Abstract
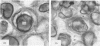
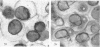
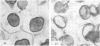
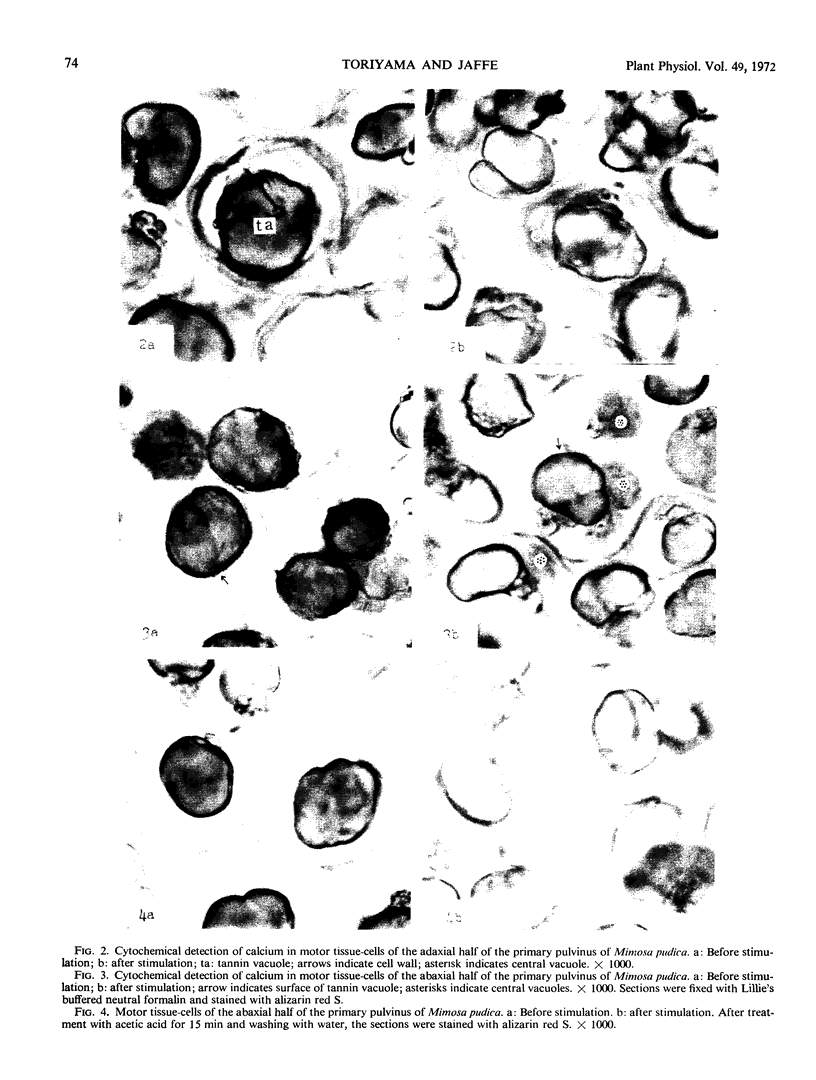
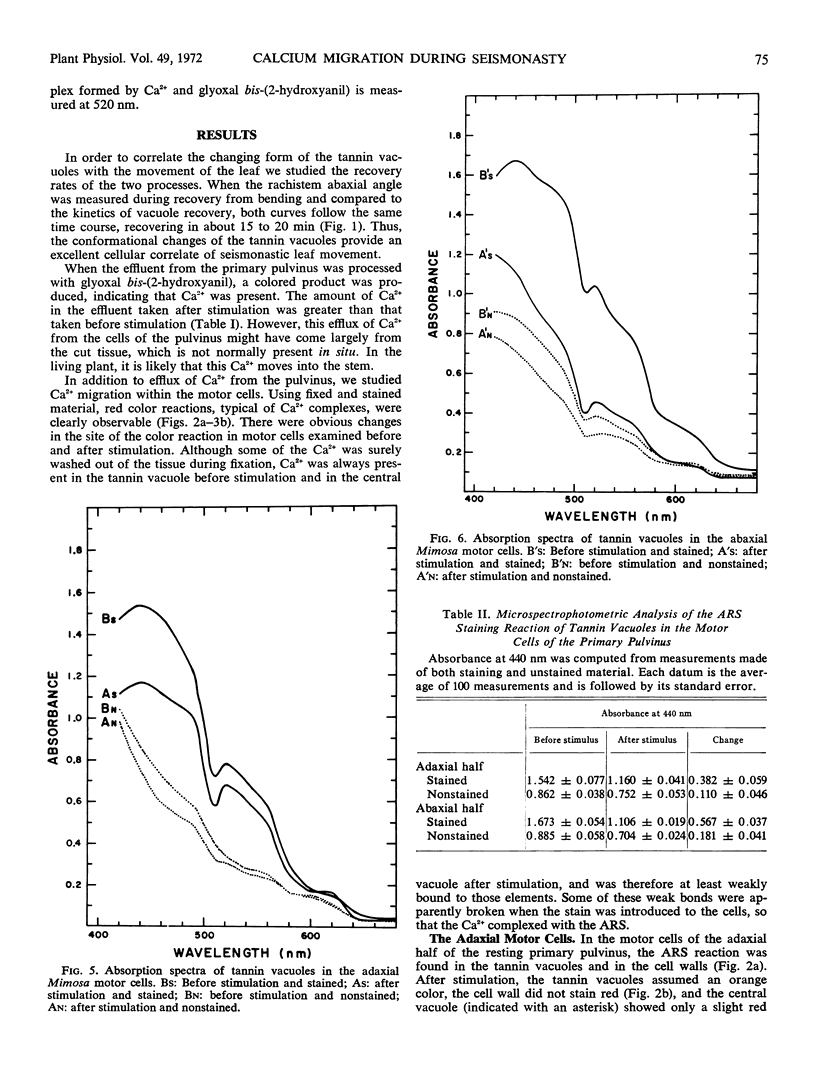
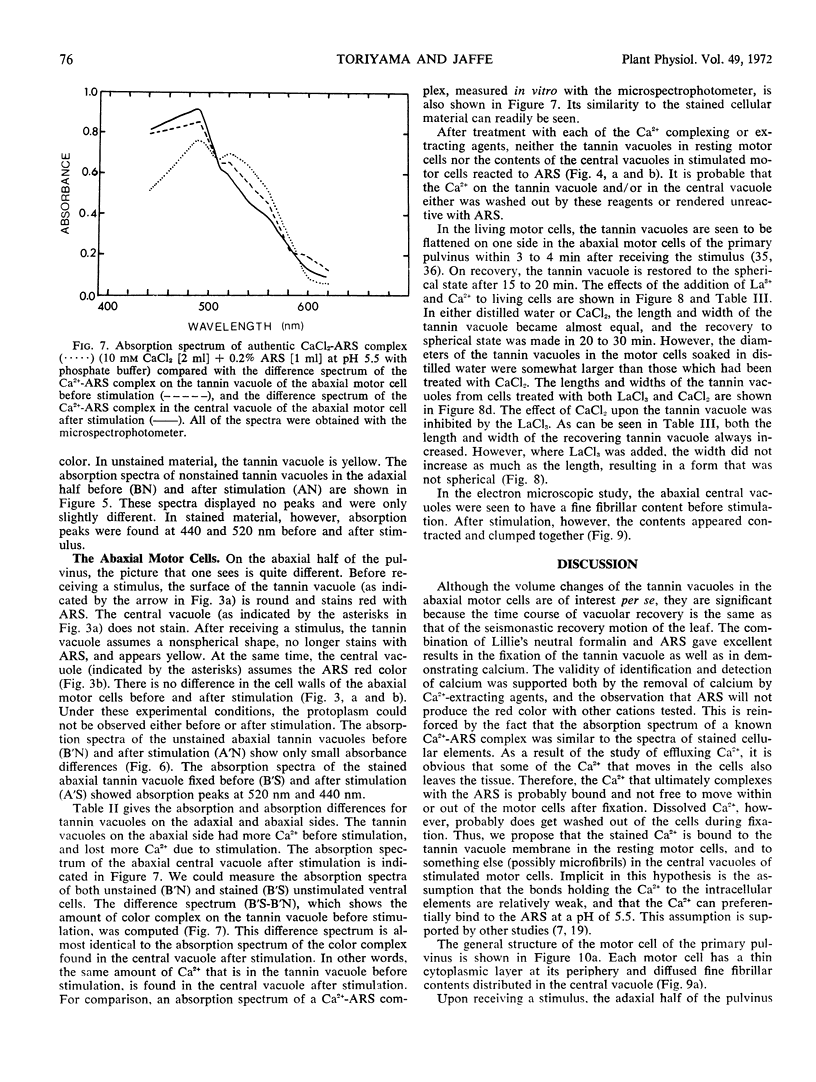
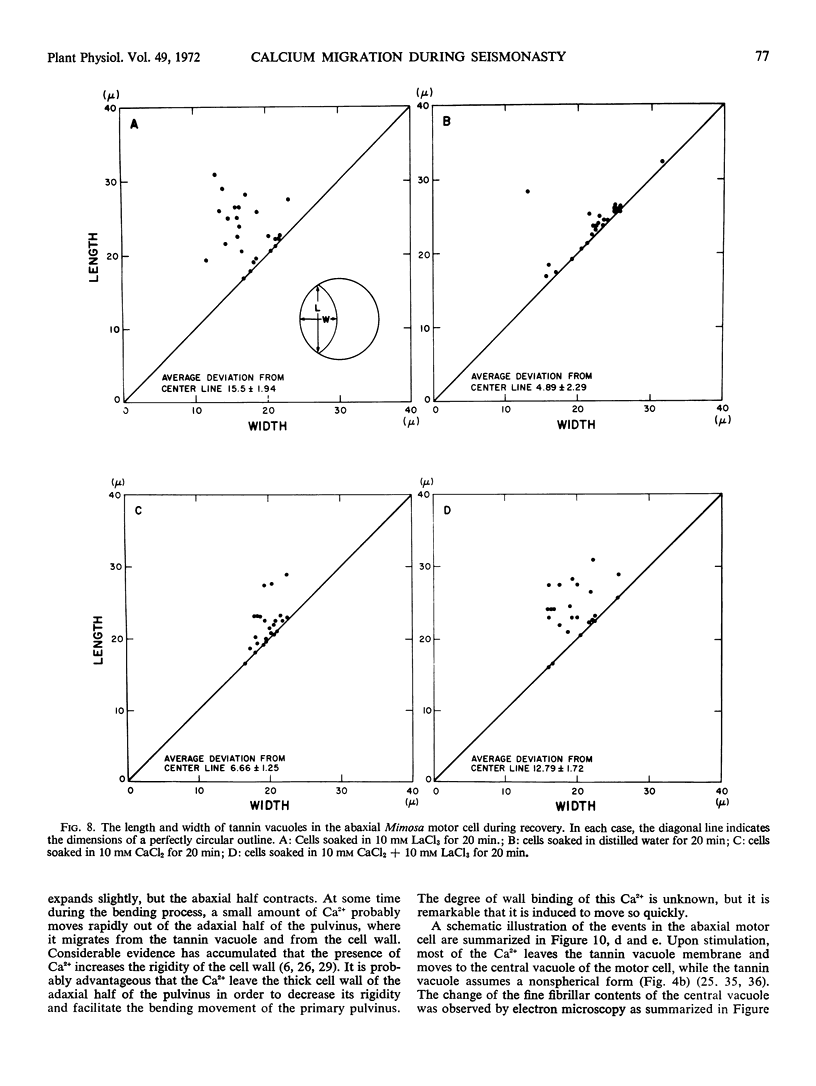
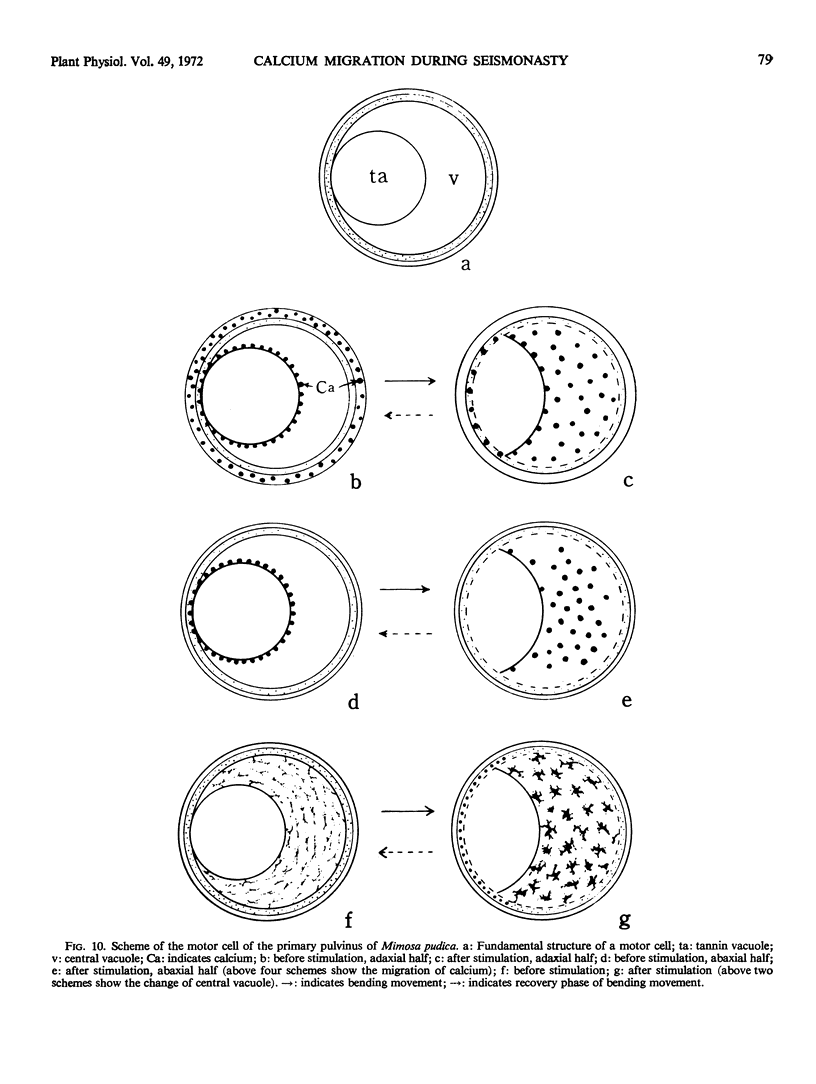
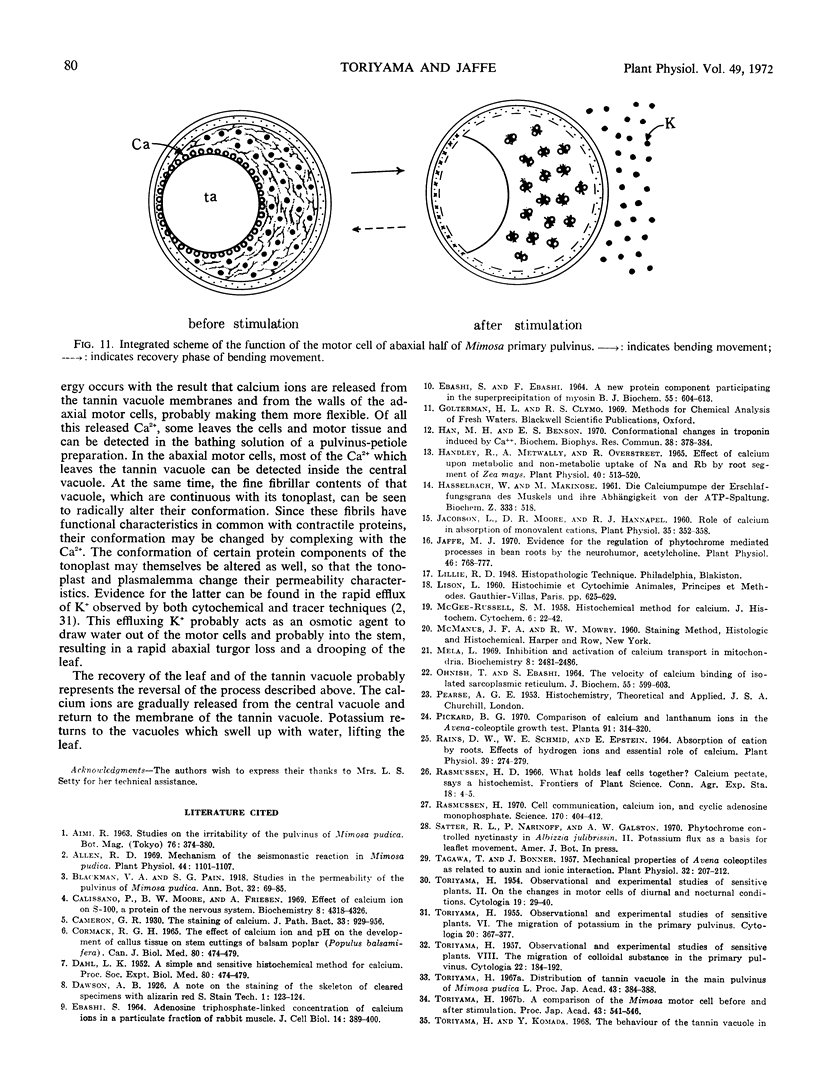
Volume and conformational changes of the contractile tannin vacuoles of the abaxial motor cells of the primary pulvinus of Mimosa pudica L. parallel the seismonastic leaf movement. Since such changes in cells and organelles of animal systems are often regulated by calcium, we studied Ca2+ movement in the motor cells and tissue. By fixation with Lillie's neutral buffered formalin, followed by staining with alizarin red sulfate (ARS), calcium was localized in the tannin vacuoles of the motor cells of the primary pulvinus. After treatment with ethylenediaminetetraacetate, 8-hydroxyquinoline, and several other calcium-complexing or extracting agents, the color reaction due to alizarin red sulfonate was no longer present. By using an analytical method, it was shown that the effluent from stimulated pulvini has significantly more Ca2+ than that from unstimulated controls. Ten millimolar LaCl3 inhibits recovery of the tannin vacuole in vivo in 10 mm CaCl2 or in distilled water. Quantitative data obtained by microspectrophotometry demonstrated calcium migration during the bending movement of the primary pulvinus. In the adaxial motor cells a small amount of calcium migrates from the tannin vacuole, and calcium on the cell wall moves to the central vacuole. In the abaxial half, a large amount of calcium from the tannin vacuole moves to the central vacuole of the motor cell. It is probable that the calcium binds to the microfibrillar contents of the central vacuole. These observations support the contention that Ca2+ migrates between the surface of the tannin vacuole and the inside of the central vacuole. The recovery and maintenance of the tannin vacuole in the spherical form may play a role in maintaining turgor in the motor cells of the abaxial half of the primary pulvinus of Mimosa.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D. Mechanism of the Seismonastic Reaction in Mimosa pudica. Plant Physiol. 1969 Aug;44(8):1101–1107. doi: 10.1104/pp.44.8.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calissano P., Moore B. W., Friesen A. Effect of calcium ion on S-100, a protein of the nervous system. Biochemistry. 1969 Nov;8(11):4318–4326. doi: 10.1021/bi00839a015. [DOI] [PubMed] [Google Scholar]
- DAHL L. K. A simple and sensitive histochemical method for calcium. Proc Soc Exp Biol Med. 1952 Jul;80(3):474–479. doi: 10.3181/00379727-80-19661. [DOI] [PubMed] [Google Scholar]
- EBASHI S., EBASHI F. A NEW PROTEIN COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF MYOSIN B. J Biochem. 1964 Jun;55:604–613. doi: 10.1093/oxfordjournals.jbchem.a127933. [DOI] [PubMed] [Google Scholar]
- Han M. H., Benson E. S. Conformational changes in troponin induced by Ca++1. Biochem Biophys Res Commun. 1970 Feb 6;38(3):378–384. doi: 10.1016/0006-291x(70)90724-2. [DOI] [PubMed] [Google Scholar]
- Handley R., Metwally A., Overstreet R. Effects of Ca Upon Metabolic and Nonmetabolic Uptake of Na and Rb by Root Segments of Zea mays. Plant Physiol. 1965 May;40(3):513–520. doi: 10.1104/pp.40.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Moore D. P., Hannapel R. J. Role of Calcium in Absorption of Monovalent Cations. Plant Physiol. 1960 May;35(3):352–358. doi: 10.1104/pp.35.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M. J. Evidence for the regulation of phytochrome-mediated processes in bean roots by the neurohumor, acetylcholine. Plant Physiol. 1970 Dec;46(6):768–777. doi: 10.1104/pp.46.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGEE-RUSSELL S. M. Histochemical methods for calcium. J Histochem Cytochem. 1958 Jan;6(1):22–42. doi: 10.1177/6.1.22. [DOI] [PubMed] [Google Scholar]
- OHNISHI T., EBASHI S. THE VELOCITY OF CALCIUM BINDING OF ISOLATED SARCOPLASMIC RETICULUM. J Biochem. 1964 Jun;55:599–603. doi: 10.1093/oxfordjournals.jbchem.a127932. [DOI] [PubMed] [Google Scholar]
- Rains D. W., Schmid W. E., Epstein E. Absorption of Cations by Roots. Effects of Hydrogen Ions and Essential Role of Calcium. Plant Physiol. 1964 Mar;39(2):274–278. doi: 10.1104/pp.39.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Tagawa T., Bonner J. Mechanical Properties of the Avena Coleoptile As Related to Auxin and to Ionic Interactions. Plant Physiol. 1957 May;32(3):207–212. doi: 10.1104/pp.32.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C. Blockade of membrane calcium fluxes by lanthanum in relation to vascular smooth muscle contractility. Arch Int Physiol Biochim. 1969 Oct;77(4):710–716. doi: 10.3109/13813456909059783. [DOI] [PubMed] [Google Scholar]
- Viets F. G. CALCIUM AND OTHER POLYVALENT CATIONS AS ACCELERATORS OF ION ACCUMULATION BY EXCISED BARLEY ROOTS. Plant Physiol. 1944 Jul;19(3):466–480. doi: 10.1104/pp.19.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., De Weer P. Lanthanum inhibition of 45Ca efflux from the squid giant axon. Nature. 1970 May 23;226(5247):760–761. doi: 10.1038/226760a0. [DOI] [PubMed] [Google Scholar]