Abstract
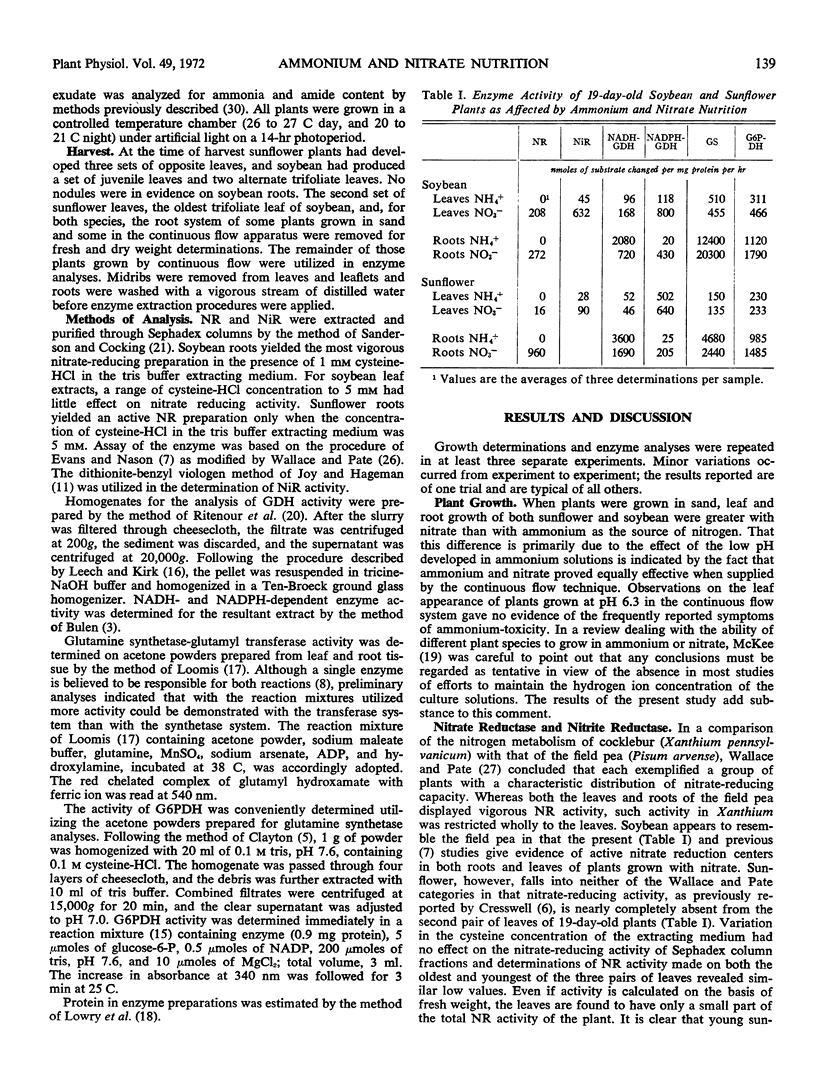
Under conditions of controlled pH, nitrate and ammonium are equally effective in supporting the growth of young soybean (Glycine max var. Bansei) and sunflower (Helianthus annuus L. var., Mammoth Russian) plans. Soybean contains an active nitrate reductase in roots and leaves, but the low specific activity of this enzyme in sunflower leaves indicates a dependency upon the roots for nitrate reduction. Suppression of nitrate reductase activity in sunflower leaves may be due to high concentrations of ammonia received from the roots. Nitrate reductase activity in leaves of nitrate-supplied soybean and sunflower follows closely the distribution of nitrate reductase. For the roots of both species, glutamic acid dehydrogenase activity was greater with ammonium than with nitrate. The glutamic acid dehydrogenase of ammonium roots is wholly NADH-dependent, whereas that of nitrate roots is active with NADH and NADPH. In leaves, an NADPH-dependent glutamic acid dehydrogenase appears to be responsible for the assimilation of translocated ammonia and ammonia formed by nitrate reduction.
In soybean roots ammonia is actively incorporated into amides, much of which remains in the roots. Sunflower roots are less active in amide formation but transfer much of it, together with ammonia, into the shoots. Glutamine synthetase activity in leaves is 20- to 40-fold lower than in roots.
Glucose-6-phosphate dehydrogenase activity appears to be correlated with the activity of the nitrate reducing system in roots, but not in leaves.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULEN W. A. The isolation and characterization of glutamic dehydrogenase from corn leaves. Arch Biochem Biophys. 1956 May;62(1):173–183. doi: 10.1016/0003-9861(56)90100-x. [DOI] [PubMed] [Google Scholar]
- BUTT V. S., BEEVERS H. The regulation of pathways of glucose catabolism in maize roots. Biochem J. 1961 Jul;80:21–27. doi: 10.1042/bj0800021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Nason A. Pyridine Nucleotide-Nitrate Reductase from Extracts of Higher Plants. Plant Physiol. 1953 Apr;28(2):233–254. doi: 10.1104/pp.28.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W. Glutamate dehydrogenase changes in lemna not due to enzyme induction. Plant Physiol. 1971 Mar;47(3):445–446. doi: 10.1104/pp.47.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W., Hageman R. H. The purification and properties of nitrite reductase from higher plants, and its dependence on ferredoxin. Biochem J. 1966 Jul;100(1):263–273. doi: 10.1042/bj1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W. Nitrogen metabolis of Lemna minor. II. Enzymes of nitrate assimilation and some aspects of their regulation. Plant Physiol. 1969 Jun;44(6):849–853. doi: 10.1104/pp.44.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby E. A., Mengel K. Ionic balance in different tissues of the tomato plant in relation to nitrate, urea, or ammonium nutrition. Plant Physiol. 1967 Jan;42(1):6–14. doi: 10.1104/pp.42.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Hageman R. H. The occurrence of nitrate reductase in apple leaves. Plant Physiol. 1969 Jan;44(1):110–114. doi: 10.1104/pp.44.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leech R. M., Kirk P. R. An NADP-dependent L-glutamate dehydrogenase from chloroplasts of Vicia faba L. Biochem Biophys Res Commun. 1968 Aug 21;32(4):685–690. doi: 10.1016/0006-291x(68)90293-3. [DOI] [PubMed] [Google Scholar]
- Loomis W. D. Amide Metabolism in Higher Plants. III. Distribution of Glutamyl Tranferase and Glutamine Synthetase Activity. Plant Physiol. 1959 Sep;34(5):541–546. doi: 10.1104/pp.34.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson G. W., Cocking E. C. Enzymic Assimilation of Nitrate in Tomato Plants. I. Reduction of Nitrate to Nitrite. Plant Physiol. 1964 May;39(3):416–422. doi: 10.1104/pp.39.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. The regulation of glutamine synthesis in microorganisms. Annu Rev Microbiol. 1970;24:501–524. doi: 10.1146/annurev.mi.24.100170.002441. [DOI] [PubMed] [Google Scholar]
- Weissman G. S. Effect of Ammonium and Nitrate Nutrition on Protein Level and Exudate Composition. Plant Physiol. 1964 Nov;39(6):947–952. doi: 10.1104/pp.39.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman G. S. Influence of ammonium and nitrate nutrition on the pyridine and adenine nucleotides of soybean and sunflower. Plant Physiol. 1972 Feb;49(2):142–145. doi: 10.1104/pp.49.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]


