Google Scholar]
- 4.Abbott SL, Cheung WKW, Janda JM. The genus Aeromonas: biochemical characteristics, atypical reactions, and phenotypic identification schemes. Journal of Clinical Microbiology. 2003;41(6):2348–2357. doi: 10.1128/JCM.41.6.2348-2357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Murcia AJ, Benlloch S, Collins MD. Phylogenetic interrelationships of members of the genera Aeromonas and Plesiomonas as determined by 16S ribosomal DNA sequencing: lack of congruence with results of DNA-DNA hybridizations. International Journal of Systematic Bacteriology. 1992;42(3):412–421. doi: 10.1099/00207713-42-3-412. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Murcia AJ, Esteve C, Garay E, Collins MD. Aeromonas allosaccharophila sp. nov., a new mesophilic member of the genus Aeromonas . FEMS Microbiology Letters. 1992;91(3):199–205. doi: 10.1016/0378-1097(92)90698-n. [DOI] [PubMed] [Google Scholar]
- 7.Figueras MJ, Guarro J, Martinez-Murcia A, Graf J. Use of restriction fragment length polymorphism of the PCR-amplified 16S rRNA gene for the identification of Aeromonas spp. Journal of Clinical Microbiology. 2000;38(5):2023–2025. doi: 10.1128/jcm.38.5.2023-2025.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yáñez MA, Catalán V, Apráiz D, Figueras MJ, Martínez-Murcia AJ. Phylogenetic analysis of members of the genus Aeromonas based on gyrB gene sequences. International Journal of Systematic and Evolutionary Microbiology. 2003;53(3):875–883. doi: 10.1099/ijs.0.02443-0. [DOI] [PubMed] [Google Scholar]
- 9.Soler L, Yáñez MA, Chacon MR, et al. Phylogenetic analysis of the genus Aeromonas based on two housekeeping genes. International Journal of Systematic and Evolutionary Microbiology. 2004;54(5):1511–1519. doi: 10.1099/ijs.0.03048-0. [DOI] [PubMed] [Google Scholar]
- 10.Esteve C, Gutierrez MC, Ventosa A. Aeromonas encheleia sp. nov., isolated from European Eels. International Journal of Systematic Bacteriology. 1995;45(3):462–466. doi: 10.1099/00207713-45-3-462. [DOI] [PubMed] [Google Scholar]
- 11.Huys G, Kämpfer P, Altwegg M, et al. Aeromonas popoffii sp. nov., a mesophilic bacterium isolated from drinking water production plants and reservoirs. International Journal of Systematic Bacteriology. 1997;47(4):1165–1171. doi: 10.1099/00207713-47-4-1165. [DOI] [PubMed] [Google Scholar]
- 12.Pidiyar V, Kaznowski A, Narayan NB, Patole M, Shouche YS. Aeromonas culicicola sp. nov., from the midgut of Culex quinquefasciatus . International Journal of Systematic and Evolutionary Microbiology. 2002;52(5):1723–1728. doi: 10.1099/00207713-52-5-1723. [DOI] [PubMed] [Google Scholar]
- 13.Harf-Monteil C, Flèche AL, Riegel P, et al. Aeromonas simiae sp. nov., isolated from monkey faeces. International Journal of Systematic and Evolutionary Microbiology. 2004;54(2):481–485. doi: 10.1099/ijs.0.02786-0. [DOI] [PubMed] [Google Scholar]
- 14.Miñana-Galbis D, Farfán M, Fusté MC, Lorén JG. Aeromonas molluscorum sp. nov., isolated from bivalve molluscs. International Journal of Systematic and Evolutionary Microbiology. 2004;54(6):2073–2078. doi: 10.1099/ijs.0.63202-0. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Murcia AJ. Phylogenetic positions of Aeromonas encheleia, Aeromonas popoffii, Aeromonas DNA hybridization Group 11 and Aeromonas Group 501. International Journal of Systematic Bacteriology. 1999;49(4):1403–1408. doi: 10.1099/00207713-49-4-1403. [DOI] [PubMed] [Google Scholar]
- 16.Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clinical Microbiology Reviews. 2010;23(1):35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirov SM. Aeromonas and Plesiomonas species. In: Doyle MP, Beuchat LR, Montville TJ, editors. Food Microbiology, Fundamentals and Frontiers. Washington, DC, USA: ASM Press; 1997. pp. 265–287. [Google Scholar]
- 18.Majeed KN, Egan AF, Mac Rae IC. Production of exotoxins by Aeromonas spp. at 5°C. Journal of Applied Bacteriology. 1990;69(3):332–337. doi: 10.1111/j.1365-2672.1990.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 19.Janda JM. Recent advances in the study of the taxonomy, pathogenicity, and infectious syndromes associated with the genus Aeromonas . Clinical Microbiology Reviews. 1991;4(4):397–410. doi: 10.1128/cmr.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph SW, Carnahan A. The isolation, identification, and systematics of the motile Aeromonas species. Annual Review of Fish Diseases. 1994;4:315–343. [Google Scholar]
- 21.Austin B, Austin DA, Dalsgaard I, et al. Characterization of atypical Aeromonas salmonicida by different methods. Systematic and Applied Microbiology. 1998;21(1):50–64. doi: 10.1016/s0723-2020(98)80008-8. [DOI] [PubMed] [Google Scholar]
- 22.Janda JM, Abbott SL. Evolving concepts regarding the genus Aeromonas: an expanding panorama of species, disease presentations, and unanswered questions. Clinical Infectious Diseases. 1998;27(2):332–344. doi: 10.1086/514652. [DOI] [PubMed] [Google Scholar]
- 23.Vila J, Ruiz J, Gallardo F, et al. Aeromonas spp. and traveler’s diarrhea: clinical features and antimicrobial resistance. Emerging Infectious Diseases. 2003;9(5):552–555. doi: 10.3201/eid0905.020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Presley SM, Rainwater TR, Austin GP, et al. Assessment of pathogens and toxicants in New Orleans, LA following Hurricane Katrina. Environmental Science and Technology. 2006;40(2):468–474. doi: 10.1021/es052219p. [DOI] [PubMed] [Google Scholar]
- 25.Yu HB, Zhang YL, Lau YL, et al. Identification and characterization of putative virulence genes and gene clusters in Aeromonas hydrophila PPD134/91. Applied and Environmental Microbiology. 2005;71(8):4469–4477. doi: 10.1128/AEM.71.8.4469-4477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reith ME, Singh RK, Curtis B, et al. The genome of Aeromonas salmonicida subsp. salmonicida A449: insights into the evolution of a fish pathogen. BMC Genomics. 2008;9, article 427 doi: 10.1186/1471-2164-9-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seshadri R, Joseph SW, Chopra AK, et al. Genome sequence of Aeromonas hydrophila ATCC 7966T: jack of all trades. Journal of Bacteriology. 2006;188(23):8272–8282. doi: 10.1128/JB.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Liu Y, Zhou Z, et al. Complete genome sequence of Aeromonas veronii strain B565. Journal of Bacteriology. 2011;193(13):3389–3390. doi: 10.1128/JB.00347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts IS. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annual Review of Microbiology. 1996;50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- 30.Merino S, Tomás JM. Encyclopedia of Life Sciences. 3rd edition. New York, NY, USA: John Wiley & Sons; 2010. Bacterial capsules and evasion of immune responses. http://www.els.net/ [Google Scholar]
- 31.Wang Z, Larocque S, Vinogradov E, et al. Structural studies of the capsular polysaccharide and lipopolysaccharide O-antigen of Aeromonas salmonicida strain 80204-1 produced under in vitro and in vivo growth conditions. European Journal of Biochemistry. 2004;271(22):4507–4516. doi: 10.1111/j.1432-1033.2004.04410.x. [DOI] [PubMed] [Google Scholar]
- 32.Shaw DH, Lee YZ, Squires MJ, Luderitz O. Structural studies on the O-antigen of Aeromonas salmonicida . European Journal of Biochemistry. 1983;131(3):633–638. doi: 10.1111/j.1432-1033.1983.tb07310.x. [DOI] [PubMed] [Google Scholar]
- 33.Garrote A, Bonet R, Merino S, Simon-Pujol MD, Congregado F. Occurrence of a capsule in Aeromonas salmonicida . FEMS Microbiology Letters. 1992;74(2-3):127–131. doi: 10.1016/0378-1097(92)90417-m. [DOI] [PubMed] [Google Scholar]
- 34.Garduño RA, Thornton JC, Kay WW. Aeromonas salmonicida grown in vivo . Infection and Immunity. 1993;61(9):3854–3862. doi: 10.1128/iai.61.9.3854-3862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merino S, Alberti S, Tomás JM. Aeromonas salmonicida resistance to complement-mediated killing. Infection and Immunity. 1994;62(12):5483–5490. doi: 10.1128/iai.62.12.5483-5490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merino S, Aguilar A, Rubires X, Simon-Pujol D, Congregado F, Tomás JM. The role of the capsular polysaccharide of Aeromonas salmonicida in the adherence and invasion of fish cell lines. FEMS Microbiology Letters. 1996;142(2-3):185–189. doi: 10.1111/j.1574-6968.1996.tb08428.x. [DOI] [PubMed] [Google Scholar]
- 37.Martínez MJ, Simon-Pujol D, Congregado F, Merino S, Rubires X, Tomás JM. The presence of capsular polysaccharide in mesophilic Aeromonas hydrophila serotypes O:11 and O:34. FEMS Microbiology Letters. 1995;128(1):69–74. doi: 10.1111/j.1574-6968.1995.tb07502.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YL, Arakawa E, Leung KY. Novel Aeromonas hydrophila PPD134/91 genes involved in O-antigen and capsule biosynthesis. Infection and Immunity. 2002;70(5):2326–2335. doi: 10.1128/IAI.70.5.2326-2335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang YL, Lau YL, Arakawa E, Leung KY. Detection and genetic analysis of group II capsules in Aeromonas hydrophila . Microbiology. 2003;149(4):1051–1060. doi: 10.1099/mic.0.26144-0. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh AS, Young KD. Helical disposition of proteins and lipopolysaccharide in the outer membrane of Escherichia coli . Journal of Bacteriology. 2005;187(6):1913–1922. doi: 10.1128/JB.187.6.1913-1922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikaido H. Outer membrane. In: Neidhardt FC, Curtiss R, Ingraham JL, et al., editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. Washington, DC, USA: ASM Press; 1996. pp. 29–47. [Google Scholar]
- 42.Whitfield C, Valvano MA. Biosynthesis and expression of cell-surface polysaccharides in gram-negative bacteria. Advances in Microbial Physiology. 1993;35:135–246. doi: 10.1016/s0065-2911(08)60099-5. [DOI] [PubMed] [Google Scholar]
- 43.Pluschke G, Mercer A, Kusecek B. Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infection and Immunity. 1983;39(2):599–608. doi: 10.1128/iai.39.2.599-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pluschke G, Achtman M. Degree of antibody-independent activation of the classical complement pathway by K1 Escherichia coli differs with O antigen type and correlates with virulence of meningitis in newborns. Infection and Immunity. 1984;43(2):684–692. doi: 10.1128/iai.43.2.684-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joiner KA. Complement evasion by bacteria and parasites. Annual Review of Microbiology. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- 46.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annual Review of Biochemistry. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samuel G, Reeves P. Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydrate Research. 2003;338(23):2503–2519. doi: 10.1016/j.carres.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Valvano MA. Export of O-specific lipopolysaccharide. Frontiers in Bioscience. 2003;8:s452–s471. doi: 10.2741/1079. [DOI] [PubMed] [Google Scholar]
- 49.Valvano MA. In: Comprehensive Natural Products Chemistry II. Vol. 6: Carbohydrates, Nucleosides and Nucleic Acids. Mander LN, Liu HW, editors. Oxford, UK: Elsevier; 2010. pp. 297–314. [Google Scholar]
- 50.Price NP, Momany FA. Modeling bacterial UDP-HexNAc: polyprenol-P HexNAc-1-P transferases. Glycobiology. 2005;15(9):29–42. doi: 10.1093/glycob/cwi065. [DOI] [PubMed] [Google Scholar]
- 51.Merino S, Jiménez N, Molero R, Bouamama L, Regué M, Tomás JM. A UDP-HexNAc:polyprenol-P GalNAc-1-P transferase (WecP) representing a new subgroup of the enzyme family. Journal of Bacteriology. 2011;193(8):1943–1952. doi: 10.1128/JB.01441-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 53.Lüderitz O, Galanos C, Lehmann V, Mayer H, Rietschel ET, Weckesser J. Chemical structure and biological activities of lipid A’s from various bacterial families. Naturwissenschaften. 1978;65(11):578–585. doi: 10.1007/BF00364907. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Li J, Altman E. Structural characterization of the lipid A region of Aeromonas salmonicida subsp. salmonicida lipopolysaccharide. Carbohydrate Research. 2006;341(17):2816–2825. doi: 10.1016/j.carres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 55.Cuthbertson L, Kos V, Whitfield C. ABC transporters involved in export of cell surface glycoconjugates. Microbiology and Molecular Biology Reviews. 2010;74(3):341–362. doi: 10.1128/MMBR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knirel YA, Vinogradov E, Jiménez N, Merino S, Tomás JM. Structural studies on the R-type lipopolysaccharide of Aeromonas hydrophila . Carbohydrate Research. 2004;339(4):787–793. doi: 10.1016/j.carres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Li J, Vinogradov E, Altman E. Structural studies of the core region of Aeromonas salmonicida subsp. salmonicida lipopolysaccharide. Carbohydrate Research. 2006;341(1):109–117. doi: 10.1016/j.carres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Jiménez N, Canals R, Lacasta A, et al. Molecular analysis of three Aeromonas hydrophila AH-3 (serotype O34) lipopolysaccharide core biosynthesis gene clusters. Journal of Bacteriology. 2008;190(9):3176–3184. doi: 10.1128/JB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiménez N, Lacasta A, Vilches S, et al. Genetics and proteomics of Aeromonas salmonicida lipopolysaccharide core biosynthesis. Journal of Bacteriology. 2009;191(7):2228–2236. doi: 10.1128/JB.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raetz CRH. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles. In: Neidhardt FC, Curtiss R III, Ingraham JL, et al., editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd edition. Washington, DC, USA: ASM Press; 1996. pp. 1035–1063. [Google Scholar]
- 61.Sakazaki R, Shimada T. O-serogrouping scheme for mesophilic Aeromonas strains. Japanese Journal of Medical Science and Biology. 1984;37(5-6):247–255. doi: 10.7883/yoken1952.37.247. [DOI] [PubMed] [Google Scholar]
- 62.Thomas LV, Gross RJ, Cheasty T, Rowe B. Extended serogrouping scheme for motile, mesophilic Aeromonas species. Journal of Clinical Microbiology. 1990;28(5):980–984. doi: 10.1128/jcm.28.5.980-984.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janda JM, Abbott SL, Khashe S, Kellogg GH, Shimada T. Further studies on biochemical characteristics and serologic properties of the genus Aeromonas . Journal of Clinical Microbiology. 1996;34(8):1930–1933. doi: 10.1128/jcm.34.8.1930-1933.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merino S, Camprubi S, Tomás JM. Effect of growth temperature on outer membrane components and virulence of Aeromonas hydrophila strains of serotype O:34. Infection and Immunity. 1992;60(10):4343–4349. doi: 10.1128/iai.60.10.4343-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aguilar A, Merino S, Rubires X, Tomás JM. Influence of osmolarity on lipopolysaccharides and virulence of Aeromonas hydrophila serotype O:34 strains grown at 37°C. Infection and Immunity. 1997;65(4):1245–1250. doi: 10.1128/iai.65.4.1245-1250.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merino S, Rubires X, Aguilar A, et al. Mesophilic Aeromonas sp. serogroup O:11 resistance to complement- mediated killing. Infection and Immunity. 1996;64(12):5302–5309. doi: 10.1128/iai.64.12.5302-5309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knirel YA, Shashkov AS, Senchenkova SN, Merino S, Tomás JM. Structure of the O-polysaccharide of Aeromonas hydrophila O:34; A case of random O-acetylation of 6-deoxy-L-talose. Carbohydrate Research. 2002;337(15):1381–1386. doi: 10.1016/s0008-6215(02)00136-2. [DOI] [PubMed] [Google Scholar]
- 68.Wang Z, Vinogradov E, Larocque S, Harrison BA, Li J, Altman E. Structural and serological characterization of the O-chain polysaccharide of Aeromonas salmonicida strains A449, 80204 and 80204-1. Carbohydrate Research. 2005;340(4):693–700. doi: 10.1016/j.carres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 69.Dooley JSG, Lallier R, Shaw DH, Trust TJ. Electrophoretic and immunochemical analyses of the lipopolysaccharides from various strains of Aeromonas hydrophila . Journal of Bacteriology. 1985;164(1):263–269. doi: 10.1128/jb.164.1.263-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Z, Liu X, Li J, Altman E. Structural characterization of the O-chain polysaccharide of Aeromonas caviae ATCC 15468 lipopolysaccharide. Carbohydrate Research. 2008;343(3):483–488. doi: 10.1016/j.carres.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 71.Jiménez N, Canals R, Saló MT, Vilches S, Merino S, Tomás JM. The Aeromonas hydrophila wb*O34 gene cluster: genetics and temperature regulation. Journal of Bacteriology. 2008;190(12):4198–4209. doi: 10.1128/JB.00153-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. Journal of Leukocyte Biology. 2003;74(4):479–485. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 73.Gumenscheimer M, Mitov I, Galanos C, Freudenberg MA. Beneficial or deleterious effects of a preexisting hypersensitivity to bacterial components on the course and outcome of infection. Infection and Immunity. 2002;70(10):5596–5603. doi: 10.1128/IAI.70.10.5596-5603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nature Immunology. 2002;3(7):667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 75.Hamann L, Alexander C, Stamme C, Zähringer U, Schumann RR. Acute-phase concentrations of lipopolysaccharide (LPS)-binding protein inhibit innate immune cell activation by different LPS chemotypes via different mechanisms. Infection and Immunity. 2005;73(1):193–200. doi: 10.1128/IAI.73.1.193-200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang Z, Georgel P, Du X, et al. CD14 is required for MyD88-independent LPS signaling. Nature Immunology. 2005;6(6):565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 77.Caroff M, Karibian D. Structure of bacterial lipopolysaccharides. Carbohydrate Research. 2003;338(23):2431–2447. doi: 10.1016/j.carres.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 78.Kapp A, Freudenberg M, Galanos C. Induction of human granulocyte chemiluminescence by bacterial lipopolysaccharides. Infection and Immunity. 1987;55(3):758–761. doi: 10.1128/iai.55.3.758-761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Freudenberg MA, Galanos C. Metabolism of LPS in vivo . In: Ryan JL, Morrison DC, editors. Bacterial Endotoxic Lipopolysaccharides, Immunopharmacology and Pathophysiology. Boca Raton, Fla, USA: CRC Press; 1992. pp. 275–294. [Google Scholar]
- 80.Morrison DC. Bacterial endotoxins and pathogenesis. Reviews of Infectious Diseases. 1983;5:S733–S747. doi: 10.1093/clinids/5.supplement_4.s733. [DOI] [PubMed] [Google Scholar]
- 81.Merino S, Rubires X, Knochel S, Tomás JM. Emerging pathogens: Aeromonas spp. International Journal of Food Microbiology. 1995;28(2):157–168. doi: 10.1016/0168-1605(95)00054-2. [DOI] [PubMed] [Google Scholar]
- 82.Albertí S, Álvarez D, Merino S, et al. Analysis of complement C3 deposition and degradation on Klebsiella pneumoniae . Infection and Immunity. 1996;64(11):4726–4732. doi: 10.1128/iai.64.11.4726-4732.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuhn HM, Meier-Dieter U, Mayer H. ECA, the enterobacterial common antigen. FEMS Microbiology Reviews. 1988;54(3):195–222. doi: 10.1111/j.1574-6968.1988.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 84.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli . Annual Review of Biochemistry. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 85.Grant WD, Sutherland IW, Wilkinson JF. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae . Journal of Bacteriology. 1969;100(3):1187–1193. doi: 10.1128/jb.100.3.1187-1193.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Preston JF, III, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. Journal of Bacteriology. 2004;186(9):2724–2734. doi: 10.1128/JB.186.9.2724-2734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zogaj X, Nimtz M, Rohde M, Bokranz W, Römling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Molecular Microbiology. 2001;39(6):1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]
- 88.Merino S, Bouamama L, Knirel YA, Senchenkova SN, Regué M, Tomás JM. Aeromonas surface glucan attached through the O-Antigen ligase represents a new way to obtain UDP-Glucose. PLoS ONE. 2012;7(5, article e35707) doi: 10.1371/journal.pone.0035707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agladze K, Wang X, Romeo T. Spatial periodicity of Escherichia coli K-12 biofilm microstructure initiates during a reversible, polar attachment phase of development and requires the polysaccharide adhesin PGA. Journal of Bacteriology. 2005;187(24):8237–8246. doi: 10.1128/JB.187.24.8237-8246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sutherland IW. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology. 2001;147(1):3–9. doi: 10.1099/00221287-147-1-3. [DOI] [PubMed] [Google Scholar]
- 91.Kumari J, Sahoo PK. Dietary β-1,3 glucan potentiates innate immunity and disease resistance of Asian catfish, Clarias batrachus (L.) Journal of Fish Diseases. 2006;29(2):95–101. doi: 10.1111/j.1365-2761.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 92.Rodríguez I, Chamorro R, Novoa B, Figueras A. β-Glucan administration enhances disease resistance and some innate immune responses in zebrafish (Danio rerio) Fish and Shellfish Immunology. 2009;27(2):369–373. doi: 10.1016/j.fsi.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 93.Beveridge TJ, Pouwels PH, Sara M, et al. Functions of S-layers. FEMS Microbiology Reviews. 1997;20:99–149. doi: 10.1111/j.1574-6976.1997.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 94.Kay WW, Buckley JT, Ishiguro EE, Phipps BM, Monette JP, Trust TJ. Purification and disposition of a surface protein associated with virulence of Aeromonas salmonicida . Journal of Bacteriology. 1981;147(3):1077–1084. doi: 10.1128/jb.147.3.1077-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kay WW, Phipps BM, Ishiguro EE, Trust TJ. Surface layer virulence A-proteins from Aeromonas salmonicida strains. Canadian Journal of Biochemistry and Cell Biology. 1984;62(11):1064–1071. doi: 10.1139/o84-137. [DOI] [PubMed] [Google Scholar]
- 96.Belland RJ, Trust TJ. Cloning of the gene for the surface array protein of Aeromonas salmonicida and evidence linking loss of expression with genetic deletion. Journal of Bacteriology. 1987;169(9):4086–4091. doi: 10.1128/jb.169.9.4086-4091.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dooley JS, Trust TJ. Surface protein composition of Aeromonas hydrophila strains virulent for fish: identification of a surface array protein. Journal of Bacteriology. 1988;170(2):499–506. doi: 10.1128/jb.170.2.499-506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noonan B, Trust TJ. The synthesis, secretion and role in virulence of the paracrystalline surface protein layers of Aeromonas salmonicida and A. hydrophila . FEMS Microbiology Letters. 1997;154(1):1–7. doi: 10.1111/j.1574-6968.1997.tb12616.x. [DOI] [PubMed] [Google Scholar]
- 99.Esteve C, Alcaide E, Canals R, et al. Pathogenic Aeromonas hydrophila serogroup O:14 and O:81 strains with an S layer. Applied and Environmental Microbiology. 2004;70(10):5898–5904. doi: 10.1128/AEM.70.10.5898-5904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chu S, Cavaignac S, Feutrier J, et al. Structure of the tetragonal surface virulence array protein and gene of Aeromonas salmonicida . The Journal of Biological Chemistry. 1991;266(23):15258–15265. [PubMed] [Google Scholar]
- 101.Kay WW, Phipps BM, Ishiguro EE, Trust TJ. Porphyrin binding by the surface array virulence protein of Aeromonas salmonicida . Journal of Bacteriology. 1985;164(3):1332–1336. doi: 10.1128/jb.164.3.1332-1336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Phipps BM, Kay WW. Immunoglobulin binding by the regular surface array of Aeromonas salmonicida . The Journal of Biological Chemistry. 1988;263(19):9298–9303. [PubMed] [Google Scholar]
- 103.Messner P, Steiner K, Zarschler K, Schäffer C. S-layer nanoglycobiology of bacteria. Carbohydrate Research. 2008;343(12):1934–1951. doi: 10.1016/j.carres.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schäffer C, Messner P. Surface-layer glycoproteins: an example for the diversity of bacterial glycosylation with promising impacts on nanobiotechnology. Glycobiology. 2004;14(8):31–42. doi: 10.1093/glycob/cwh064. [DOI] [PubMed] [Google Scholar]
- 105.Messner P. Prokaryotic glycoproteins: unexplored but important. Journal of Bacteriology. 2004;186(9):2517–2519. doi: 10.1128/JB.186.9.2517-2519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kokka RP, Vedros NA, Janda JM. Electrophoretic analysis of the surface components of autoagglutinating surface array protein-positive and surface array protein-negative Aeromonas hydrophila and Aeromonas sobria . Journal of Clinical Microbiology. 1990;28(10):2240–2247. doi: 10.1128/jcm.28.10.2240-2247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wooldridge KG, Williams PH. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiology Reviews. 1993;12(4):325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 108.Stintzi A, Raymond KN. Amonabactin-mediated iron acquisition from transferrin and lactoferrin by Aeromonas hydrophila: direct measurement of individual microscopic rate constants. Journal of Biological Inorganic Chemistry. 2000;5(1):57–66. doi: 10.1007/pl00010655. [DOI] [PubMed] [Google Scholar]
- 109.Beyers BR, Massad G, Barghouthi S, Arceneaux JEL. Iron acquisition and virulence in the motile aeromonads: siderophore-dependent and -independent systems. Experientia. 1991;47(5):416–418. [PubMed] [Google Scholar]
- 110.Telford JR, Raymond KN. Coordination chemistry of the amonabactins, Bis(catecholate) siderophores from Aeromonas hydrophila . Inorganic Chemistry. 1998;37(18):4578–4583. doi: 10.1021/ic980090x. [DOI] [PubMed] [Google Scholar]
- 111.Massad G, Arceneaux JEL, Byers BR. Diversity of siderophore genes encoding biosynthesis of 2,3- dihydroxybenzoic acid in Aeromonas spp. BioMetals. 1994;7(3):227–236. doi: 10.1007/BF00149553. [DOI] [PubMed] [Google Scholar]
- 112.Najimi M, Lemos ML, Osorio CR. Identification of iron regulated genes in the fish pathogen Aeromonas salmonicida subsp. salmonicida: genetic diversity and evidence of conserved iron uptake systems. Veterinary Microbiology. 2009;133(4):377–382. doi: 10.1016/j.vetmic.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 113.Ebanks RO, Dacanay A, Goguen M, Pinto DM, Ross NW. Differential proteomic analysis of Aeromonas salmonicida outer membrane proteins in response to low iron and in vivo growth conditions. Proteomics. 2004;4(4):1074–1085. doi: 10.1002/pmic.200300664. [DOI] [PubMed] [Google Scholar]
- 114.Najimi M, Lemos ML, Osorio CR. Identification of heme uptake genes in the fish pathogen Aeromonas salmonicida subsp. salmonicida . Archives of Microbiology. 2008;190(4):439–449. doi: 10.1007/s00203-008-0391-5. [DOI] [PubMed] [Google Scholar]
- 115.Braun V. Bacterial iron transport related to virulence. Contributions to Microbiology. 2005;12:210–233. doi: 10.1159/000081697. [DOI] [PubMed] [Google Scholar]
- 116.De Lorenzo V, Wee S, Herrero M, Neilands JB. Operator sequences of the aerobactin operon of plasmid colV-K30 binding the ferric uptake regulation (fur) repressor. Journal of Bacteriology. 1987;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hirst ID, Ellis AE. Iron-regulated outer membrane proteins of Aeromonas salmonicida are important protective antigens in Atlantic salmon against furunculosis. Fish and Shellfish Immunology. 1994;4(1):29–45. [Google Scholar]
- 118.Asao T, Kinoshita Y, Kozaki S, Uemura T, Sakaguchi G. Purification and some properties of Aeromonas hydrophila hemolysin. Infection and Immunity. 1984;46(1):122–127. doi: 10.1128/iai.46.1.122-127.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chopra AK, Houston CW. Enterotoxins in Aeromonas-associated gastroenteritis. Microbes and Infection. 1999;1(13):1129–1137. doi: 10.1016/s1286-4579(99)00202-6. [DOI] [PubMed] [Google Scholar]
- 120.Ferguson MR, Xu XJ, Houston CW, et al. Hyperproduction, purification, and mechanism of action of the cytotoxic enterotoxin produced by Aeromonas hydrophila . Infection and Immunity. 1997;65(10):4299–4308. doi: 10.1128/iai.65.10.4299-4308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu XJ, Ferguson MR, Popov VL, Houston CW, Peterson JW, Chopra AK. Role of a cytotoxic enterotoxin in Aeromonas-mediated infections: development of transposon and isogenic mutants. Infection and Immunity. 1998;66(8):3501–3509. doi: 10.1128/iai.66.8.3501-3509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Galindo CL, Gutierrez C, Jr., Chopra AK. Potential involvement of galectin-3 and SNAP23 in Aeromonas hydrophila cytotoxic enterotoxin-induced host cell apoptosis. Microbial Pathogenesis. 2006;40(2):56–68. doi: 10.1016/j.micpath.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 123.Howard SP, Buckley JT. Molecular cloning and expression in Escherichia coli of the structural gene for the hemolytic toxin aerolysin from Aeromonas hydrophila . MGG Molecular & General Genetics. 1986;204(2):289–295. doi: 10.1007/BF00425512. [DOI] [PubMed] [Google Scholar]
- 124.Buckley JT, Howard SP, Chopra AK, Houston CW. The cytotoxic enterotoxin of Aeromonas hydrophila is aerolysin (multiple letters) Infection and Immunity. 1999;67(1):466–467. doi: 10.1128/iai.67.1.466-467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tanoue N, Takahashi A, Okamoto K, et al. A pore-forming toxin produced by Aeromonas sobria activates cAMP-dependent C1-secretory pathways to cause diarrhea. FEMS Microbiology Letters. 2005;242(2):195–201. doi: 10.1016/j.femsle.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 126.Sha J, Kozlova EV, Chopra AK. Role of various enterotoxins in Aeromonas hydrophila-induced gastroenteritis: generation of enterotoxin gene-deficient mutants and evaluation of their enterotoxic activity. Infection and Immunity. 2002;70(4):1924–1935. doi: 10.1128/IAI.70.4.1924-1935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chopra AK, Xu XJ, Ribardo D, et al. The cytotoxic enterotoxin of Aeromonas hydrophila induces proinflammatory cytokine production and activates arachidonic acid metabolism in macrophages. Infection and Immunity. 2000;68(5):2808–2818. doi: 10.1128/iai.68.5.2808-2818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shenkar R, Abraham E. Mechanisms of lung neutrophil activation after hemorrhage or endotoxemia: roles of reactive oxygen intermediates, NF-κB, and cyclic AMP response element binding protein. Journal of Immunology. 1999;163(2):954–962. [PubMed] [Google Scholar]
- 129.Howard SP, Garland WJ, Green MJ, Buckley JT. Nucleotide sequence of the gene for the hole-forming toxin aerolysin of Aeromonas hydrophila . Journal of Bacteriology. 1987;169(6):2869–2871. doi: 10.1128/jb.169.6.2869-2871.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chakraborty T, Montenegro MA, Sanyal SC, et al. Cloning of enterotoxin gene from Aeromonas hydrophila provides conclusive evidence of production of a cytotonic enterotoxin. Infection and Immunity. 1984;46(2):435–441. doi: 10.1128/iai.46.2.435-441.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Potomski J, Burke V, Robinson J, Fumarola D, Miragliotta G. Aeromonas cytotonic enterotoxin cross reactive with cholera toxin. Journal of Medical Microbiology. 1987;23(2):179–186. doi: 10.1099/00222615-23-2-179. [DOI] [PubMed] [Google Scholar]
- 132.Thelestam M, Ljungh A. Membrane-damaging and cytotoxic effects on human fibroblasts of alpha- and beta-hemolysins from Aeromonas hydrophila . Infection and Immunity. 1981;34(3):949–956. doi: 10.1128/iai.34.3.949-956.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leung KY, Stevenson RMW. Tn5-induced protease-deficient strains of Aeromonas hydrophila with reduced virulence for fish. Infection and Immunity. 1988;56(10):2639–2644. doi: 10.1128/iai.56.10.2639-2644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pemberton JM, Kidd SP, Schmidt R. Secreted enzymes of Aeromonas . FEMS Microbiology Letters. 1997;152(1):1–10. doi: 10.1111/j.1574-6968.1997.tb10401.x. [DOI] [PubMed] [Google Scholar]
- 135.Chuang YC, Chiou SF, Su JH, Wu ML, Chang MC. Molecular analysis and expression of the extracellular lipase of Aeromonas hydrophila MCC-2. Microbiology. 1997;143(3):803–812. doi: 10.1099/00221287-143-3-803. [DOI] [PubMed] [Google Scholar]
- 136.Merino S, Aguilar A, Nogueras MM, Regue M, Swift S, Tomás JM. Cloning, sequencing, and role in virulence of two phospholipases (A1 and C) from mesophilic Aeromonas sp. serogroup O:34. Infection and Immunity. 1999;67(8):4008–4013. doi: 10.1128/iai.67.8.4008-4013.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala’Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiology and Molecular Biology Reviews. 2004;68(4):692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kostakioti M, Newman CL, Thanassi DG, Stathopoulos C. Mechanisms of protein export across the bacterial outer membrane. Journal of Bacteriology. 2005;187(13):4306–4314. doi: 10.1128/JB.187.13.4306-4314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Palmer T, Berks BC. Moving folded proteins across the bacterial cell membrane. Microbiology. 2003;149(3):547–556. doi: 10.1099/mic.0.25900-0. [DOI] [PubMed] [Google Scholar]
- 140.Michiels T, Wattiau P, Brasseur R, Ruysschaert JM, Cornelis G. Secretion of Yop proteins by yersiniae. Infection and Immunity. 1990;58(9):2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Galán JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284(5418):1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 142.Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiology and Molecular Biology Reviews. 1998;62(2):379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lloyd SA, Norman M, Rosqvist R, Wolf-Watz H. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Molecular Microbiology. 2001;39(2):520–531. doi: 10.1046/j.1365-2958.2001.02271.x. [DOI] [PubMed] [Google Scholar]
- 144.Burr SE, Stuber K, Wahli T, Frey J. Evidence for a type III secretion system in Aeromonas salmonicida subsp. salmonicida . Journal of Bacteriology. 2002;184(21):5966–5970. doi: 10.1128/JB.184.21.5966-5970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu HB, Rao PSS, Lee HC, et al. A type III secretion system is required for Aeromonas hydrophila AH-1 pathogenesis. Infection and Immunity. 2004;72(3):1248–1256. doi: 10.1128/IAI.72.3.1248-1256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vilches S, Urgell C, Merino S, et al. Complete type III secretion system of a mesophilic Aeromonas hydrophila strain. Applied and Environmental Microbiology. 2004;70(11):6914–6919. doi: 10.1128/AEM.70.11.6914-6919.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sha J, Pillai L, Fadl AA, Galindo CL, Erova TE, Chopra AK. The type III secretion system and cytotoxic enterotoxin alter the virulence of Aeromonas hydrophila . Infection and Immunity. 2005;73(10):6446–6457. doi: 10.1128/IAI.73.10.6446-6457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burr SE, Stuber K, Frey J. The ADP-ribosylating toxin, AexT, from Aeromonas salmonicida subsp. salmonicida is translocated via a type III secretion pathway. Journal of Bacteriology. 2003;185(22):6583–6591. doi: 10.1128/JB.185.22.6583-6591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fehr D, Casanova C, Liverman A, et al. AopP, a type III effector protein of Aeromonas salmonicida, inhibits the NF-κB signalling pathway. Microbiology. 2006;152(9):2809–2818. doi: 10.1099/mic.0.28889-0. [DOI] [PubMed] [Google Scholar]
- 150.Fehr D, Burr SE, Gibert M, D’Alayer J, Frey J, Popoff MR. Aeromonas exoenzyme T of Aeromonas salmonicida is a bifunctional protein that targets the host cytoskeleton. The Journal of Biological Chemistry. 2007;282(39):28843–28852. doi: 10.1074/jbc.M704797200. [DOI] [PubMed] [Google Scholar]
- 151.Vilches S, Wilhelms M, Yu HB, Leung KY, Tomás JM, Merino S. Aeromonas hydrophila AH-3 AexT is an ADP-ribosylating toxin secreted through the type III secretion system. Microbial Pathogenesis. 2008;44(1):1–12. doi: 10.1016/j.micpath.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 152.Sha J, Wang SF, Suarez G, et al. Further characterization of a type III secretion system (T3SS) and of a new effector protein from a clinical isolate of Aeromonas hydrophila-part I. Microbial Pathogenesis. 2007;43(4):127–146. doi: 10.1016/j.micpath.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 153.Pukatzki S, Ma AT, Sturtevant D, et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(39):15508–15513. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pukatzki S, McAuley SB, Miyata ST. The type VI secretion system: translocation of effectors and effector-domains. Current Opinion in Microbiology. 2009;12(1):11–17. doi: 10.1016/j.mib.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 156.Schwarz S, Hood RD, Mougous JD. What is type VI secretion doing in all those bugs? Trends in Microbiology. 2010;18(12):531–537. doi: 10.1016/j.tim.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bernard CS, Brunet YR, Gueguen E, Cascales E. Nooks and crannies in type VI secretion regulation. Journal of Bacteriology. 2010;192(15):3850–3860. doi: 10.1128/JB.00370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Suarez G, Sierra JC, Sha J, et al. Molecular characterization of a functional type VI secretion system from a clinical isolate of Aeromonas hydrophila . Microbial Pathogenesis. 2008;44(4):344–361. doi: 10.1016/j.micpath.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Han S, Craig JA, Putnam CD, Carozzi NB, Tainer JA. Evolution and mechanism from structures of an ADP-ribosylating toxin and NAD complex. Nature Structural Biology. 1999;6(10):932–936. doi: 10.1038/13300. [DOI] [PubMed] [Google Scholar]
- 160.Burke V, Cooper M, Robinson J. Hemagglutination patterns of Aeromonas spp. in relation to biotype and source. Journal of Clinical Microbiology. 1984;19(1):39–43. doi: 10.1128/jcm.19.1.39-43.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Proft T, Baker EN. Pili in Gram-negative and Gram-positive bacteria—structure, assembly and their role in disease. Cellular and Molecular Life Sciences. 2009;66(4):613–635. doi: 10.1007/s00018-008-8477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kirov SM, Jacobs I, Hayward LJ, Hapin RH. Electron microscopic examination of factors influencing the expression of filamentous surface structures on clinical and environmental isolates of Aeromonas veronii biotype sobria . Microbiology and Immunology. 1995;39(5):329–338. doi: 10.1111/j.1348-0421.1995.tb02209.x. [DOI] [PubMed] [Google Scholar]
- 163.Pepe CM, Eklund MW, Strom MS. Cloning of an Aeromonas hydrophila type IV pilus biogenesis gene cluster: complementation of pilus assembly functions and characterization of a type IV leader peptidase/N-methyltransferase required for extracellular protein secretion. Molecular Microbiology. 1996;19(4):857–869. doi: 10.1046/j.1365-2958.1996.431958.x. [DOI] [PubMed] [Google Scholar]
- 164.Barnett TC, Kirov SM, Strom MS, Sanderson K. Aeromonas spp. possess at least two distinct type IV pilus families. Microbial Pathogenesis. 1997;23(4):241–247. doi: 10.1006/mpat.1997.0152. [DOI] [PubMed] [Google Scholar]
- 165.Kirov SM, Barnett TC, Pepe CM, Strom MS, John Albert M. Investigation of the role of type IV Aeromonas pilus (Tap) in the pathogenesis of Aeromonas gastrointestinal infection. Infection and Immunity. 2000;68(7):4040–4048. doi: 10.1128/iai.68.7.4040-4048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hadi N, Yang Q, Barnett TC, Tabei SMB, Kirov SM, Shaw JG. Bundle-forming pilus locus of Aeromonas veronii bv. Sobria. Infection and Immunity. 2012;80(4):1351–1360. doi: 10.1128/IAI.06304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boyd JM, Dacanay A, Knickle LC, et al. Contribution of type IV pili to the virulence of Aeromonas salmonicida subsp. salmonicida in Atlantic salmon (Salmo salar L.) Infection and Immunity. 2008;76(4):1445–1455. doi: 10.1128/IAI.01019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Quinn DM, Atkinson HM, Bretag AH, et al. Carbohydrate-reactive, pore-forming outer membrane proteins of Aeromonas hydrophila . Infection and Immunity. 1994;62(9):4054–4058. doi: 10.1128/iai.62.9.4054-4058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Merino S, Tomás JM. Pili and Flagella: Current Research and Future Trends. Norfolk, UK: Caister Academic Press; 2009. Lateral flagella systems; pp. 173–190. [Google Scholar]
- 170.Macnab RM. How bacteria assemble flagella. Annual Review of Microbiology. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- 171.McCarter LL. Polar flagellar motility of the Vibrionaceae . Microbiology and Molecular Biology Reviews. 2001;65(3):445–462. doi: 10.1128/MMBR.65.3.445-462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rabaan AA, Gryllos I, Tomás JM, Shaw JG. Motility and the polar flagellum are required for Aeromonas caviae adherence to HEp-2 cells. Infection and Immunity. 2001;69(7):4257–4267. doi: 10.1128/IAI.69.7.4257-4267.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gavín R, Rabaan AA, Merino S, Tomás JM, Gryllos I, Shaw JG. Lateral flagella of Aeromonas species are essential for epithelial cell adherence and biofilm formation. Molecular Microbiology. 2002;43(2):383–397. doi: 10.1046/j.1365-2958.2002.02750.x. [DOI] [PubMed] [Google Scholar]
- 174.Canals R, Ramirez S, Vilches S, et al. Polar flagellum biogenesis in Aeromonas hydrophila . Journal of Bacteriology. 2006;188(2):542–555. doi: 10.1128/JB.188.2.542-555.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Berg HC. The rotary motor of bacterial flagella. Annual Review of Biochemistry. 2003;72:19–54. doi: 10.1146/annurev.biochem.72.121801.161737. [DOI] [PubMed] [Google Scholar]
- 176.Terashima H, Fukuoka H, Yakushi T, Kojima S, Homma M. The Vibrio motor proteins, MotX and MotY, are associated with the basal body of Na+-driven flagella and required for stator formation. Molecular Microbiology. 2006;62(4):1170–1180. doi: 10.1111/j.1365-2958.2006.05435.x. [DOI] [PubMed] [Google Scholar]
- 177.Wilhelms M, Vilches S, Molero R, Shaw JG, Tomás JM, Merino S. Two redundant sodium-driven stator motor proteins are involved in Aeromonas hydrophila polar flagellum rotation. Journal of Bacteriology. 2009;191(7):2206–2217. doi: 10.1128/JB.01526-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Karlyshev AV, Linton D, Gregson NA, Wren BW. A novel paralogous gene family involved in phase-variable flagella-mediated motility in Campylobacter jejuni . Microbiology. 2002;148(2):473–480. doi: 10.1099/00221287-148-2-473. [DOI] [PubMed] [Google Scholar]
- 179.Molero R, Wilhelms M, Infanzón B, Tomás JM, Merino S. Aeromonas hydrophila motY is essential for polar flagellum function, and requires coordinate expression of motX and Pom proteins. Microbiology. 2011;157(10):2772–2784. doi: 10.1099/mic.0.049544-0. [DOI] [PubMed] [Google Scholar]
- 180.Stewart BJ, McCarter LL. Lateral flagellar gene system of Vibrio parahaemolyticus . Journal of Bacteriology. 2003;185(15):4508–4518. doi: 10.1128/JB.185.15.4508-4518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Canals R, Altarriba M, Vilches S, et al. Analysis of the lateral flagellar gene system of Aeromonas hydrophila AH-3. Journal of Bacteriology. 2006;188(3):852–862. doi: 10.1128/JB.188.3.852-862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chilcott GS, Hughes KT. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar typhimurium and Escherichia coli . Microbiology and Molecular Biology Reviews. 2000;64(4):694–708. doi: 10.1128/mmbr.64.4.694-708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dasgupta N, Wolfgang MC, Goodman AL, et al. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa . Molecular Microbiology. 2003;50(3):809–824. doi: 10.1046/j.1365-2958.2003.03740.x. [DOI] [PubMed] [Google Scholar]
- 184.Kim YK, McCarter LL. Cross-regulation in Vibrio parahaemolyticus: compensatory activation of polar flagellar genes by the lateral flagellar regulator LafK. Journal of Bacteriology. 2004;186(12):4014–4018. doi: 10.1128/JB.186.12.4014-4018.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wilhelms M, Molero R, Shaw JG, Tomás JM, Merino S. Transcriptional hierarchy of Aeromonas hydrophila polar-flagellum genes. Journal of Bacteriology. 2011;193(19):5179–5190. doi: 10.1128/JB.05355-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Syed KA, Beyhan S, Correa N, et al. The Vibrio cholerae flagellar regulatory hierarchy controls expression of virulence factors. Journal of Bacteriology. 2009;191(21):6555–6570. doi: 10.1128/JB.00949-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ramos HC, Rumbo M, Sirard JC. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends in Microbiology. 2004;12(11):509–517. doi: 10.1016/j.tim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 188.Lillehoj EP, Kim BT, Kim KC. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. American Journal of Physiology. 2002;282(4):L751–L756. doi: 10.1152/ajplung.00383.2001. [DOI] [PubMed] [Google Scholar]
- 189.Girón JA, Torres AG, Freer E, Kaper JB. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Molecular Microbiology. 2002;44(2):361–379. doi: 10.1046/j.1365-2958.2002.02899.x. [DOI] [PubMed] [Google Scholar]
- 190.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 191.Verma A, Arora SK, Kuravi SK, Ramphal R. Roles of specific amino acids in the N terminus of Pseudomonas aeruginosa flagellin and of flagellin glycosylation in the innate immune response. Infection and Immunity. 2005;73(12):8237–8246. doi: 10.1128/IAI.73.12.8237-8246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochimica et Biophysica Acta. 1999;1473(1):4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 193.Moens S, Vanderleyden J. Glycoproteins in prokaryotes. Archives of Microbiology. 1997;168(3):169–175. doi: 10.1007/s002030050484. [DOI] [PubMed] [Google Scholar]
- 194.Mescher MF, Strominger JL. Purification and characterization of a prokaryotic glycoprotein from the cell envelope of Halobacterium salinarium . The Journal of Biological Chemistry. 1976;251(7):2005–2014. [PubMed] [Google Scholar]
- 195.Sleytr UB. Heterologous reattachment of regular arrays of glycoproteins on bacterial surfaces. Nature. 1975;257(5525):400–402. doi: 10.1038/257400a0. [DOI] [PubMed] [Google Scholar]
- 196.Sleytr UB, Thorne KJI. Chemical characterization of the regularly arranged surface layers of Clostridium thermosaccharolyticum and Clostridium thermohydrosulfuricum . Journal of Bacteriology. 1976;126(1):377–383. doi: 10.1128/jb.126.1.377-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Logan SM. Flagellar glycosylation—a new component of the motility repertoire? Microbiology. 2006;152(5):1249–1262. doi: 10.1099/mic.0.28735-0. [DOI] [PubMed] [Google Scholar]
- 198.Szymanski CM, Ruijin Y, Ewing CP, Trust TJ, Guerry P. Evidence for a system of general protein glycosylation in Campylobacter jejuni . Molecular Microbiology. 1999;32(5):1022–1030. doi: 10.1046/j.1365-2958.1999.01415.x. [DOI] [PubMed] [Google Scholar]
- 199.Nothaft H, Szymanski CM. Protein glycosylation in bacteria: sweeter than ever. Nature Reviews Microbiology. 2010;8(11):765–778. doi: 10.1038/nrmicro2383. [DOI] [PubMed] [Google Scholar]
- 200.Samatey FA, Imada K, Nagashima S, et al. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature. 2001;410(6826):331–337. doi: 10.1038/35066504. [DOI] [PubMed] [Google Scholar]
- 201.McNally DJ, Hui JPM, Aubry AJ, et al. Functional characterization of the flagellar glycosylation locus in Campylobacter jejuni 81-176 using a focused metabolomics approach. The Journal of Biological Chemistry. 2006;281(27):18489–18498. doi: 10.1074/jbc.M603777200. [DOI] [PubMed] [Google Scholar]
- 202.Logan SM, Hui JPM, Vinogradov E, et al. Identification of novel carbohydrate modifications on Campylobacter jejuni 11168 flagellin using metabolomics-based approaches. FEBS Journal. 2009;276(4):1014–1023. doi: 10.1111/j.1742-4658.2008.06840.x. [DOI] [PubMed] [Google Scholar]
- 203.Szymanski CM, Logan SM, Linton D, Wren BW. Campylobacter—a tale of two protein glycosylation systems. Trends in Microbiology. 2003;11(5):233–238. doi: 10.1016/s0966-842x(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 204.Schoenhofen IC, Lunin VV, Julien JP, et al. Structural and functional characterization of PseC, an aminotransferase involved in the biosynthesis of pseudaminic acid, an essential flagellar modification in Helicobacter pylori. The Journal of Biological Chemistry. 2006;281(13):8907–8916. doi: 10.1074/jbc.M512987200. [DOI] [PubMed] [Google Scholar]
- 205.Guerry P, Ewing CP, Schirm M, et al. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Molecular Microbiology. 2006;60(2):299–311. doi: 10.1111/j.1365-2958.2006.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Taguchi F, Shibata S, Suzuki T, et al. Effects of glycosylation on swimming ability and flagellar polymorphic transformation in Pseudomonas syringae pv. tabaci 6605. Journal of Bacteriology. 2008;190(2):764–768. doi: 10.1128/JB.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gryllos I, Shaw JG, Gavín R, Merino S, Tomás JM. Role of flm locus in mesophilic Aeromonas species adherence. Infection and Immunity. 2001;69(1):65–74. doi: 10.1128/IAI.69.1.65-74.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schirm M, Schoenhofen IC, Logan SM, Waldron KC, Thibault P. Identification of unusual bacterial glycosylation by tandem mass spectrometry analyses of intact proteins. Analytical Chemistry. 2005;77(23):7774–7782. doi: 10.1021/ac051316y. [DOI] [PubMed] [Google Scholar]
- 209.Rabaan AA, Gryllos I, Tomás JM, Shaw JG. Motility and the polar flagellum are required for Aeromonas caviae adherence to HEp-2 cells. Infection and Immunity. 2001;69(7):4257–4267. doi: 10.1128/IAI.69.7.4257-4267.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tabei SMB, Hitchen PG, Day-Williams MJ, et al. An Aeromonas caviae Genomic island ism required for both O-antigen lipopolysaccharide biosynthesis and flagellinlycosylation. Journal of Bacteriology. 2009;191(8):2851–2863. doi: 10.1128/JB.01406-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Canals R, Vilches S, Wilhelms M, Shaw JG, Merino S, Tomaás JM. Non-structural flagella genes affecting both polar and lateral flagella-mediated motility in Aeromonas hydrophila . Microbiology. 2007;153(4):1165–1175. doi: 10.1099/mic.0.2006/000687-0. [DOI] [PubMed] [Google Scholar]
- 212.Wilhelms M, Fulton KM, Twine SM, Toma's JM, Merino S. Differential glycosylation of polar and lateral flagellins in Aeromonas hydrophila AH-3. The Journal of Biological Chemistry. 2012;287(33):27851–27862. doi: 10.1074/jbc.M112.376525. [DOI] [PMC free article] [PubMed] [Google Scholar]


