Abstract
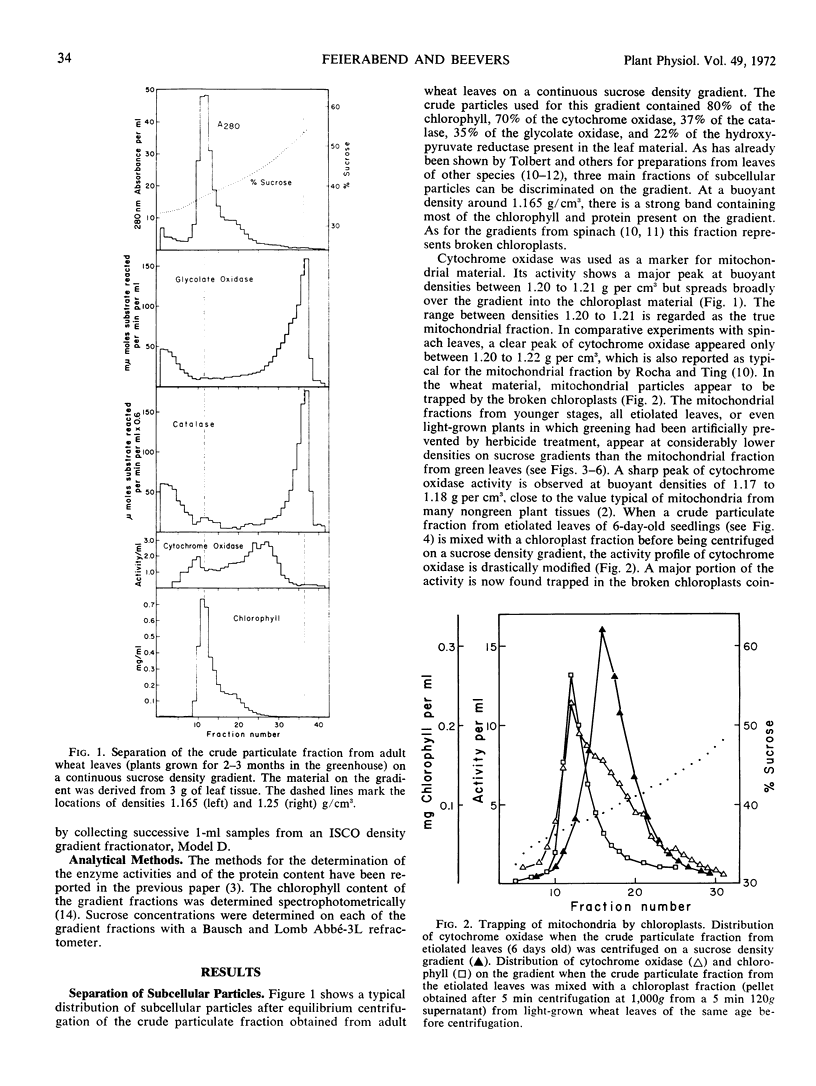
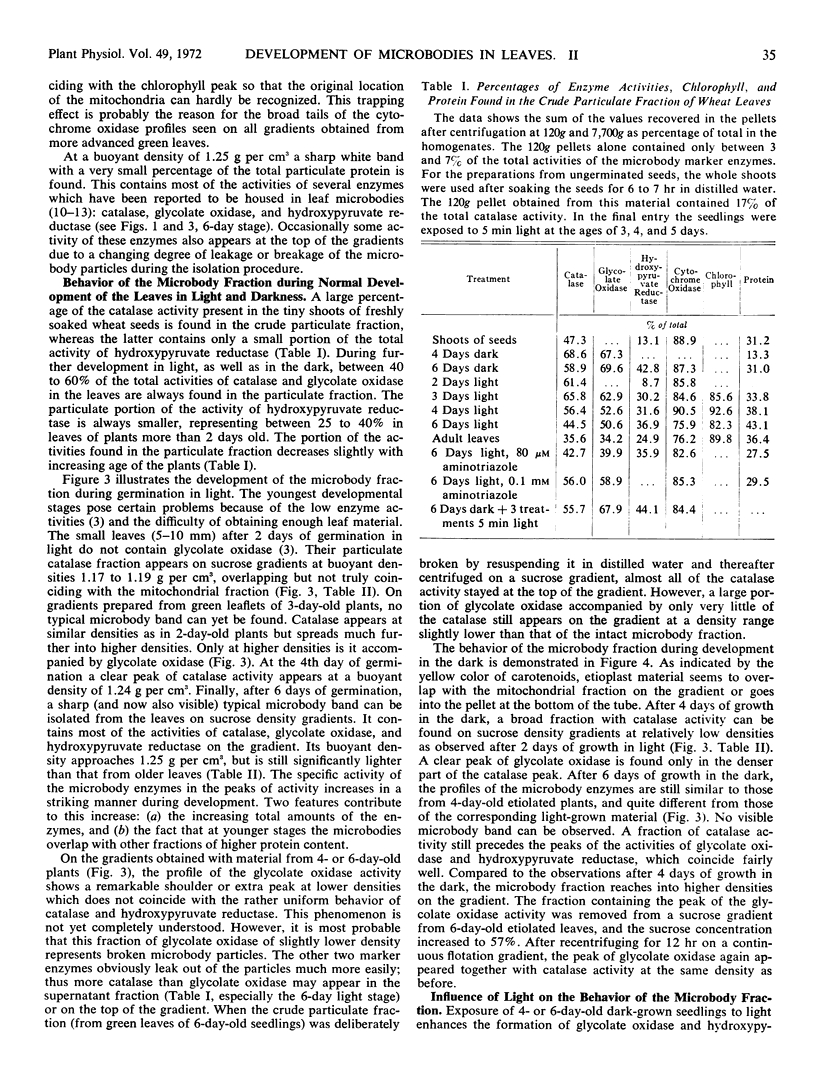
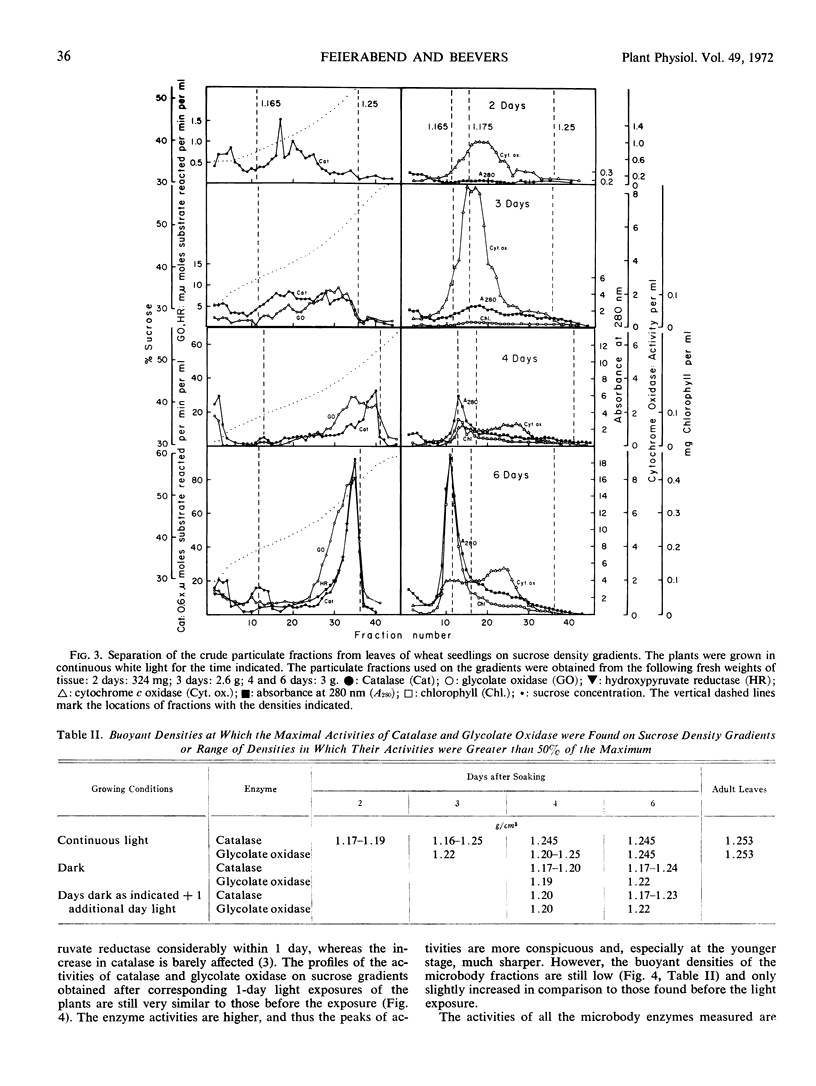
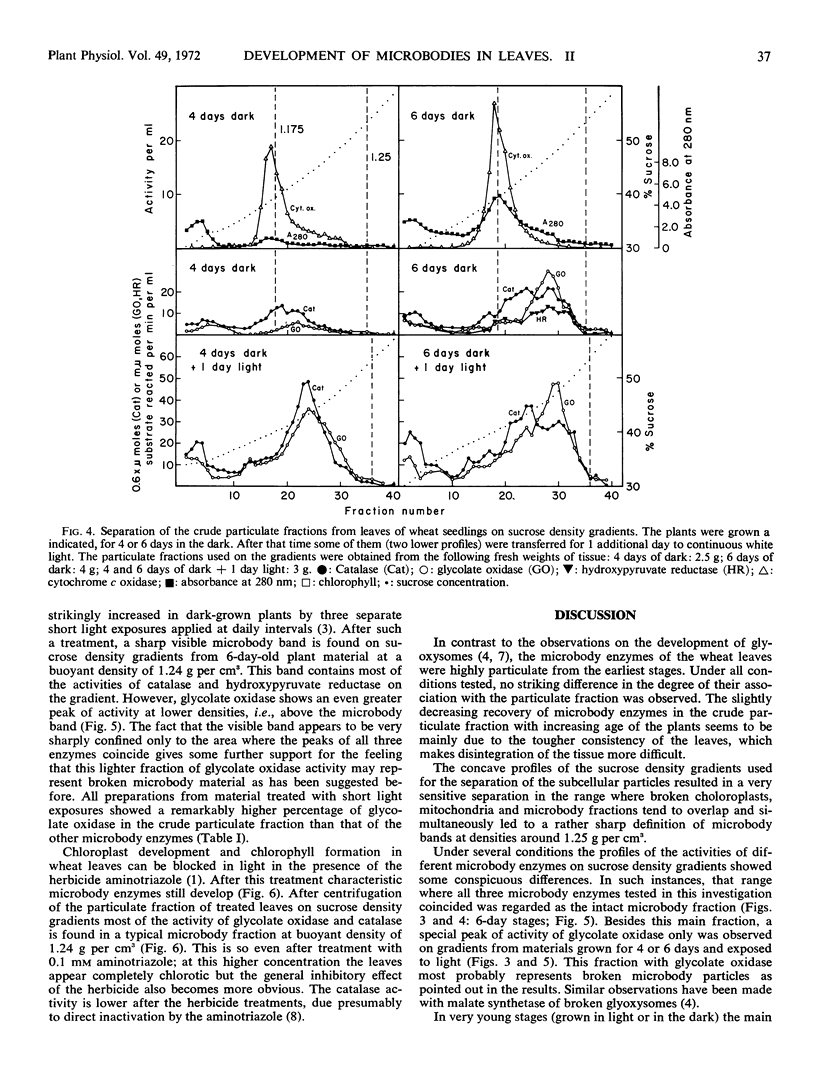
Crude particulate fractions from wheat leaves (Triticum vulgare L.) were separated on continuous sucrose density gradients, resulting in: broken chloroplasts, a mitochondrial fraction (indicated by cytochrome c oxidase), and microbodies. The visible band of the microbody fraction from adult leaves appears at a buoyant density of 1.25 grams per cm3 and contains most of the activities of catalase, glycolate oxidase, and hydroxypyruvate reductase on the gradient. In the shoots of freshly soaked seeds, catalase is already highly particulate. During further development in light or in darkness, 40 to 60% of the total activities of catalase and glycolate oxidase and 25 to 40% of the total activity of hydroxypyruvate reductase are always found in the particulate fractions of the leaves. In young developmental stages, the peaks of the activity profiles of the microbody enzymes appear on sucrose gradients at relatively low densities, first between 1.17 to 1.20 grams per cm3. During development in light, the buoyant density of the microbody fraction shifts to the final value of 1.25 grams per cm3. However, even after 1 week of growth in the dark, the microbody fraction from etiolated leaves was observed at buoyant densitites 1.17 to 1.24 grams per cm3 and did not appear as a defined visible band. A characteristic visible microbody band at a buoyant density 1.24 grams per cm3 was found when the dark-grown seedlings received only three separate 5-minute exposures to white light. A similar peak was also obtained from light-grown leaves in which chloroplast development had been blocked by 3-amino-1,2,4-triazole.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels P. G., Matsuda K., Siegel A., Weier T. E. Chloroplastic ribosome formation: inhibition by 3-amino-1,2,4-triazole. Plant Physiol. 1967 May;42(5):736–741. doi: 10.1104/pp.42.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental studies on microbodies in wheat leaves : I. Conditions influencing enzyme development. Plant Physiol. 1972 Jan;49(1):28–32. doi: 10.1104/pp.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. C., Taylor J. M. Effects of organic acids on ion uptake and retention in barley roots. Plant Physiol. 1970 Oct;46(4):538–542. doi: 10.1104/pp.46.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo G. P., Longo C. P. The development of glyoxysomes in maize scutellum: changes in morphology and enzyme compartmentation. Plant Physiol. 1970 Oct;46(4):599–604. doi: 10.1104/pp.46.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A., SCHEJTER A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J. 1960 Feb;74:339–348. doi: 10.1042/bj0740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K. Leaf peroxisomes and their relation to photorespiration and photosynthesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):325–341. doi: 10.1111/j.1749-6632.1969.tb43119.x. [DOI] [PubMed] [Google Scholar]



