Abstract
Background
We investigated the hypothesis that postconditioning by FTY720 (FTY) in isolated perfused mouse hearts is independent of the sphingosine 1-phosphate (S1P) pathway.
Material/Methods
Ex vivo hearts were exposed to postconditioning (POST) by either ischemia or FTY720. Protection against ischemia/reperfusion (IR) injury was measured by recovery of left ventricular developed pressure (LVDP) and infarct size.
Results
FTY effectively postconditioned (POST) ex vivo hearts against ischemia/reperfusion (IR) injury as measured by recovery of LVDP and a low infarct size. FTY protection, unlike S1P but like sphingosine (Sph), was insensitive to inhibition of S1P G-Protein Coupled Receptors (GPCRs) or inhibition of PI3 kinase. Protection by FTY and Sph was however blocked by inhibitors of PKA and PKG. Thus, FTY follows the same cardioprotective pathway as Sph. This was further supported by studies of FTY POST in knockout (KO) mice lacking the SphK2 form of Sph kinase that is needed for phosphorylation of FTY to an S1P analog. In the absence of SphK2, FTY (and Sph) POST was still cardioprotective. This differed from the effect of SphK2 KO on protection by ischemic POST (IPOST). IPOST was not effective in KO hearts. To see if the GPCR signaling pathway to protection is normal in KO hearts, we looked at POST by GPCR agonists S1P and adenosine. Both provided effective protection even in KO hearts suggesting that the problem with IPOST in KO hearts is a low level of S1P available for release during IPOST. Thus, pharmacologic POST with FTY or Sph, like adenosine and S1P, is unaffected in the KO.
Conclusions
FTY720 administered in vivo might behave in a dual manner showing both S1P-like effects and sphingosine-like effects. It appears that the latter may have been overlooked and may be the more important in aging hearts.
Keywords: cardioprotection, FTY720, ischemia/reperfusion injury, postconditioning, sphingosine, sphingosine kinase 2, sphingosine 1-phosphate
Background
Injury to the heart can result from prolonged periods of ischemia that extend beyond 20 min [1,2]. Much of the early damage arises at reperfusion and thus is referred to as ischemia/reperfusion (I/R) injury. Protection against I/R injury can be obtained by applying short periods of I/R that either precede long term ischemia (ischemic preconditioning, IPC) or are initiated immediately at the start of full reperfusion (ischemic postconditioning, IPOST). These protective regimens have the potential to be effective if implemented over the initial 90 min of ischemia [2]; after this time irreversible events occur [3]. One mechanism for IPC and IPOST involves cellular agonists that are released from myocytes via pannexin-1/P2X7 channels [4,5] in response to brief ischemia. These agonists bind to G-protein coupled receptors (GPCRs) initiating a protective response [6–9]. Sphingosine-1-phosphate (S1P) has been shown to be one of these important endogenous cardioprotectants and its effects are mediated by binding to specific cell surface S1P GPCRs [9–13].
Of great interest is the sphingosine analog FTY720, which is an important immunomodulatory drug that also has cardiovascular effects [14,15]. Because FTY720-phosphate is an effective S1P analog, it has been presumed that FTY720 only functions as an S1P pro-drug [14,15]. However, it has recently been shown that sphingosine also promotes an effective cardioprotective pathway, one that is very different from the S1P pathway [16,17]. This raises the question of whether FTY in its unphosphorylated form might also be able protect via the sphingosine pathway.
Sphingosine is phosphorylated to form S1P in an ATP dependent reaction catalyzed by sphingosine kinase. Sphingosine kinase has two isoforms (SphK1 and SphK2) and both are present in heart [18–20]. SphK1 appears to be a protective kinase, as over expression of SphK1 increases intracellular S1P content and promotes cell growth and survival [21–23]. Indeed, study of SphK1 null (KO) mice demonstrated that the absence of SphK1 sensitizes the myocardium to I/R injury and results in the loss of both IPC and IPOST [24,25]. In a study of hearts from SphK2 KO mice it was shown that they are also more sensitive to I/R injury in an isolated perfused heart model, and have a diminished response to IPC [26]. Gomez et al. [27] also reported a decreased responsiveness to IPC in SphK2 knockout mice hearts in vivo. Of interest for the current study is the fact that FTY720 is predominantly phosphorylated by the SphK2 form [14,28,29], and thus should not be phosphorylated in SphK2 KO hearts.
Our first objective was to determine whether cardioprotection using FTY720 more closely resembled S1P or sphingosine. We demonstrate that in an ex vivo heart the acute cardioprotective properties of FTY720 resemble sphingosine not S1P, and that these cardioprotective effects are even seen in knockout hearts that possess an inactivated SphK2 gene (SphK2 KO). Thus, in the absence of the SphK2 form of sphingosine kinase, which is the form that catalyzes FTY720 phosphorylation, FTY720 is still an effective cardioprotectant.
Also important is the recent suggestion that SphK2 directed S1P synthesis in mitochondria is important for proper assembly and function of the respiratory chain [30] and that deletion of SphK2 leads to increased susceptibility to mitochondrial permeability transition [27]. Because of the importance of the membrane permeability transition in I/R injury [31], it seemed possible that SphK2 could contribute to cardioprotection in ways unrelated to its role in providing S1P for binding to extracellular receptors. Discerning the role of SphK2 is important particularly with respect to aging as we have found that SphK2 activity, but not SphK1, decreases with aging [16].
Thus, a second objective of the present study was to utilize SphK2 knockout mice to determine if SphK2 affects sensitivity to myocardial IR injury or cardioprotection via a pathway unrelated to S1P release and binding to GPCRs. To do this, we have looked at an alternate pathway to cardioprotection. Sphingosine can also pre- and post-condition the ex vivo heart but, unlike S1P, it is independent of the S1P-GPCRs and subsequent PI3 kinase (PI3K) driven pathway [16,17]. Instead, sphingosine utilizes a PKA and PKG dependent pathway [17], and we have investigated this pathway in SphK2 KO hearts
Material and Methods
D-Erythro-sphingosine and D-Erythro-sphingosine 1-phosphate (S1P) were obtained from Biomol Research Laboratories. FTY720 was obtained from Cayman Chemicals. L-Erythro-sphingosine was obtained from Sigma. The S1P1 receptor antagonist VPC23019 was obtained from Avanti Polar Lipids. The protein kinase A (PKA) inhibitor PKA-I 14–22 amide myristoylated, and the protein kinase G (PKG) inhibitor KT5823 were obtained from Calbiochem. The PI3Kinase inhibitor wortmannin was obtained from Sigma.
Animals
This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academic Press, Washington DC, 1996), and all procedures were approved by the Institutional Animal Care and Use Committee of the San Francisco Department of Veterans Affairs Medical Center. Male C57BL/6 mice (3–4 months, ca. 25 g) were obtained from Jackson Labs. Mice in which exons 2–5 of the SphK2 gene had been deleted [26] were bred from a colony obtained from Drs. Shaun Coughlin and Rajita Pappu (Cardiovascular Research Institute, University of California, San Francisco) and are referred to as SphK2 null (KO) mice. These mice, and their wild-type littermates (WT), were 3 to 4 months of age at the time of study. Genotyping using PCR to confirm the absence of exons 2–5 of SphK2 DNA was routinely performed on tail biopsies of 3–4-week-old mice as described previously [26].
Previous studies [26] demonstrated that baseline parameters for WT and SphK2 KO hearts are not different. Thus, comparison of WT and KO mice revealed no differences in body weight, heart weight, heart rate, or left ventricular developed pressure (LVDP) at baseline. The KO mice exhibit no evident phenotype, breed normally, have normal vascular development, and live a normal lifespan.
Langendorff isolated perfused heart preparation
Mice were heparinized (500 U/kg, IP) and anesthetized with sodium pentobarbital (60 mg/kg, IP). Hearts were rapidly excised, washed in ice-cold arresting solution (NaCl 120 mM, KCl 30 mM), and cannulated via the aorta. Hearts were perfused at 70 mm Hg on a modified Langendorff apparatus at 37°C as previously described [22]. Hemodynamic function was monitored by measuring left ventricular developed pressure (LVDP), LV end-diastolic pressure (LVEDP), and ±dP/dt via a Mylar pressure sensor inserted into the left ventricle. During periods of global ischemia, the hearts were lowered into a thermostated chamber to maintain a heart temperature of 37°C.
Ischemia-reoxygenation (IR) and ischemic postconditioning (IPOST) protocols
The protocol for IR experiments consisted of a 20 minute equilibration period, followed by 50 minutes of global ischemia and 40 minutes of reperfusion (reoxygenation). For IPOST, hearts were equilibrated for 20 minutes, subjected to 50 min of index ischemia, and then reperfusion initiated with four short cycles of IPOST consisting of 5 sec of global ischemia and 5 sec of reperfusion. This was followed immediately by 40 min of reperfusion. LVEDP was initially set at 5 mmHg during equilibration and subsequent changes measured. Infarct size was measured at the end of the 40 min of reperfusion by TTC staining. Hearts were sectioned, placed in 1% TTC at 37° for 10 min, and then placed in 10% formalin. The fixed sections were then photographed and infarct size determined by computer analysis [32]. Infarct size is expressed as percent of the area at risk which for global ischemia is the whole heart.
Statistical analysis
The data are presented as mean ±SEM and in all cases the sample size is n ≥4. The significance of the differences in mean values for hemodynamics, infarction size, between groups was evaluated by one-way ANOVA, followed by post-hoc testing (Newman-Keuls). Differences in SphK activity between WT and SphK2 KO tissues were evaluated by Student’s t test. P<0.05 (n=4) was considered statistically significant.
Results
Cardioprotection against ischemia/reoxygenation injury by FTY720
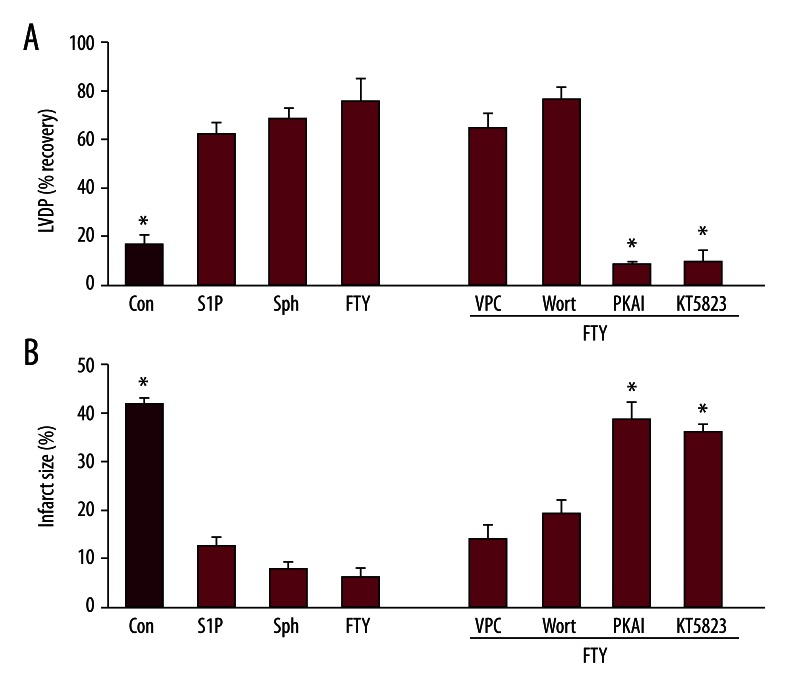
We have previously shown [17] that sphingosine can pre- and post-condition the ex vivo heart, and that it does so via a different pathway than S1P, i.e., via a PKA and PKG dependent pathway independent of S1P GPCRs. FTY720 is a sphingolipid which in its phosphorylated form is an important S1P analog. However, we postulated that in an ex vivo model the absence of extensive systemic phosphorylation would reveal the potential for FTY720 to behave as a sphingosine analog. We therefore examined postconditioning (POST) by FTY720 in the isolated perfused mouse heart to determine if its mechanism of cardioprotection resembled sphingosine. Ex vivo hearts from C57BL/6 mice were equilibrated for 20 min, exposed to a 50 min index ischemia, and reperfusion for 40 min in the presence or absence of FTY720. Study of the concentration dependence revealed that optimum protection against IR injury was achieved at 0.6 μM FTY. The recovery of LVDP was improved from 17±5% in untreated hearts to 76±9% in FTY720 treated hearts, and the infarct size was reduced from 42±1% to 6±2% (Figure 1). This is significant (p<0.05) cardioprotection and is equal to that provided by 0.4 μM sphingosine or 0.4 μM S1P (Figure 1).
Figure 1.
Characterization of FTY720 postconditioning against ischemia-reperfusion injury in ex vivo B6 mouse hearts. Ex vivo hearts from C57BL/6 mice were exposed to 50 min of ischemia followed by 40 min of reoxygenation either in the absence of any postconditioning (Control) or with pharmacologic postconditioning using 0.4 μM S1P or sphingosine (Sph), or 0.6 μM FTY720 (FTY), and in the indicated cases FTY plus the antagonist VPC23019 (VPC), wortmannin (W), PKA-I, or KT5823. Data are expressed as mean ± s.e.m. * P<0.05 vs. -PC. (A) Hemodynamic function as measured by maximum left ventricular developed pressure (LVDP) achieved during 40 min of reperfusion expressed as % recovery relative to the pre-ischemic value. (B) Infarct size expressed as percent of the area at risk (which is the total heart area in this global ischemia model) determined at the end of the 40 min of reperfusion.
To determine which pathway to protection, S1P or sphingosine, is involved in FTY720 protection of ex vivo hearts, we studied a number of specific antagonists previously used to characterize S1P and sphingosine cardioprotection [17]. All of these antagonists, KT5823, PKA-I, VPC23019, and wortmannin were previously shown to be without effect on ex vivo heart hemodynamics [17]. We first used the S1P1–3 receptor antagonist VPC23019. When ischemia is followed by reperfusion in the presence of 0.4 μM FTY720 plus 1 μM VPC23019, recovery of LVDP was the same (65±6%) as FTY720 alone and the infarct size 14±3%) was not statistically different (p>0.05, Figure 1). Further, since the S1P pathway is sensitive to inhibition of PI3K by 1 μM wortmannin, we tested its effect on FTY720 protection. As with VPC23019, co-treatment at reperfusion with 0.4 μM FTY720 and 1 μM wortmannin did not significantly alter protection relative to FTY720 alone (77±5% LVDP, 19±3% infarct size). Thus, FTY720 does not act via the S1P GPCR pathway in ex vivo hearts.
Alternatively, inhibitors of sphingosine cardioprotection were found to be inhibitory to FTY720 protection. Thus, 0.2 μM KT5823, an inhibitor of PKG, and 0.1 μM PKA-I, an inhibitor of PKA, both were able to substantially reduce the cardioprotective capacity of FTY720 (Figure 1). KT5823 reduced the recovery of LVDP to 10±3% and increased the infarct size to 36±1%, while PKA-I reduced the recovery of LVDP to 8±2% and increased the infarct size to 39±1%. Thus, FTY720 effectively postconditions the ex vivo mouse heart and does so exclusively via a PKA/PKG dependent pathway in the same manner as sphingosine, a pathway that appears to be independent of S1P-related effects.
We also tested the L-isomer of erythro-sphingosine as a postconditioning cardioprotectant. For D-erythro-sphingosine, the optimum concentration for postconditioning is 0.4 μM, but L-erythro-sphingosine was not very effective at 0.4 μM. However, it was determined that the optimum concentration for postconditioning was 1 μM, and at this concentration L-erythro-sphingosine resulted in a 59±15% recovery of LVDP with only an 11±2% infarct size, which is similar though not equivalent protection to D-erythro-sphingosine.
Deletion of SphK2 prevents cardioprotection during ischemia/reoxygenation injury and during ischemic and S1P postconditioning
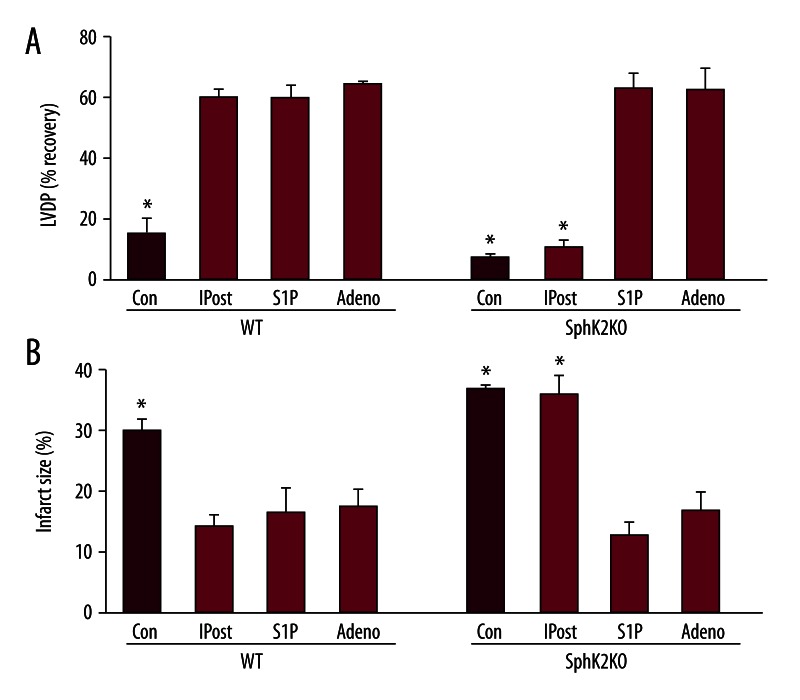
We next investigated the role of SphK2 in IR injury and in protection by ischemic and pharmacologic POST. Previous characterization of the SphK2 null mice (KO) and wild-type (WT) mice revealed that SphK2 protein and activity is absent from the KO hearts [25]. This is accompanied by a 53% decrease in sphingosine kinase activity in both cytosolic and particulate fractions. Hemodynamic function was not different at baseline between KO and WT hearts [26]. Both WT and KO hearts were subjected to 50 minutes of global ischemia and 40 minutes of reperfusion. The recovery of LVDP at the end of IR is shown in Figure 2. On average, there was lower recovery of LVDP in KO mouse hearts (8±1%) than in WT hearts (16±5%), and KO hearts showed a larger infarct size (37±1% vs. 30±2%). Thus, IR appeared to cause more serious damage to KO hearts relative to hearts from WT mice. These results are consistent with our previous findings [22].
Figure 2.
Infarct sizes and recovery of LVDP in wild-type (WT) and SphK2 KO (KO) hearts in ischemia-reperfusion injury and ischemic postconditioning. Equilibrated ex vivo hearts were exposed to 50 min of ischemia followed by 40 min of reperfusion either without postconditioning (Con) or with IPOST, S1P (0.4 μM) or adenosine (Adeno, 0.4 μM) postconditioning in wild-type (WT) and SphK2 knockout (SphK2 KO) hearts. Panel (A) shows LVDP and (B) infarct size expressed as percent of the area at risk at the end of 40 min of reperfusion. Data are expressed as mean ± s.e.m. * P<0.05 vs. all others.
As shown in Figure 2, IPOST increased cardiac protection in WT hearts. IPOST of WT hearts greatly improved recovery of LVDP (61±3%) compared to untreated WT hearts without IPOST (16±5%). Infarct size was far smaller in IPOST treated WT hearts (14±2%) compared with IR alone (30±2%). However, with KO hearts IPOST did not provide protection. Infarct size was not significantly diminished (36±3% compared to 37±1% for untreated). Further, recovery of LVDP was not significantly different between IPOST treated and untreated KO hearts (8±1% for no IPOST vs. 11±2% for IPOST (p>0.05).
POST by S1P also was investigated in WT and KO hearts. The data in Figure 2 indicate that WT mouse hearts can be pharmacologically postconditioned with 0.4 μM S1P. S1P improved LVDP recovery (60±4% vs. 16±5% for untreated, p<0.05). There is also significantly reduced infarction size (17±4% vs. 30±2% in WT, p<0.05). More importantly, the data in Figure 2 show that pharmacological POST by S1P also is not altered in SphK2 null mouse hearts. Thus, there was a 64±2% recovery of LVDP (as compared to 8±1% in untreated hearts) and the infarction size was significantly decreased (13±2% vs. 37±1% untreated).
The success of exogenous S1P in SphK2 KO hearts suggests that the only limitation to ischemic POST is the need for adequate S1P release to fully stimulate S1P receptors. This finding also suggests that S1P does not have intracellular interactions that are important for POST. However, it could just be that exogenous S1P bolsters intracellular S1P levels. To clarify this, we also investigated pharmacologic POST by adenosine, which also postconditions via a GPCR linked pathway [33] but does so without affecting intracellular S1P. As shown in Figure 2, 0.4 μM adenosine effectively postconditioned not only the WT heart (65±1% LVDP and 17±3% infarct size) but also KO hearts (63±8% LVDP and 17±3% infarct size). This indicates that the signaling pathway for pharmacologic postconditioning by GPCRs is unaltered in SphK2 KO hearts.
Protection by exogenous sphingosine and FTY720 in SphK2 KO hearts
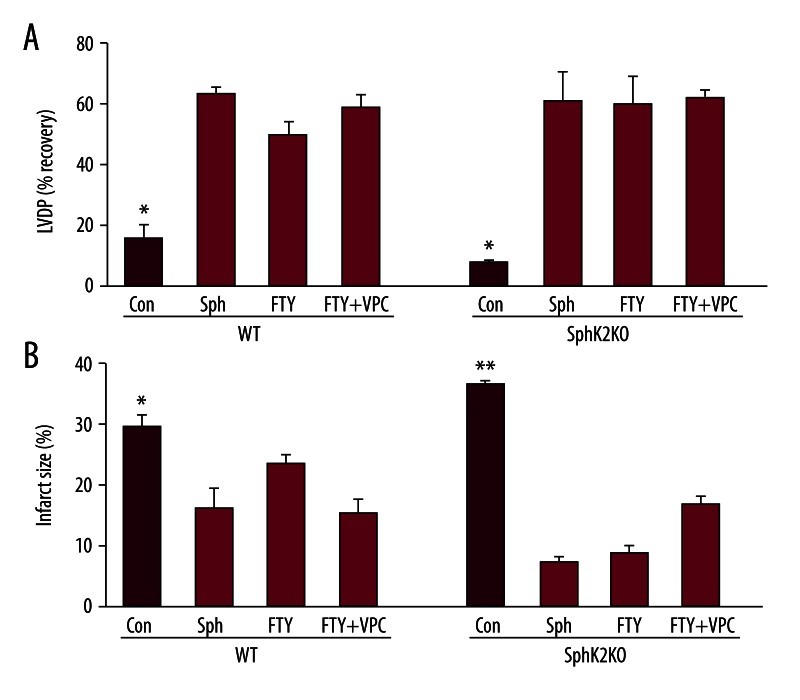
We next examined the effect of loss of SphK2 on pharmacologic POST by sphingosine. The data in Figure 3 indicate that WT hearts can be effectively pharmacologically postconditioned with 0.4 μM sphingosine, as we previously reported [17]. Sphingosine improved LVDP recovery (64±2% vs. 16±3% in untreated WT), and significantly reduced infarction size (16±3% vs. 30±2% in untreated WT). The data in Figure 3 also show that SphK2 KO mouse hearts can also be effectively postconditioned by exogenous sphingosine. However, a slightly higher concentration (0.6 μM) is required for optimum protection. At 0.6 μM sphingosine, recovery of LVDP in KO hearts was 62±13% and the infarct size was exceedingly low (8±1%). This suggests that the signaling pathway for pharmacologic POST by the sphingosine pathway is not significantly altered in SphK2 KO hearts.
Figure 3.
Effect of postconditioning with sphingosine and FTY720 on infarct sizes and recovery of LVDP in wild-type (WT) and SphK2 KO (KO) hearts. Ex vivo wild-type (WT) and SphK2 knockout (SphK2 KO) hearts were equilibrated for 20 min and then exposed to 50 min of ischemia followed by 40 min of reoxygenation with or without pharmacological postconditioning with 0.4 μM FTY720 (FTY) or 0.6 μM sphingosine (Sph), or FTY720 plus 1 μM VPC 23019. Panel (A) shows LVDP and (B) infarct size at the end of 40 min of reperfusion. Data are expressed as mean ± s.e.m. * P<0.05 vs. all others.
Since the SK2 form is responsible for the phosphorylation of FTY720, the effect of the knockout on FTY720 POST was of particular interest. Based on the data in Figure 1, one would expect it to behave in a manner similar to sphingosine unless phosphorylation is very important. As shown in Figure 3, WT mouse hearts can be pharmacologically postconditioned with FTY720 (50±7% LVDP and 24±1% infarct size), though it was slightly less efficient than sphingosine. In KO hearts, we found that FTY720 provided very effective postconditioning. The recovery of LVDP in FTY720 treated KO hearts was comparable (60±9%) to that for WT hearts, while the infarct size was actually significantly better (9±1% vs. 24±1% in WT, p<0.05). This protection in KO hearts was not eliminated by the S1P receptor antagonist VPC23019 which also rules out FTY720-phosphate effects.
Discussion
FTY720 has been presumed to function as a pro-drug for FTY720-phosphate which is an S1P mimic [14]. However, we felt that unphosphorylated FTY720 could, due to its similarity to sphingosine, also be an effective cardioprotectant. Thus, in the absence of the extensive systemic phosphorylation that occurs in vivo, we tested the hypothesis that in the ex vivo heart FTY720 would behave at least in part as a sphingosine analog. Indeed, we found that, like sphingosine cardioprotection, protection by FTY720 is insensitive to S1P receptor antagonists. Inhibition of PI3 kinase, which is downstream of the S1P receptors, was also without effect. This suggests that FTY720 does not act like S1P, which means that prior phosphorylation of FTY720 is not a prerequisite to acute protection by this agent. This was confirmed by the study of the SphK2 KO heart which lacks the form of sphingosine kinase responsible for FTY720 phosphorylation [30,31] and yet is still able to be effectively postconditioned by FTY720. FTY720 further resembles sphingosine in that antagonists of PKA or PKG block its cardioprotection. Clearly, in the ex vivo heart, FTY720 is protecting exclusively via the same PKA/PKG dependent pathway as sphingosine.
Our studies of the SphK2 KO hearts have also demonstrated that SphK2 is essential for successful ischemic postconditioning. SphK2 may be important for maintaining adequate levels of intracellular S1P sufficient to support a high level of S1P release during conditioning, which then would provide for maximum extracellular signaling via GPCRs. The importance of SphK2 in the maintenance of S1P levels is supported by studies in cardiomyocytes from SphK2 KO hearts that revealed significantly reduced levels of S1P [30]. Thus, the simplest explanation for the failure of SphK2 KO mice to respond to ischemic POST is that there is insufficient intracellular S1P to achieve threshold levels of S1P release for triggering maximum cardioprotection.
However, SphK2 has also been reported to have intracellular effects on the mitochondrial respiratory chain that might be important in I/R injury and contribute to the failure of IPC and IPOST in SphK2 KO hearts [27,30]. If this were the case, then even pharmacologic POST by exogenous GPCR agonists such as S1P or adenosine should be compromised. They were not. Both S1P and adenosine effectively postconditioned KO hearts which suggests that SphK2 effects on mitochondrial function do not impact POST. This indicates that a primary reason for failure of IPOST in SphK2 KO hearts is indeed a lower level of S1P release during conditioning and thus a diminished stimulation of GPCRs resulting in reduced effectiveness.
In contrast to IPOST, pharmacologic POST appears to be normal in SphK2 KO hearts, and FTY720 is as effective as S1P or sphingosine. Since FTY720 does not have the predisposition to toxicity associated with sphingosine, it would suggest that acute FTY720 might be the better agent to use for POST, even in the elderly where the SphK2 levels are greatly reduced [16]. The data suggest that FTY720 administered in vivo acts in a dual manner showing both S1P-like effects and sphingosine-like effects. Indeed, following feeding of FTY720, the concentrations of phosphorylated and unphosphorylated FTY720 in plasma were similar [34]. Thus, it appears that the sphingosine-like effects of FTY720 in cardioprotection may have been overlooked. This would not be readily apparent or significant in young hearts where POST by the S1P and sphingosine pathways is equivalently effective. However, in aging hearts the S1P pathway is deficient while the sphingosine pathway is not (16) so FTY720 would be more effective in its unphosphorylated form in aging hearts.
Conclusions
Our data reveal that FTY720 effectively postconditions the ex vivo heart and does so by the same mechanism as sphingosine. Studies of SphK2 KO hearts further revealed that this occurs even in the absence of capacity for FTY720 phosphorylation, which indicates that POST is unrelated to FTY720-phosphate. Based on existing data, it appears that in vivo FTY720 can be expected to exhibit a mix of S1P and sphingosine like effects with the latter being more effective in aging hearts.
Acknowledgements
The authors thank Drs. Shaun Coughlin and Rajita Pappu for supplying the SphK2 null mice.
Footnotes
Source of support: This work was supported by a grant from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (DAV); and by the National Institutes of Health (Grant 1RO1 HL090606 to JSK)
References
- 1.Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Ann Revs Med. 1991;42:225–46. doi: 10.1146/annurev.me.42.020191.001301. [DOI] [PubMed] [Google Scholar]
- 2.Vessey DA, Li L, Kelley M, Karliner JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375:425–29. doi: 10.1016/j.bbrc.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Cath Cardiovasc Interventions. 2008;72:950–57. doi: 10.1002/ccd.21715. [DOI] [PubMed] [Google Scholar]
- 4.Vessey DA, Li L, Kelley M. Pannexin-1/P2X7 purinergic receptor channels mediate the release of cardioprotectants induced by ischemic pre- and postconditioning. J Cardiovasc Pharmacol Ther. 2010;15:190–95. doi: 10.1177/1074248409360356. [DOI] [PubMed] [Google Scholar]
- 5.Vessey DA, Li L, Kelley M. Ischemic preconditioning requires opening of pannexin-1/P2X7 channels not only during preconditioning but again after index ischemia at full reperfusion. Mol Cell Biochem. 2011;351:77–84. doi: 10.1007/s11010-011-0713-9. [DOI] [PubMed] [Google Scholar]
- 6.Hendriks-Balk MC, Peters SLM, et al. Regulation of G protein-coupled receptor signaling: Focus on the cardiovascular system and regulator of G protein signaling proteins. Eur J Pharmacol. 2008;585:278–91. doi: 10.1016/j.ejphar.2008.02.088. [DOI] [PubMed] [Google Scholar]
- 7.Schulz R, Cohen MV, Behrends M, et al. Signal transduction of ischemic preconditioning. Cardiovasc Res. 2001;52:181–98. doi: 10.1016/s0008-6363(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 8.Gross ER, Gross GJ. Ligand triggers of classical preconditioning and postconditioning. Cardiovasc Res. 2006;70:212–21. doi: 10.1016/j.cardiores.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009;297:H1429–35. doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signaling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy S, Kane KA, Pyne NJ, Pyne S. Targeting sphingosine-1-phosphate signaling for cardioprotection. Curr Opin Pharmacol. 2009;9:194–201. doi: 10.1016/j.coph.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Karliner JS, Brown JH. Lipid signaling in cardiovascular pathophysiology. Cardiovasc Res. 2009;82:171–74. doi: 10.1093/cvr/cvp096. [DOI] [PubMed] [Google Scholar]
- 13.Verziji D, Peters SLM, Alewijnse AE. Sphingosine-1-phosphate receptors: zooming in on ligand-induced intracellular trafficking and its functional implications. Mol Cells. 2010;29:99–104. doi: 10.1007/s10059-010-0041-z. [DOI] [PubMed] [Google Scholar]
- 14.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Egom EEA, Ke Y, Musa H, et al. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Molec Cell Cardiol. 2010;48:406–14. doi: 10.1016/j.yjmcc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vessey DA, Kelley M, Li L, Huang Y. Sphingosine protects aging hearts from ischemia/reperfusion injury. Superiority to sphingosine 1-phosphate and ischemic pre- and post-conditioning. Oxid Med Cell Longev. 2009;2:146–51. doi: 10.4161/oxim.2.3.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vessey DA, Li L, Kelley, et al. Sphingosine can pre- and post-condition the heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Molec Toxicol. 2008;22:113–18. doi: 10.1002/jbt.20227. [DOI] [PubMed] [Google Scholar]
- 18.Kohama T, Olivera A, Edsall L, et al. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–28. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Sugiura M, Nava VE, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–20. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 20.Vessey DA, Kelley M, Zhang JQ, et al. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Molec Toxicol. 2007;21:273–79. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- 21.Limaye V, Li X, Hahn C, et al. Sphingosine kinase -1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood. 2005;105:3169–77. doi: 10.1182/blood-2004-02-0452. [DOI] [PubMed] [Google Scholar]
- 22.Olivera A, Kohama T, Edsall L, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–58. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edsall LC, Cuvillier O, Twitty S, et al. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J Neurochem. 2001;76:1573–84. doi: 10.1046/j.1471-4159.2001.00164.x. [DOI] [PubMed] [Google Scholar]
- 24.Jin Z-Q, Zhang J, Huang Y, Hoover HE, et al. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Jin Z-Q, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79:134–40. doi: 10.1093/cvr/cvn065. [DOI] [PubMed] [Google Scholar]
- 26.Vessey DA, Li L, Jin Z-Q, et al. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygentation injury and diminishes responsiveness to ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:961059. doi: 10.1155/2011/961059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez L, Paillard M, Price M, et al. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Basic Res Cardiol. 2011;106:600–12. doi: 10.1007/s00395-011-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez T, Estrada-Hernandez T, Paik JH, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–90. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 29.Paugh SW, Payne SG, Barbour SE, et al. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Letters. 2003;554:189–93. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 30.Strub GM, Paillard M, Liang J, et al. Sphingosine 1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011;25:600–12. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hausenloy DJ, Ong SB, Yellon DM. The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol. 2009;104:189–202. doi: 10.1007/s00395-009-0010-x. [DOI] [PubMed] [Google Scholar]
- 32.Vessey DA, Kelley M, Li L, et al. Role of sphingosine kinase in protection of heart against ischemia reperfusion injury. Med Sci Monit. 2006;12(10):BR318–24. [PubMed] [Google Scholar]
- 33.Headrick JP, Lasley RD. Adenosine receptors and reperfusion injury of the heart. Handb Exp Pharmacol. 2009;193:189–214. doi: 10.1007/978-3-540-89615-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sensken S-C, Bode C, Graler MH. Accumulation of Fingolimod (FTY720) in lymphoid tissues contributes to prolonged efficacy. J Pharmacol Exp Ther. 2009;328:963–69. doi: 10.1124/jpet.108.148163. [DOI] [PubMed] [Google Scholar]