Abstract
A review of contemporary research on the working memory system (WMS) is important, both due to the need to focus the discussion on further necessary investigations on the structure and function of this key part of the human brain, as well as to share this knowledge with clinicians. In our introduction we try to clarify the actual terminology and provide an intuitively understandable model for 3 basic cognitive operations: perception, recognition, imagery, and manipulation of recalled mental images. We emphasize the importance of knowledge of the structure and function of the WMS for the possibility to demonstrate the links between genetic polymorphisms and the prevalence to some mental disorders. We also review current knowledge of working memory dysfunction in the most common diseases and specific clinical situations such as maturation and aging. Finally, we briefly discuss methods for assessment of WMS capacity. This article establishes a kind of compendium of knowledge for clinicians who are not familiar with the structure and operation of the WMS.
Keywords: working memory, genetics of mental disorders, assessment of memory capacity, short-term memory, neural circuits of imagery
Background
Working memory is essential for most important human cognitive processes taking place in the central executive part of the brain [1]. In recent years, neuroscientists and clinicians have increasingly referred to this concept [2–4].
Impairments of Working Memory System (WMS) capacity cause the early signs of aging, manifested by difficulties in memorizing, performing planned daily activities, and solving different kinds of problems. People with mental or behavioral disorders also exhibit disturbances in the WMS. Karlsgodt et al. and Ziermans et al. drew attention to the significant role of the WMS in shaping patterns of behavior [5,6]. Ziemans et al. refer to the exploratory work of A. Baddeley and emphasize that the WMS is responsible for the retention and manipulation of information over a brief period of time [6]. The ability to interact with the environment depends on ability to keep the perceived or remembered information in an active state during the time necessary for the mental manipulation of them. The WMS is necessary for activities such as reading, writing, planning, problem solving, and coherent acting and communication [5].
The WMS capacity is related to general intellectual ability [7]. Impaired WM capacity was found in many psychiatric disorders, including schizophrenia [8–10]. Low WM capacity in children is a risk factor for later psychopathology, especially psychotic syndromes, depression, and suicidal ideation [11]. Impaired WM capacity is also an important factor in emotional disequilibrium and self-regulation [12]. A restricted self-regulation caused by low WM capacity is usually associated with behavioral problems in children, such as aggression and antisocial behavior.
Bigos et al., in an exhaustive review of modern accessible technologies relevant to investigations of the WMS, postulates that there are 3 major regions of the brain – the hippocampus, amygdales, and prefrontal cortex – which can be evaluated in individuals who have behavioral disorders, in the search for pathological changes [13,14].
Currently, there are a variety of methods enabling the evaluation of WMS structure and function. The first useful tools of these studies were based on functional magnetic resonance imaging (fMRI). Changes in blood oxygen level-dependent (BOLD) activations were demonstrated in fronto-parietal brain regions, particularly during experiments related to the visuospatial WM (VSWM) capacity [15,16]. This field of structural research was recently expanded to studies of the “conectome” [17].
Development of successful therapeutic approaches requires an intuitive understanding of the structure and function of the system to evaluate and differentiate possible causes of impairments of the WMS. Various authors differ in opinion regarding the structures and function of this system. There is also a great deal of confusion in terminology. It is necessary to clarify the meaning of terms such as episodic memory, short-term memory, and long-term memory. Another problem in mastering knowledge useful for clinicians is caused by the different models proposed for the WMS.
Karlsgodt, for instance, based her review of genetic influences on the working memory on Baddeley’s model of WMS circuitry [1,5], but this is a non-intuitive “box-type” model, which use the concept of memory buffers. In our opinion, intuitive understanding of working memory activities requires distinction of perception, recognition, and recall of mental images (imaginary). We believe that explanations of mechanisms of memory should avoid the notion of transfer of information between buffers.
Therefore, we will try to present an intuitively comprehensible model of the structure and function of WSM based on the ideas of Eric R. Kandel [18] and on the basis of our own model of neural circuitry, previously presented and already recognized by other researchers [19,20]. We hope this model will be useful for clinicians who assess the function of the system, differentiate possible damages, and try to treat the observed disorders.
Short Intuitive Definitions of Terms Related to Brain Memory
The following list of short definitions of terms used in the literature take into account all quoted references, especially the textbook by Eric R. Kandel [18].
Episodic memory is the memory of one’s own biographical events that can be precisely related in time, location, and associated emotions. Thus, it is the collection of past personal experiences that occurred at a particular time and place.
Semantic memory is the memory of meanings, understandings, and concepts related to facts, information, and general knowledge about the world. The semantic memory gives meaning to otherwise meaningless words and sentences and enables learning based on past experiences.
Short-term memory is the capacity to keep a small amount of information in mind in an active, readily available state for a short period of time. Short-term memory should be distinguished from working memory, which refers to structures and processes used for temporarily storing and manipulating information. The relationship between short-term memory and working memory is presented variously by different theories. The notion of working memory is broader and more general because it refers to structures and processes used for temporarily stored and manipulated information.
Working memory is the system that actively maintains some amount of information in the mind to enable their manipulation. This encompasses verbal and nonverbal tasks involved in reasoning and comprehension. The results of these manipulations can be available for further information-processing. Working memory tasks complete the goal-directed problem solving and actions. The information processing realized by working memory system consists of manipulations of elements recalled by short-term memory activities.
Long-term memory (LTM) refers to the unlimited, continuing memory store that can hold information over lengthy periods of time, even for an entire lifetime. Long-term memory is mainly preconscious and unconscious. Information in LTM is to a great extent outside of our awareness, but can be called into working memory to be used when needed. Some of this information is easy to recall, but some is much more difficult to access.
LTM is usually divided into declarative (explicit) memory and procedural (implicit) memory. Declarative memory includes all information that is available in the consciousness. Declarative memory can be further divided into episodic memory (specific events) and semantic memory (knowledge about the world). Procedural memory is information about the pattern of body movement and procedures for using objects in the environment.
Autobiographical memory contains information about specific personal, experienced events and personal facts. It refers to a person’s history. An individual does not remember exactly everything that has happened in his past. Autobiographical memory enables the reconstruction of the evolving process of one’s own history, but this reconstruction can be inexact and distorted.
It should be emphasized that the realization of processes described above takes place in a variety of brain structures that often overlap. Nowadays, very often there is no unanimity among neurophysiologists on the exact location of each of these processes. It is, however, important for clinicians know information related to the function of memory.
Attempts to Determine the Location of Discerned Elements of Human Brain Memory
The aim of this chapter is to describe the structure of the working memory. However, the WMS works on more basic elements of human brain memory like episodic memory, semantic memory, and autobiographical memory. Therefore, trying to describe the WMS, it is necessary to refer also to these other types of memory. Distinction of the enumerated kinds of memory is primarily for educational purposes – in fact, the underlying structures overlap. In is important also to realize that the juxtaposition of the long-term memory to short-term memory is functionally important, but it is necessary to realize that the same neuro- anatomical elements are engaged for both memory activities. Careful study of the figures presented in the next chapter will help the reader comprehend this structural overlapping. However, to avoid an overly-subjective presentation, we should first mention the latest experimental findings.
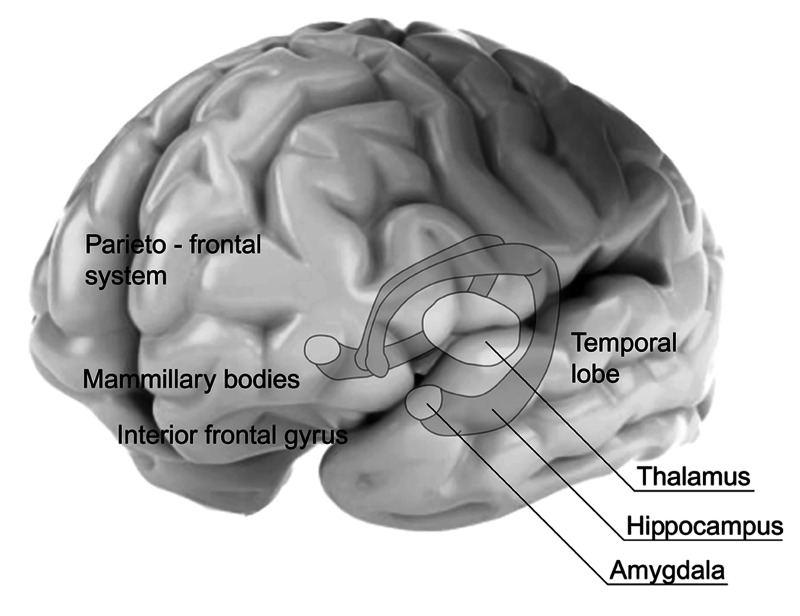
Significant progress in locating components of the human brain memory systems has been made since the implementation of imaging methods based on fMRI. Authors of early papers, which are referred to the results of investigations based on fMRI brain imaging, indicate that the regions important for the working memory are the amygdale, hippocampus, the temporo-parietal and parieto-frontal systems, and the right inferior frontal gyrus [21–23]. The anatomical relations of different parts of the memory system are presented in Figure 1.
Figure 1.
Anatomical locations of elements of the working memory system.
Moscovitch et al. argue that important distinctions exist among different types of memories and the structures that mediate them [24] and that the retention and retrieval of detailed, vivid autobiographical memories depend on the hippocampal system. Semantic memories, on the other hand, benefit from hippocampal contribution for some time before they can be retrieved independently of the hippocampus. They argue also that it is necessary to distinguish between detailed spatial memories and more schematic memories akin to semantic memory that are sufficient for navigation but not for re-experiencing the environment. According to them, the function of the hippocampus and related limbic structures is to help encode, retain, and retrieve experiences, no matter how long ago the events occurred [24].
Daselaar et al. investigated autobiographical memory retrieval [25]. During their experiments, participants recalled personal memories of auditory word cues during event-related functional magnetic resonance imaging (fMRI). The subjects pressed a button when a memory was accessed and maintained and then gave subjective ratings of emotion and reliving. The initial period engaged hippocampal and medial and right prefrontal activity, whereas the later period aroused precuneus and left prefrontal activity. Emotional arousal was correlated with the activity in the amygdale and the hippocampus during the initial period. Reliving ratings were correlated with activity in the visual cortex and ventromedial and inferior prefrontal regions during the later period. The authors emphasized that their findings indicate dynamic recruitment and contribution of emotion and sensory-related brain regions during remembering [25].
Experiments by Burianova et al. tried to delineate a common functional network that underlies autobiographical, episodic, and semantic memory retrieval. They conducted the sophisticated event-related fMRI study [26]. Experiments encompassed investigations of co called functional connectivity [17]. First, they examined the functional connectivity of 3 regions: the left hippocampus, left lingual gyrus, and right caudate nucleus. These regions shared a common pattern of connectivity. Activity in inferior frontal and middle temporal cortex bilaterally, left temporoparietal junction, and anterior and posterior cingulate gyri was positively correlated, whereas activity in posterior occipito-temporo-parietal regions was negatively correlated. The authors argue that a common neural network underlies the retrieval of declarative memories, regardless of memory content [26].
Aggleton wrote that new evidence has emerged for the existence of a series of parallel temporal-diencephalic pathways that function in a reciprocal manner, both directly and indirectly, between the hippocampal formation and the anterior thalamic nuclei [27]. These extended pathways also involve the mammillary bodies, the retrosplenial cortex, and parts of the prefrontal cortex. Recent neuropsychological findings reveal the importance of these hippocampal-anterior thalamic systems for recollective rather than familiarity-based recognition. The direct hippocampal-anterior thalamic projections are often opposed by the indirect hippocampal projections via the mammillary bodies to the thalamus [27].
Ino et al, from the Department of Neurology in Kyoto University, conducted neuroimaging studies of brain regions that are activated during retrieval of autobiographical memory [28]. They maintain that this process overlaps with the activity of the default mode network [28] and that autobiographical memory retrieval is the conscious reconstruction and recollection of personally relevant events. The neuroanatomical basis of this process has been consistently reported to be located in the retrosplenial-posterior cingulate cortex, medial temporal lobe, hippocampus, medial prefrontal cortex, and the inferior parietal lobule. The default mode network is active during rest or low-demand tasks such as independent thoughts, mind wandering, and self-referential sensory processing – processes not related to information in the actual external environment [28]. Constantinidis and Procyk remarked on the relation of the frontal and prefrontal part of the working memory network to other elements of this system [2]. They concluded: “Working memory has long been associated with the prefrontal cortex… Anatomical and physiological evidence suggests, however, that the prefrontal cortex is part of a broader network of interconnected brain areas involved in working memory. These include the parietal and temporal association areas of the cerebral cortex, cingulate and limbic areas, and subcortical structures such as the mediodorsal thalamus and the basal ganglia. Neurophysiological studies in primates confirm the involvement of areas beyond the frontal lobe and illustrate that working memory involves parallel, distributed neuronal network…” [2].
Ranganath reviewed findings from neurophysiological, neuropsychological, and neuroimaging studies of visual working memory in human and nonhuman primates [29]. He concluded that all gathered data “support a model in which visual working memory operations rely on activation of object representations in inferior temporal cortex, via top-down feedback from neocortical areas in the prefrontal and medial temporal cortex, and also from the hippocampus” [29].
The above short review of the latest research on the structure and function of particular components of brain memory is not sufficient for clinical needs; thus it is necessary to provide some generalizations that would constitute in clinicians’ minds a functional model of the working memory.
The working memory system, in order to find a solution to a problem, usually activates more elementary mechanisms of episodic, semantic, and autobiographical memory. An example is the action of the WMS during a sudden worrying situation when someone has lost a precious object and is trying to find it. In such a situation, it is necessary to recall the mental image of this lost object (semantic memory) and recall past events (autobiographical memory). It should be noted that for solving this problem it is necessary to activate, for a period of time, the different kinds of memory traces. A key mechanism that we need to understand is how the activation of a mental image is maintained. We attempt to present such a generalizing model in the next section.
Models of Neural Circuits for Perceptions, Recognition, and Mental Imaginary
The neural circuits realizing perceptions
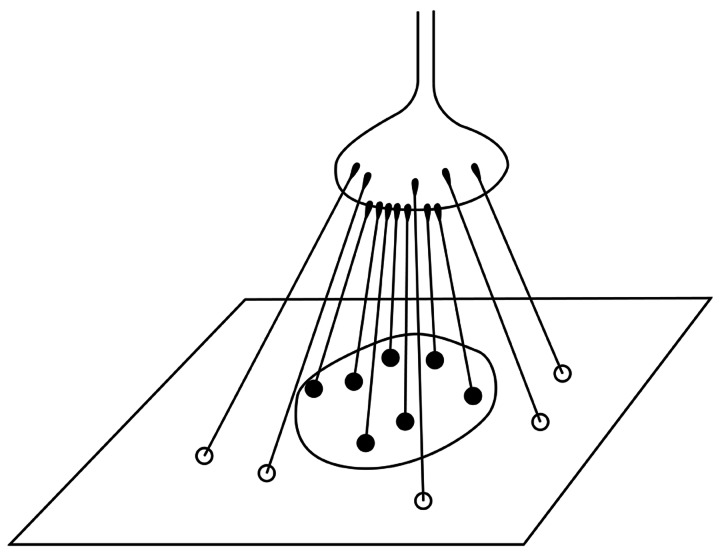
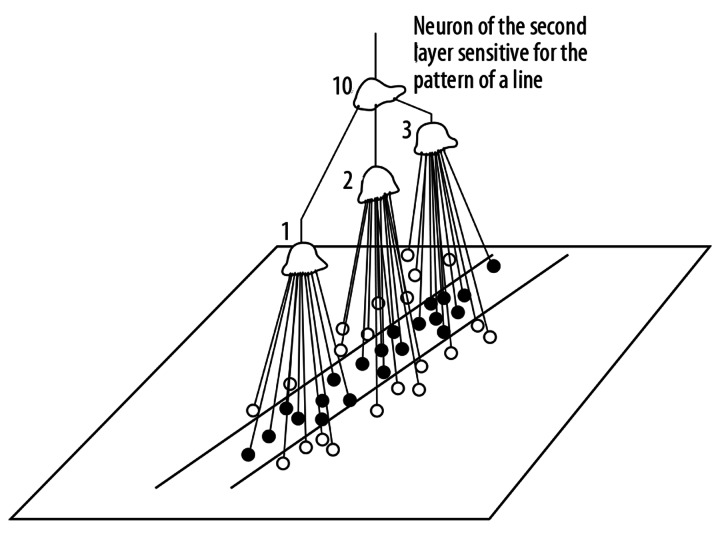
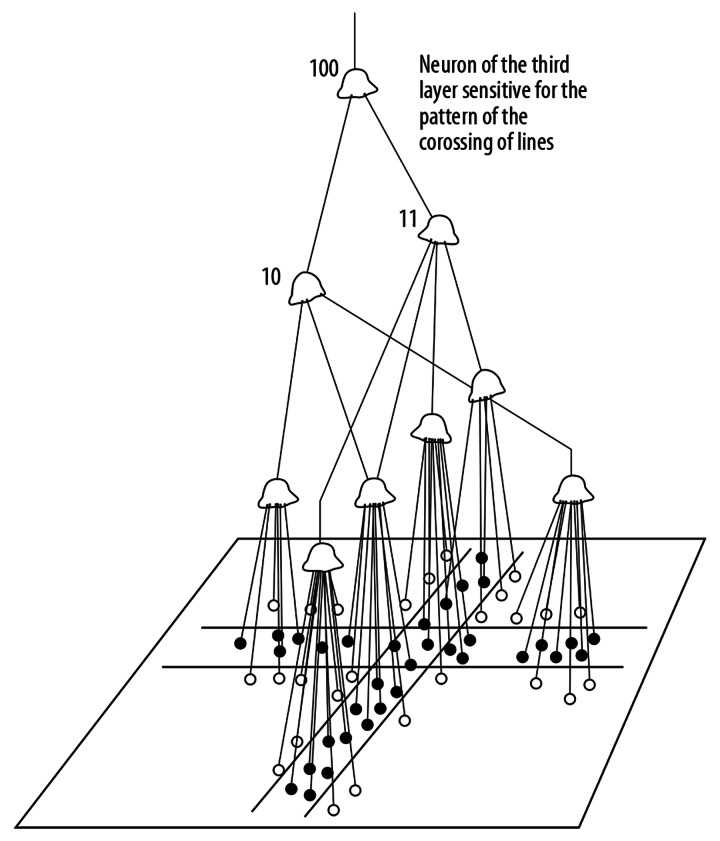
After the learning period, the neuron is sensitive to a particular pattern. For instance, if the weights of the synapses in the central region of the body of a neuron are increased but the circumferential synapses are not changed, the neuron will be sensitive to a dot pattern (Figure 2). Kuffler, many years ago, discovered cells of this type in the ophthalmic retina [30]. Hubel and Wiesel, in a series of famous experiments, proved that at higher layers of the visual integrative pathway there are neurons sensitive to more and more complex patterns [19,31]. The lateral geniculate body and the striate cortex contain neurons sensitive to rows (lines) positioned at different angles in the visual field. At the next layer, the crossing of lines existing in the visual field can be detected (Figures 3 and 4).
Figure 2.
Scheme of a neuron of retina sensitive for a dot pattern.
Figure 3.
The function of a neurons of second layer of the visual pathway.
Figure 4.
An example of the rule of function for a neuron of third layer of the visual pathway.
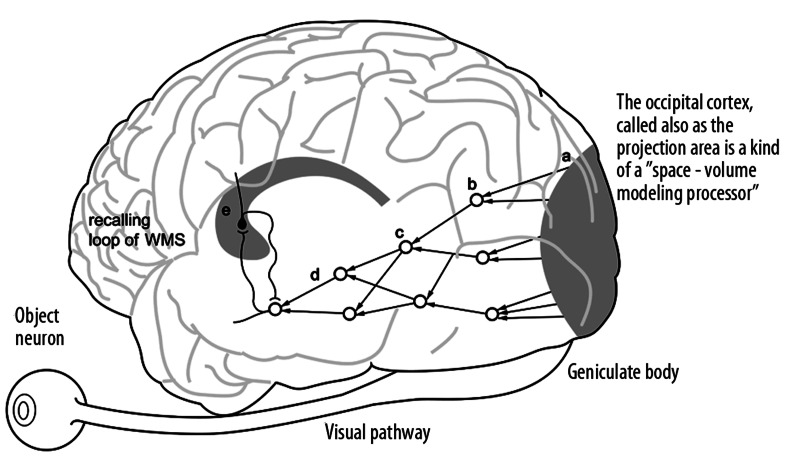
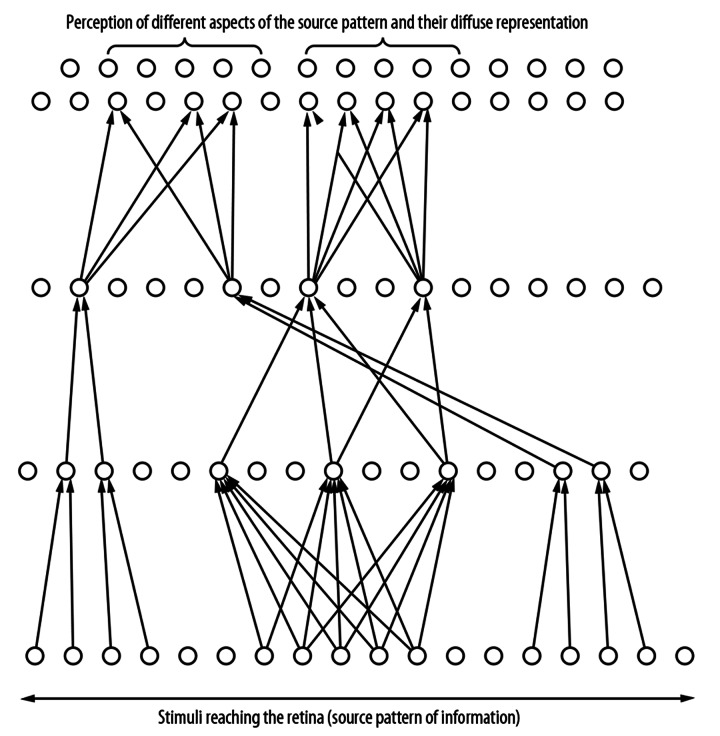
Advocates of the existence of the so-called ‘gnostic’ neurons, which represent particular known objects such as an apple, an orange, a face, and so forth, have been engaged for many years in a dispute with the adherents of more diffuse models of neural structures. The idea of such high-level ‘gnostic’ (object) neurons was first proposed by Konorski [19] and finally experimentally proven by Gross and Mishkin [19]. The visual ‘object’ neurons are located in the anterior part of the temporal lobes. Thus, the visual pathways does not end in the occipital region, but is prolonged by superior structures placed above the occipital lobe. From the anatomical point of view, the visual pathway is bent, aiming towards the anterior part of the temporal lobe (Figure 5). It turns out that the idea of ‘object neurons’ is not in fact inconsistent with the diffuse model of data processing, because any given object is represented by many such highest-level neurons, which constitute a set of multiple representations of the object [19]. Parallel processing is also realized by structures representing different aspects of the same object, such as shape, size, color, or texture (Figure 6).
Figure 5.
The visual pathways does not end in the occipital region, but is prolonged by superior structures placed in the anterior part of the temporal lobe, where so called ‘object neuron’ can be found. The object neurons are connected with the recalling loop of the working memory system.
Figure 6.
The representations of the source pattern of information on higher level of the visual pathway are dispersed and different aspect of this source pattern are perceived.
The occipital cortex is a kind of a ‘space-volume modeling processor’ [19]. This means that the activation of many neurons comprising short segments inclined at different angels relevant to the horizontal plane reproduce here a 3-dimensional replica of the external world. This replica reproduces distances and other topological and relational characteristics of the observed fragment of the external world.
The neural circuitry for long-term memory and consolidating its traces
In the late 1950s, Wilder Penfield stimulated the cortex of conscious patients during surgery for epilepsy performed under local anesthesia [19]. The patients reported vivid experiences of past events. He also demonstrated the existence of speech (language, vocal) ‘object neurons’. A written or pronounced word is, of course, also an object [19].
Brenda Milner found that neurosurgery performed on the hippocampus caused a profound and irreversible deficit of recent memory [19]. Patients lost the capacity to form new long-term memories, but previously acquired long-term memories remained intact. It was later discovered that some hippocampal neurons exhibit ‘long-term potentiation’. This means that their resting potential is steady near the threshold of activation. If such neurons form a loop with certain cortical ‘object neurons’, the circuit can easily fall into oscillations. The phenomenon of self-sustained, repetitive oscillations after the activation of an object neuron is important for the consolidation of memory traces and also for recalling mental images.
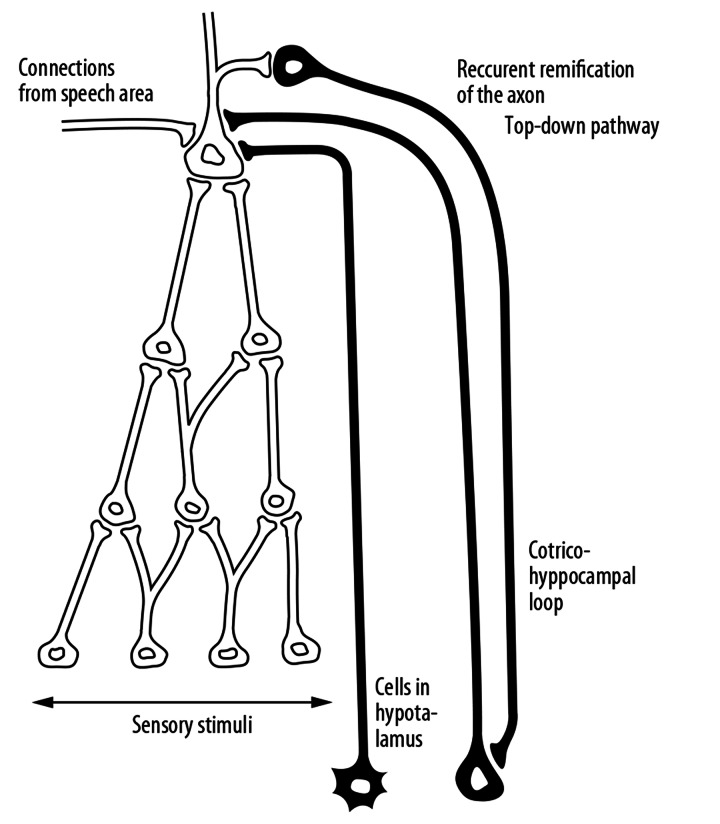
The neurosurgical experiments performed by Mishkin on monkeys, however, proved that 2 separate but cooperating loops are involved in the mechanism of the memory [19] (Figures 7 and 8). The first loop, based on the hippocampal neurons, is necessary to maintain the arousal of a mental image activated from the side of the speech area. These loops are essential for action of the short-term memory recalling for the working memory system. To understand the mechanisms of memory, it is important to realize that these loops based on neurons characterized by so-called ‘long-term potentiation’ cause recurrent excitation of neurons representing specific objects for a certain period of time necessary to perform a particular mental manipulation. The second kind of these loops involves neurons situated in hypothalamic structures, especially in the amygdales nuclei. These structures are known as centers of emotional phenomena. The activation of the cortex-hypothalamic (limbic) loops is necessary for the consolidation of memory traces, leading to the formation of long-term memory.
Figure 7.
Two kinds of connections reach so called ‘object neurons’. One of them, going from the side of hypothalamus and amygdale is active in the case of emotional arousal and take part in the consolidation of the long- term memory traces. The second connection constitutes the oscillating loop, which cause that the evoked mental image is aroused for the time necessary for the action of the working memory.
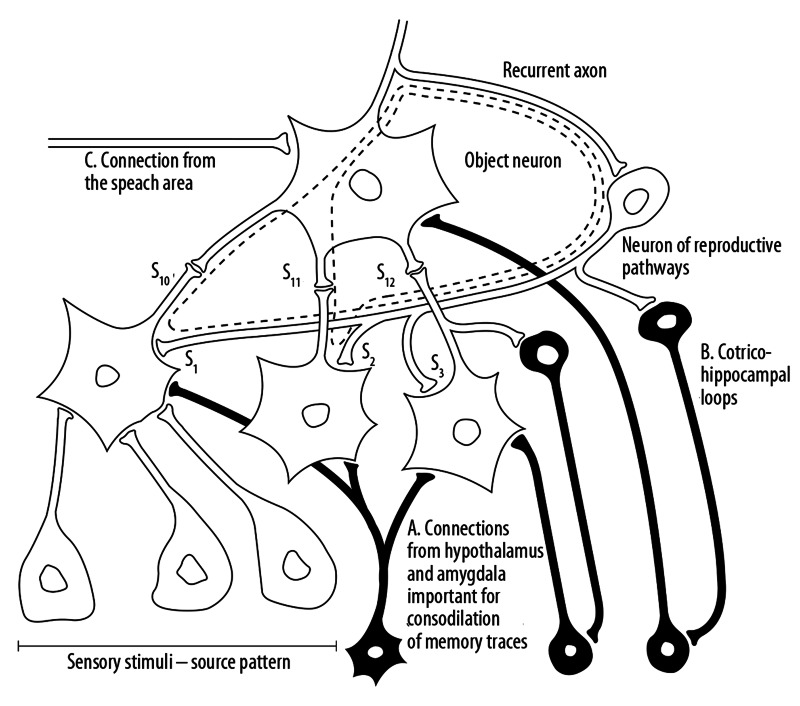
Figure 8.
The nature of the mental imaginary. A mental image is recalled from the memory, when the activation oscillate down and back to the top of the hierarchical structure owing to so called recurrent axons. Three layers of the hierarchical structure of neurons integrating sensory information, for instance – in the visual pathways. The traces of long term memory are consolidated under the influence of connections from hypothalamus and amygdale (A). There are consolidated through two mappings: weights of receiving, ascending synapses (s10, s11, s12) and synaptic weights of reproducing connections (s1, s2, s3). The second, cortico – hippocampal loop (B) is necessary for temporary recalling of the mental image. The activation of the object neuron by this indexing loop causes the recurrent reactivation of neurons in lower layers. The doted line indicate the pathways of repetitive circulations of stimuli in the upper layers of the hierarchical structure, what is the essence of mental imaginary. The object neuron can be activated also from the side of speech area (C). Mental imagery is the essence of episodic memory, short term memory and is used for working memory activity.
The neural circuitry necessary for recalling mental images and realizing mental imagery
Almost all cortical neurons have “recurrent” axons. This was demonstrated by Carpenter’s histological pictures of the cytoarchitecture of the cortex [19]. These ramifications are necessary to begin top-down pathways featured to activate the lower levels of the hierarchical structures at the moment of stimulation of an object neuron from the side of the speech area or during complex mental processes, especially problem solving. When the neuron of a known object is stimulated, next the activation returns – by means of recurrent axons or generally by reproductive pathways – to lower levels of the hierarchical structure consolidated formerly during perceptions and the learning process (Figure 8). Downward activation can even proceed to a lower projection level, such as the occipital cortex, causing vivid dreams or hallucinations.
When such a structure is activated from below by repeated perceptions, the object neuron is further stimulated by the cortex-hippocampus indexing loop. Thus, the structure of a known, recognized object is stimulated from 2 directions. It is the basis for impression that objects are known to us and recognized.
When the object neuron is stimulated from the speech area, the mental image (a remembrance) is recalled. The neural mechanism of the mental image consists of the circulation of impulses up and down along superior levels of the hierarchical structure, which is maintained by the cortex-hippocampal indexing loops.
It is also useful to be aware that above the neuronal hierarchical structures recognizing objects, there are neurons that recognize situations. Remembrances of a complex situation can be recalled from the memory by words such as: travel, holidays, harvest, wedding, dancing. The excitation of such complex images begins from activation of neurons (called the configuration neurons), located in the prefrontal lobes.
The presented model shows that as a result of WMS action always comes to realize ideas (to realize mental imagery). Therefore, the considerations of the function of WMS should be complemented by familiarizing oneself with the nature of mental imagery [19,32–35]. Huijbers et al. proved that the regions of the brain that are active during mental imagery are: the hippocampus, posterior cingulate cortex, medial, dorsolateral and ventrolateral prefrontal cortex, angular gyrus, dorsal and ventral precuneus, anterior and mid-cingulate cortex, and supramarginal gyrus [34].
Figures 2–8 help explain why we can say that the same structural elements are engaged in long-term and short-term memory. Memory traces essential for the long-term memory are formed by synaptic weights of afferent connections and recurrent pathways. In the moment of repeated perceptions, these traces enable pattern recognition. The upper layers of the same structure can be activated from the side of the speech area. A sequence of words (some sentences) activates a set of object neurons representing unknown or unusual configurations of objects; they can constitute the problem that should be solved. The maintenance of activations of this unusual configuration of imagined objects is the nature of the working memory action. The result of this manipulations, for instance a finding of some linked (related) objects, can be memorized, but very often only for a short period of time. Therefore we use the notion of the short-term memory.
Genetic Determinants of Working Memory Circuitry Function
People manifesting behavioral disturbances and mental disorders have impairments of working memory, which is important for clinicians and neuroscientists.
Recently, some researchers have pointed out that the justification of a causal relationship between the genetic factors and behavioral disorders will be more credible if we take into account changes in the WMS. These morphological, structural, or functional changes can be revealed by imaging and functional techniques [11,13,14].
According to Bigos and Ziemans, biological measures such as evaluation of the brain structure and function, are biologically more closely linked to gene activity and therefore have better at finding genetic associations [5,13,14].
Working memory is a heritable system [5,36], but we currently have only a fragmentary understanding of the genetically conditioned development of the WMS. The WMS relies on distributed neural structures. Moreover, genes may influence different parts of these structures or may influence cellular levels, neurotransmitters, or large coordination activities.
Therefore, studies of links between altered genes and different elements of the WMS should consider cellular signaling mechanisms, the so-called structural connectivity of white matter tracts and integrity of gray matter in main regions [5,6].
Recently, several review papers were published on cellular signaling dysfunctions [5,6]. These papers consider separately the dopamine, serotonin, glutamate, and GABA systems signaling pathways [5,6]. We refer readers to these reviews, but it would be useful here to mention the most striking findings.
Working memory dysfunction as a result of impairment of a neurotransmitter was first noticed, by Patricia Goldman-Rakic et al. [37–39], in relation to dopamine. Dopamine is present in the brain tissue in the form of 5 receptor subtypes: D1–D5. Goldman-Rakic et al. proved that the D1 and D2 receptors have a modulatory role in WMS [38,40]. Goldman-Rakic established the link between the disturbances of dopamine and working memory function in the course of schizophrenia [41,42].
Several genetic changes were discovered that can influence the impact of dopamine on WMS function. There are genes responsible for the catabolism of dopamine (COMT), as well as for removal of dopamine from the synapse (DAT1), and the synthesis of the D2 receptor (DRD2); some polymorphisms of these genes were found [43].
Experiments consisting on acute tryptophan depletion and studies of polymorphisms of genes related to the serotonin signaling pathway suggest a role of serotonin in proper functioning of working memory [44]. Enge et al. examined the working memory performance of 130 participants, using behavioral tests and neurophysiological indices among persons demonstrating variations within genes encoding key regulators of the serotonergic system – the serotonin transporter gene-linked polymorphic region (5-HTTLPR) – and a repeated polymorphism in the transcriptional control region of the monoamine-oxidase gene (MAOA-uVNTR). They found that carriers of 5-HTTLPR and MAOA-uVNTR alleles influence the efficient executive control of working memory-related performance measured by reaction time and error rate. They concluded that the intact serotonin signaling pathway is important in the response to stimuli and appropriate executive functioning [44].
Ziermans et al. concentrated on 18 single-nucleotide polymorphisms (SNPs) located in 6 dopaminergic candidate genes (COMT, SLC6A3/DAT1, DBH, DRD4, DRD5, MAOA) [6]. The authors measured the visuospatial working memory activity by means of functional magnetic resonance imaging.
This WMS capacity was assessed in a longitudinal study of typically developing children and adolescents. The authors evaluated the behavioral problems using the Child Behavior Checklist (CBCL). The researchers found that One SNP (rs6609257), located ~6.6 kb downstream of the monoamine oxidase A gene (MAOA) on human chromosome X, significantly affected brain activity in a network of frontal, parietal, and occipital regions. The authors tried to correlate the activity of this region with WM capacity. Persons with higher WM capacity had fewer externalizing symptoms of externalizing aggressive or oppositional symptoms. They did not, however, find direct significant correlations between rs6609257 and behavioral symptoms. These authors suggested a mediating role of WM brain activity and capacity in linking the MAOA gene to aggressive behavior during development.
Clinicians interested in the genetic determinates of working memory capacity should study sources presented by more exhaustive review papers [5].
Working Memory Assessment Methods
Capacity and proper action of WMS can be assessed by many precise methods; it can also be done in experiments on animals. The best-known method used in studies of animals is the so-called delayed response task (DRT) [5]. Performing this test, the location of a reward is shown to the animal. The animal must retain information about this location across a delay.
Action of the WMS of people is assessed by neuropsychological tasks, for example, the Wisconsin Card Sort Task (WCST) [5]. Researchers also often use the Sternberg Item Recognition Paradigm, a type of DRT [5]. A small group of items, called the “positive set”, is presented for the examined person to memorize. After the delay, a single item is presented that may or may not have been shown before. The subject is asked to respond ‘yes’ or ‘no’, indicating their recognition of the item. This procedure is repeated over several trials in which the number of items in the positive set is varied. Subjects are asked to respond as fast as they can without making errors [45].
Syndromes Characterized by Damage to the Working Memory
Schizophrenia
Indicating the most common syndromes characterized by the damages of WMS, we should first mention schizophrenia. Working memory dysfunction is one of the core components of schizophrenia. This damage is related to profound cognitive deficits observed in patients with this disorder. Developments in functional imaging have facilitated the investigation of the neural basis of these cognitive deficits [46]. These investigations demonstrated the occurrence in this disease of impairments of lateral prefrontal cortex function, constraints of adequate responses to stimuli, and disturbances of inter-regional integrations [47].
Walter et al. showed dysfunction of the lateral prefrontal cortex, superior temporal areas, and the striatum and maintained that superior temporal cortex dysfunction in patients with schizophrenia may be regarded as a schizophrenia-specific finding in terms of psychiatric diagnosis specificity [47].
Karch et al. simultaneously examined brain function using fMRI and performed tests of attention-independent working memory in schizophrenia patients and found hypoactivations in frontal, temporal, and subcortical brain regions [48].
Zilles et al. examined 31 patients with schizophrenia and 47 controls [49], testing different aspects of verbal and visuospatial working memory using modified Sternberg paradigms in a computer-based behavioral experiment. The subjects revealed significant impairment of many WMS components. These authors demonstrated that patients with schizophrenia exhibit specific and, in part, selective, WMS deficits, with evidence of dysfunctions of the underlying neural networks. In contrast to a global working memory deficit, patients with schizophrenia had differential patterns of working memory impairments.
Attention-Deficit/Hyperactivity Disorder (ADHD) and Bipolar Disorder (BPD)
Westerberg et al. emphasized that working memory was formerly hypothesized to be impaired in attention-deficit/hyperactivity disorder (ADHD) [50], and stated there have been few studies on tests measuring visuo-spatial working memory (VSWM) in ADHD. Some of these studies used paradigms including episodic memory, and others only used low memory loads. These authors used a VSWM test that had not been used previously in ADHD research. They concluded that their results show that the VSWM test is a sensitive measure of cognitive deficits in ADHD and it supports the hypothesis that deficits in VSWM are a major component of ADHD [50].
Brown et al. remarked that ADHD and BPD are often comorbid conditions [51], associated with alterations in anterior and posterior parts of the working memory system. The symptoms of each disorder are related to anatomically and functionally distinct brain regions. The authors investigated functional brain circuits by fMRI imaging in 18 adult males with both ADHD and BPD, and 18 healthy control participants matched one-to-one on age, sex, and handedness, while they performed WM assessment tests. The authors found significant hypoactivity in the subjects with ADHD and BPD towards controls across frontal and parietal regions, and they found that BPD and ADHD symptoms are related to activity in anatomically distinct regions.
Kupferschmidt and Zakzanis performed a large meta-analysis of studies on patients with BPD [52]. The meta-analysis reported on 55 functional neuroimaging studies published between 1987 and 2010, encompassing a total of 774 adult patients with BPD and 810 healthy adult controls. The authors concluded that despite heterogeneity across studies there are findings indicating limbic frontal lobe hyperactivity. However, many brain regions are involved in patients with BPD.
Major depression
Grubner et al. studied WMS impairments among patients with major depression [53]. They remarked that the literature on the presence and the extent of WM deficits is inconsistent. They performed their own investigations of 18 patients and 18 healthy controls matched for age, gender, and education. They found that patients with acute major depression showed a selective impairment in articulatory rehearsal of verbal information in working memory. By contrast, visuospatial WM was unimpaired in this sample. These authors did not find significant correlations between symptom severity and WM performance. They concluded that patients with major depression have a specific verbal WMS dysfunction [53].
Parkinson disease
Persons with Parkinson disease have working memory deficits [54,55]. Gilbert et al. tested 3 hypotheses: a limited storage capacity, an impaired executive component, and a reduction of psychomotor speed. Verbal working memory was assessed in 14 patients without dementia and 14 matched controls. They used a classical verbal span test and motor and psychomotor speed tasks. The results indicate a deficit in manipulation, with normal updating capacities. Results indicated that patients with Parkinson disease have selective working memory impairment [54].
Costa et al. tested 23 patients with Parkinson disease and 25 controls, using prospective memory tasks. The participants were asked to execute 3 actions after a 20-minute delay. Participants with Parkinson disease were less accurate than comparison participants in the prospective component of the time-based task [55].
Mild cognitive impairment and dementia syndromes
Impairment of working memory system and other types of memory in patients with different kinds of dementia is known and evident. There are many publications on these subjects [56–58]. Richard et al. described characteristic features of WM impairments in late-life depression and mild cognitive impairments [56]. MacDuffie emphasized that short-term memory impairment with some preserved semantic processing is evident in Alzheimer disease [57]. He described the core pathological process occurring in this disease: “the extent and variety of intrusions reported by these participants indicates a breakdown in their ability to monitor and constrain their recall responses, even within seconds of initial learning” [57]. Peters stated that in Alzheimer disease impaired semantic knowledge underlies the reduced verbal short-term storage capacity [58].
Changes of the capacity of working memory during aging
Many papers have been published on the influence of aging on WMS capacity. For example, Gras et al. examined the ability of older people to learn new routes [59]. They found that spatial memory involved in navigation and route learning is impaired during aging, partly due to decline of episodic and working memory components.
Overview of Practical, Clinical Significance of Knowledge About the Structure, Function, and Impairments of the Working Memory
Knowledge of the structure and function of the WMS is important for physicians, who often need to assess working memory capacity in children and youths during their development, as well as in the elderly [11,59]. The slow deterioration of the efficiency of the working memory system in old age is a physiological phenomenon. It can, however, occur early in syndromes of mild cognitive impairment [56]. Doctors should be able to notice that the mild cognitive impairments appeared in their patients too early and have pathological features. In addition to the need to evaluate physiological changes of working memory capacity, or the recognition of impairments that occurred prematurely, physicians should be able to establish a proper diagnosis of syndromes in which there is a substantial impairment of the WMS. In this article we have only briefly discussed difficulties in evaluation of working memory damages in schizophrenia [46–49], ADHD [50], bipolar disorder [51,52], major depression [53], Parkinson disease [54,55], and dementia syndromes [56–58]. Understanding the structure and function of the WMS will help doctors in practical assessment of their patients [5,45]. Clinical physicians can also designate appropriate treatment in the form of recently elaborated training methods [60–64], and may also be interested in the development of pharmacological methods influencing the capacity of working memory [65,66]. Understanding of WMS should facilitate design of further clinical studies to clarify numerous problems related to the functioning of human memory in disease states.
Conclusions
Medical education should include concepts of different types of memory, such as episodic, semantic, short-term, long-term, autobiographical, etc.
Medical education in the subject of neurophysiology should include knowledge of the essence of cognitive processes of perception, recognition, recalling from memory, mental imagery, and manipulations of recalled mental images.
Education and professional practice of clinicians should develop the ability to assess working memory capacity and knowledge about physiological processes of changes of WMS capacity during development, adolescence, and aging.
The clinician should be aware of the specificity of the working memory disorders in the course of mental disorders like schizophrenia, ADHD, episodes of depression, bipolar depressive disorder, Parkinson disease, and mild and severe dementia syndromes.
Clinicians should be familiar with already established methods for improving the efficiency of the working memory.
WMS researchers should improve models of its structure and function, taking into account the roles of different parts of the system already discerned, such as the hippocampus, amygdale, and the temporo-parietal and prefrontal and frontal part of the memory system.
WMS researchers should assemble data about genetic conditions affecting working memory disorders and their relationships to mental and behavioral disorders.
Footnotes
Source of support: Self financing
References
- 1.Baddeley A. Working memory. Science. 1992;255:556–59. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 2.Constantinidis C, Procyk E. The primate working memory networks. Cogn Affect Behav Neurosci. 2004;4:444–65. doi: 10.3758/cabn.4.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Repovs G, Baddeley A. The multi-component model of working memory: explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 4.Müller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139:51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Karlsgodt KH, Bachman P, Winkler AM, et al. Genetic influence on the working memory circuitry: behavior, structure, function and extensions to illness. Behav Brain Res. 2011;225:610–22. doi: 10.1016/j.bbr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziermans T, Dumontheil I, Roggeman C, et al. Working memory brain activity and capacity link MAOA polymorphism to aggressive behavior during development. Transl Psychiatry. 2012;2:e85. doi: 10.1038/tp.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cogn Sci. 2003;7:547–52. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull. 1997;23:437–58. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- 9.Honey GD, Fletcher PC. Investigating principles of human brain function underlying working memory: what insights from schizophrenia? Neuroscience. 2006;139:59–71. doi: 10.1016/j.neuroscience.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 10.Walter H, Vasic N, Höse A, Spitzer M, Wolf RC. Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: evidence from event-related fMRI. Neuroimage. 2007;35:1551–61. doi: 10.1016/j.neuroimage.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 11.Spronk M, Vogel EK, Jonkman LM. Electrophysiological evidence for immature processing capacity and filtering in visuospatial working memory in adolescents. PLoS One. 2012;7:e42262. doi: 10.1371/journal.pone.0042262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweizer S, Hampshire A, Dalgleish T. Extending brain-training to the affective domain: increasing cognitive and affective executive control through emotional working memory training. PLoS One. 2011;6:e24372. doi: 10.1371/journal.pone.0024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigos KL, Hariri AR. Neuroimaging: technologies at the interface of genes, brain, and behavior. Neuroimaging Clin N Am. 2007;17:459–67. doi: 10.1016/j.nic.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigos KL, Weinberger DR. Imaging genetics – days of future past. Neuroimage. 2010;53:804–9. doi: 10.1016/j.neuroimage.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Thomas KM, King SW, Franzen PL, et al. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–38. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- 16.Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- 17.Fornito A, Bullmore ET. Connectomic intermediate phenotypes for psychiatric disorders. Front Psychiatry. 2012;3:32. doi: 10.3389/fpsyt.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kandel ER. In Search of Memory: The Emergence of a New Science of Mind. Norton & Company; New York: 2007. [Google Scholar]
- 19.Brodziak A. Neurophysiology of the mental image. Med Sci Monit. 2001;7(3):534–38. [PubMed] [Google Scholar]
- 20.Pąchalska M, Talar J, Brodziak A, MacQueen BD. Disturbances of mental image processing in post-stroke patients with left and right hemisphere damage. Med Sci Monit. 2001;7(4):716–24. [PubMed] [Google Scholar]
- 21.Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Levine B, Turner GR, Tisserand D, et al. The functional neuroanatomy of episodic and semantic autobiographical remembering: a prospective functional MRI study. Cogn Neurosci. 2004;16:1633–46. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg DL, Rice HJ, Cooper JJ, et al. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–74. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Moscovitch M, Rosenbaum RS, Gilboa A, et al. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daselaar SM, Rice HJ, Greenberg DL, et al. The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cereb Cortex. 2008;18:217–29. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- 26.Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage. 2010;49:865–74. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- 27.Aggleton JP, O’Mara SM, Vann SD, et al. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31:2292–30. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ino T, Nakai R, Azuma T, et al. Brain activation during autobiographical memory retrieval with special reference to default mode network. Open Neuroimag J. 2011;5:14–23. doi: 10.2174/1874440001105010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranganath C. Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–89. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 30.Kuffluer SW. Discharge patterns and functional organization of mammalian retina. J Neurophisiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- 31.Hubel TH, Wiesel TN. Receptive fields of single neuron in the cat’s striate cortex. J Physiol. 1959;148:574–91. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartolomeo P. The neural correlates of visual mental imagery: an ongoing debate. Cortex. 2008;44:107–8. doi: 10.1016/j.cortex.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Daselaar SM, Porat Y, Huijbers W, Pennartz CM. Modality-specific and modality-independent components of the human imagery system. Neuroimage. 2010;52:677–85. doi: 10.1016/j.neuroimage.2010.04.239. [DOI] [PubMed] [Google Scholar]
- 34.Huijbers W, Pennartz CM, Rubin DC, Daselaar SM. Imagery and retrieval of auditory and visual information: neural correlates of successful and unsuccessful performance. Neuropsychologia. 2011;49:1730–40. doi: 10.1016/j.neuropsychologia.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 35.Zvyagintsev M, Clemens B, Chechko N, et al. Brain networks underlying mental imagery of auditory and visual information. Eur J Neurosci. 2013 doi: 10.1111/ejn.12140. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behav Genet. 2001;31:615–24. doi: 10.1023/a:1013353613591. [DOI] [PubMed] [Google Scholar]
- 37.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251(4996):947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 38.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376(6541):572–75. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 39.Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA. 1996;93:13473–80. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303(5659):853–56. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 41.Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46:650–61. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- 42.Goldman-Rakic PS, Castner SA, Svensson TH, et al. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology. 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 43.Markett SA, Montag C, Reuter M. The association between dopamine DRD2 polymorphism and working memory capacity is modulated by a functional polymorphism on the nicotinic receptor gene CHRNA4. J Cogn Neurosci. 2010;22:1944–54. doi: 10.1162/jocn.2009.21354. [DOI] [PubMed] [Google Scholar]
- 44.Enge S, Fleischhauer M, Lesch KP, et al. Serotonergic modulation in executive functioning: linking genetic variations to working memory performance. Neuropsychologia. 2011;49:3776–85. doi: 10.1016/j.neuropsychologia.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 45.The Sternberg Short-Term Memory Experiment. http://web.uct.ac.za/depts/psychology/psy300/sternb.html.
- 46.Honey GD, Fletcher PC. Investigating principles of human brain function underlying working memory: what insights from schizophrenia? Neuroscience. 2006;139:59–71. doi: 10.1016/j.neuroscience.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 47.Walter H, Vasic N, Höse A, et al. Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: evidence from event-related fMRI. Neuroimage. 2007;35:1551–61. doi: 10.1016/j.neuroimage.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 48.Karch S, Leicht G, Giegling I, et al. Inefficient neural activity in patients with schizophrenia and nonpsychotic relatives of schizophrenic patients: evidence from a working memory task. J Psychiatr Res. 2009;43:1185–94. doi: 10.1016/j.jpsychires.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Zilles D, Gruber E, Falkai P, Gruber O. Patients with schizophrenia show deficits of working memory maintenance components in circuit-specific tasks. Eur Arch Psychiatry Clin Neurosci. 2010;260:519–25. doi: 10.1007/s00406-010-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westerberg H, Hirvikoski T, Forssberg H, Klingberg T. Visuo-spatial working memory span: a sensitive measure of cognitive deficits in children with ADHD. Child Neuropsychol. 2004;10:155–61. doi: 10.1080/09297040409609806. [DOI] [PubMed] [Google Scholar]
- 51.Brown A, Biederman J, Valera E, et al. Working memory network alterations and associated symptoms in adults with ADHD and Bipolar Disorder. J Psychiatr Res. 2012;46:476–83. doi: 10.1016/j.jpsychires.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kupferschmidt DA, Zakzanis KK. Toward a functional neuroanatomical signature of bipolar disorder: quantitative evidence from the neuroimaging literature. Psychiatry Res. 2011;193:71–79. doi: 10.1016/j.pscychresns.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Gruber O, Zilles D, Kennel J, et al. A systematic experimental neuropsychological investigation of the functional integrity of working memory circuits in major depression. Eur Arch Psychiatry Clin Neurosci. 2011;261:179–84. doi: 10.1007/s00406-010-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert B, Belleville S, Bherer L, Chouinard S. Study of verbal working memory in patients with Parkinson’s disease. Neuropsychology. 2005;19:106–14. doi: 10.1037/0894-4105.19.1.106. [DOI] [PubMed] [Google Scholar]
- 55.Costa A, Peppe A, Caltagirone C, Carlesimo GA. Prospective memory impairment in individuals with Parkinson’s disease. Neuropsychology. 2008;22:283–92. doi: 10.1037/0894-4105.22.3.283. [DOI] [PubMed] [Google Scholar]
- 56.Richard E, Reitz C, Honig LH, et al. Late-life depression, mild cognitive impairment, and dementia. Arch Neurol. 2012;31:1–7. doi: 10.1001/jamaneurol.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacDuffie KE, Atkins AS, Flegal KE, et al. Memory distortion in Alzheimer’s disease: deficient monitoring of short- and long-term memory. Neuropsychology. 2012;26:509–16. doi: 10.1037/a0028684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters F, Majerus S, De Baerdemaeker J, et al. Impaired semantic knowledge underlies the reduced verbal short-term storage capacity in Alzheimer’s disease. Neuropsychologia. 2009;47:3067–73. doi: 10.1016/j.neuropsychologia.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Gras D, Daniel MP, Labiale G, et al. Effect of aging on real route memorization: the role of working memory and episodic memory. Geriatr Psychol Neuropsychiatr Vieil. 2012;10:463–70. doi: 10.1684/pnv.2012.0370. [DOI] [PubMed] [Google Scholar]
- 60.Klingberg T. Training and plasticity of working memory. Trends Cogn Sci. 2010;14:317–24. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Dahlin E, Bäckman L, Neely AS, Nyberg L. Training of the executive component of working memory: subcortical areas mediate transfer effects. Restor Neurol Neurosci. 2009;27:405–19. doi: 10.3233/RNN-2009-0492. [DOI] [PubMed] [Google Scholar]
- 62.Salminen T, Strobach T, Schubert T. On the impacts of working memory training on executive functioning. Front Hum Neurosci. 2012;6:166. doi: 10.3389/fnhum.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osaka M, Otsuka Y, Osaka N. Verbal to visual code switching improves working memory in older adults: an fMRI study. Front Hum Neurosci. 2012;6:24. doi: 10.3389/fnhum.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langdon KD, Corbett D. Improved working memory following novel combinations of physical and cognitive activity. Neurorehabil Neural Repair. 2012;26:523–32. doi: 10.1177/1545968311425919. [DOI] [PubMed] [Google Scholar]
- 65.Wallace DL, Vytlacil JJ, Nomura EM, et al. The dopamine agonist bromocriptine differentially affects fronto-striatal functional connectivity during working memory. Front Hum Neurosci. 2011;5:32. doi: 10.3389/fnhum.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibbs SE, D’Esposito M. A functional MRI study of the effects of bromocriptine, a dopamine receptor agonist, on component processes of working memory. Psychopharmacology. 2005;180:644–53. doi: 10.1007/s00213-005-0077-5. [DOI] [PubMed] [Google Scholar]