Abstract
Both metaxbolic syndrome (MS) and atrial fibrillation (AF) are associated with increased cardiovascular disease morbidity and mortality. This analysis evaluates the association between MS and AF in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. MS was defined using criteria recommended in the joint interim statement from several international societies. AF was defined in two ways - by electrocardiogram (ECG) and/or self-report and by ECG alone. Among individuals with 0, 1, 2, 3, 4 and 5 MS components, the prevalence of AF by ECG and/or self-report was 5.5%, 7.7%, 8.2%, 9.2%, 9.6% and 11.5%, respectively (p-trend<0.001). After multivariable adjustment, each of the MS components except serum triglycerides was significantly associated with AF. The multivariable-adjusted odds ratio for AF, defined by ECG and/or or self-reported history, comparing those with versus without MS was 1.20 (95% CI: 1.10 – 1.29). Results were consistent when AF was defined by ECG alone (OR=1.15, 95% CI: 0.92 – 1.39). In conclusion, MS is associated with an increased prevalence of AF. Further studies investigating a potential mechanism for this excess risk are warranted.
Keywords: Atrial Fibrillation/epidemiology, Metabolic Syndrome X/epidemiology, Adults, Humans, Cohort Studies
Many metabolic syndrome (MS) components (i.e., elevated blood pressure1-3, high glucose2, 4-7, dyslipidemia8,9, abdominal adiposity10) are also risk factors for atrial fibrillation (AF). However, there are limited data evaluating the association between MS and AF. Quantifying the burden of AF among individuals with MS might provide justification for further studies investigating potential mechanisms of excess cardiovascular disease risk in this population. Accordingly, the goal of this analysis was to evaluate the association of MS with AF in a large population-based national study of US adults. To do so, we analyzed data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study.
Methods
The REGARDS study is a national, population-based, observational study of African-American and white US adults ≥ 45 years of age. Details of the study design and recruitment have been published previously.11 In brief, the study was designed to oversample African-Americans and residents of the geographic region referred to as the “Stroke Belt”, which consists of North and South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana.
The REGARDS study enrolled 30,239 participants between June 2003 and October 2007. Individuals without electrocardiographic (ECG; n=708), blood pressure (n=78), serum glucose (n=1,123), serum lipids (n=544), or waist circumference (n=108) data were excluded from the current analysis. Additionally, participants who did not answer the question about having a prior diagnosis of AF (n=24) and those not fasting (n=3,638) or missing fasting status (n=105) and having poor quality ECGs (n=261) were excluded, leaving data from 23,650 participants for analysis. The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided written consent.
Data were collected through a computer-assisted telephone interview followed by an in-home examination. Of relevance to the current analysis, the following demographic and behavioral information was collected during the interview: age, sex, race, education, annual household income, frequency of physical activity, smoking status, non-steroidal anti-inflammatory (NSAID) use, past history of stroke, and current use of antihypertensive and anti-diabetes medication. The in-home examination included clinical measurements, an ECG, and the collection of a fasting blood sample and urine sample. Left ventricular hypertrophy was defined on the basis of Cornell voltage criteria as described previously.12 Clinical data (height, weight, waist circumference, blood pressure) were collected following standardized protocols. Two blood pressure measurements were taken and averaged for analysis. Using a spot urine, the albumin to creatinine ratio was calculated and categorized as no albuminuria (< 30 mg/g), microalbuminuria (30 to 299 mg/g) or macroalbuminuria (≥ 300 mg/g). Estimated glomerular filtration rate (eGFR) was calculated via the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.13 C-reactive protein was measured using a high-sensitivity particle-enhanced immunonepholometric assay with levels ≥ 3 mg/L defined as elevated.
MS was defined using criteria recommended in the joint interim statement of the International Diabetes Foundation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity.14 Specifically, elevated blood pressure was defined by a systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 85 mmHg, or current antihypertensive medication use; low HDL-cholesterol was defined as < 40 mg/dL in men and < 50 mg/dL in women; high serum triglycerides was defined as ≥ 150 mg/dL; elevated fasting plasma glucose was defined as ≥ 100 mg/dL or antidiabetes medication use; and abdominal obesity was defined as a waist circumference > 102 cm in men and > 88 cm in women. MS was defined as the presence of ≥ 3 of the 5 components. In a secondary analysis, C-reactive protein level was included as a sixth component, with CRP-MetS defined as ≥ 3 of these six components.15
During the initial stages of the REGARDS study, AF was detected from a 7-lead ECG recording using a single mid-sternal chest lead (n=6,507). However, a standard 12-lead ECG was used later in the study (n=17,143). Since ECG-diagnosed AF is not affected by the number of leads, participants with 7-and 12-lead ECGs were included in this analysis. All ECGs were read and coded at a central reading center by trained personnel masked to the clinical data collected in the REGARDS study. For the primary analysis, AF was defined via self-report and/or ECG. This method has been shown to be more sensitive to detection of AF than standard 12-lead ECGs alone.16 Further, self-report is a common method for AF ascertainment in epidemiologic studies, and the associations of morbidity and mortality with self-reported AF are similar to those with ECG-detected AF.17,18 A self-reported history of AF was defined as answering “yes” to the following question: “Has a physician or a health professional ever told you that you had atrial fibrillation?” Analyses were also conducted using AF determined by ECG alone as the outcome.
The percentage of REGARDS study participants having 0, 1, 2, 3, 4, or 5 MS components was calculated. Characteristics of the study participants with and without MS were determined. The statistical significance of differences in variables across MS status was determined using t-tests, chi-square tests, or Mann-Whitney tests, as appropriate. The prevalence of AF was calculated for study participants with and without each MS component and according to number of MS components. Odds ratios for AF associated with each MS component were calculated using logistic regression. These were calculated unadjusted and after adjustment for age, sex, race, education, income, physical inactivity, smoking, NSAID use, eGFR, microalbuminuria, macroalbuminuria, and left ventricular hypertrophy. Due to the strong association between stroke and AF, a sensitivity analysis was conducted by repeating the main analyses after excluding REGARDS study participants with a history of stroke. We performed secondary analyses using C-reactive protein level as a sixth MS component (CRP-MS).15 First, the prevalence of AF was calculated for participants with and without elevated C-reactive protein. Then, we calculated the prevalence of AF in those with or without CRP-MS. Additionally, the odds ratio for AF associated with elevated C-reactive protein and for CRP-MS was calculated after adjustment as described above. For an additional secondary analysis, the multivariable adjusted odds ratios for AF associated with each component, number of components, and MS were calculated for whites and African-Americans separately. Effect modification by race was assessed by including multiplicative interaction terms in the regression model (e.g., race * MS). All analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC).
Results
Overall, 39.8% of study participants had MS. The prevalence of 0, 1, 2, 3, 4, and 5 MS components was 12.4%, 22.5%, 25.2%, 21.7%, 13.4%, and 4.8%, respectively. Characteristics of the study population by MS status are shown in Table 1.
Table 1. Characteristics of REGARDS study participants with and without the metabolic syndrome.
| Variable | Metabolic Syndrome | P-value | |
|---|---|---|---|
| Yes (n=9,421) | No (n=14,229) | ||
| Age (years) | 64.7 (9.0) | 64.5 (9.7) | <0.001 |
| Women | 58.4% | 53.1% | <0.001 |
| African American | 44.6% | 36.8% | <0.001 |
| High school education | 85.5% | 90.4% | <0.001 |
| Income <$20,000 | 24.4% | 15.8% | <0.001 |
| Height (inches) | 66.7 (4.4) | 66.8 (4.0) | <0.001 |
| Weight (kg) | 94.0 (19.8) | 77.8 (16.2) | <0.001 |
| Body mass index (kg/m2) | 32.6 (6.1) | 27.0 (5.0) | <0.001 |
| Physically inactive | 40.0% | 29.5% | <0.001 |
| Currently smoke | 14.9% | 14.0% | 0.07 |
| NSAID* use in the past month | 16.6% | 13.2% | <0.001 |
| Albumin to creatinine ratio (mg/g) | 8.7 (5.1, 22.0) | 6.5 (4.3, 12.2) | <0.001 |
| Microalbuminuria | 15.8% | 8.7% | <0.001 |
| Macroalbuminuria | 4.2% | 1.5% | <0.001 |
| Estimated GFR (mL/min/1.73m2) | 83.6 (22.0) | 86.8 (18.2) | <0.001 |
| C-reactive protein (mg/L) | 3.2 (1.5, 6.8) | 1.6 (0.8, 3.8) | <0.001 |
| Systolic blood pressure (mm Hg) | 132.1 (16.5) | 124.2 (16.0) | <0.001 |
| Diastolic blood pressure (mm Hg) | 78.3 (9.9) | 75.5 (9.7) | <0.001 |
| Currently using antihypertensive medication | 72.9% | 38.6% | <0.001 |
| Plasma glucose (mg/dL) | 114.9 (38.8) | 93.0 (20.0) | <0.001 |
| Serum HDL cholesterol (mg/dL) | 44.5 (12.7) | 57.4 (16.0) | <0.001 |
| Serum LDL cholesterol (mg/dL) | 113.4 (36.3) | 116.0 (33.5) | <0.001 |
| Currently using lipid-lowering medication | 40.6% | 28.2% | <0.001 |
| Serum triglycerides (mg/dL) | 150.0 (103.0, 199.0) | 94.0 (72.0, 122.0) | <0.001 |
| Waist circumference (cm) | 104.9 (14.0) | 89.7 (13.2) | <0.001 |
Numbers in table are mean (standard deviation) or percent except albumin to creatinine ratio, C-reactive protein, and serum triglycerides, which are median (25th, 75th percentiles)
NSAID: non-steroidal anti-inflammatory drugs
Microalbuminuria defined as an albumin to creatinine ratio of 30 to 300 mg/g
Macroalbuminuria defined as an albumin to creatinine ratio ≥300 mg/g
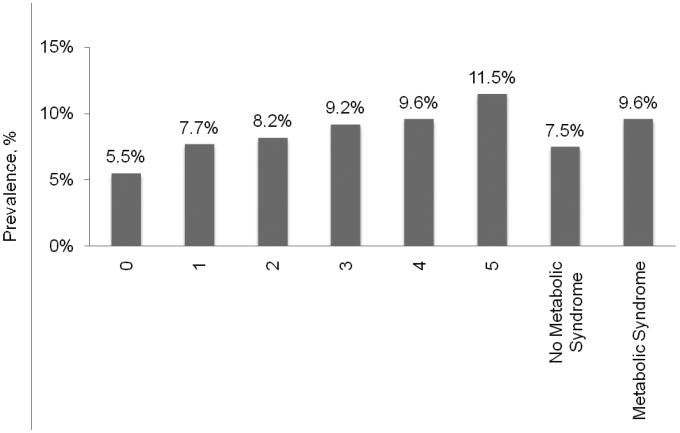
The prevalence of AF defined by ECG and/or self-report was 8.3%. The prevalence of AF was higher in participants with versus without each MS component (Table 2). AF was also more common among those with a greater number of MS components present (Figure 1; p-trend<0.001). Overall, the prevalence of AF by ECG and/or self-report was 9.6% and 7.5% for those with and those without MS, respectively (p<0.001).
Table 2. Prevalence of atrial fibrillation by electrocardiogram and/or self-report among study participants with and without each component of the metabolic syndrome.
| Atrial Fibrillation by electrogardiogram and/or self-report | ||
|---|---|---|
|
| ||
| N (Prevalence) | P-Value | |
| Systolic/diastolic blood pressure ≥ 130/85 mmHg or use of antihypertensive medication | ||
| Yes | 15,904 (9.2%) | <0.001 |
| No | 7,746 (6.6%) | |
| Serum HDL-cholesterol <40 mg/dL in men and <50 mg/dL in women | ||
| Yes | 8,292 (9.4%) | <0.001 |
| No | 15,358 (7.7%) | |
| Serum triglycerides ≥ 150 mg/dl | ||
| Yes | 6,192 (9.0%) | 0.03 |
| No | 17,458 (8.1%) | |
| Serum glucose ≥ 100 mg/dL or use of glucose lowering medication | ||
| Yes | 9,172 (9.4%) | <0.001 |
| No | 14,478 (7.7%) | |
| Waist circumference ≥ 102 cm in males and ≥ 88 cm in females | ||
| Yes | 11,381 (8.9%) | 0.001 |
| No | 12,269 (7.8%) | |
| Metabolic Syndrome | ||
| Yes | 9,421 (9.6%) | <0.001 |
| No | 14,229 (7.5%) | |
Components of the metabolic syndrome include systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥ 85 mmHg or use of antihypertensive medication, serum HDL-cholesterol <40 mg/dL in men and <50 mg/dL in women, serum triglycerides ≥ 150 mg/dl, serum glucose ≥ 100 mg/dL or use of glucose lowering medication, and waist circumference ≥ 102 cm in males and ≥ 88 cm in females.
Figure 1. Prevalence of atrial fibrillation by electrocardiogram and or self-report according to number of metabolic syndrome components.
p-trend comparing number of components <0.001
p-value comparing metabolic syndrome vs. no metabolic syndrome <0.001
Each MS component was associated with an increased odds ratio for AF by ECG and/or self-report in crude analyses (Table 3). After multivariable adjustment, these associations were attenuated, but remained statistically significant for each component except elevated triglycerides. As the number of MS components increased, the odds ratios for AF increased, in crude analyses and after multivariable adjustment (each p-trend<0.001). The crude and multivariable adjusted odds ratios for AF comparing those with, to those without, MS were 1.32 (95% CI: 1.20 – 1.45) and 1.20 (95% CI: 1.10 – 1.29), respectively. In a sensitivity analysis excluding REGARDS study participants with prevalent stroke the multivariable adjusted odds ratio for AF was 1.23 (95% CI: 1.13 – 1.33).
Table 3. Crude and multivariable adjusted odds ratios for atrial fibrillation by electrocardiogram and/or self-report associated with individual or multiple components of the metabolic syndrome.
| Variable | Odds Ratio (95% CI) for Atrial Fibrillation by Electrocardiogram and/or Self-report | ||
|---|---|---|---|
|
| |||
| Crude | Multivariable adjustedˆ | Multivariable adjustedˆˆ | |
| Systolic/diastolic blood pressure ≥130/85 mmHg§ | 1.43 (1.29-1.59)*** | 1.30 (1.19-1.41)*** | 1.21 (1.10-1.32)*** |
| Serum HDL-cholesterol <40 mg/dL in men and <50 mg/dL in women | 1.24 (1.13-1.36)*** | 1.22 (1.12-1.32)*** | 1.17 (1.07-1.27)** |
| Serum triglycerides ≥150 mg/dL | 1.12 (1.01-1.24)* | 1.08 (0.97-1.19) | 1.04 (0.93-1.14) |
| Plasma glucose ≥100mg/dL+ | 1.25 (1.14-1.37)*** | 1.22 (1.13-1.32)*** | 1.17 (1.07-1.27)** |
| Waist circumference ≥102 cm in men and ≥ 88 cm in women | 1.17 (1.06-1.28)** | 1.17 (1.07-1.27)** | 1.14 (1.04-1.23)* |
| 2 Risk Factors‡ | 1.19 (1.05-1.35)** | 1.15 (1.02-1.28)* | 1.12 (0.99-1.25) |
| 3 Risk Factors‡ | 1.36 (1.20-1.55)*** | 1.30 (1.17-1.43)*** | 1.22 (1.09-1.36)** |
| 4 Risk Factors‡ | 1.42 (1.23-1.65)*** | 1.35 (1.20-1.50)*** | 1.26 (1.11-1.41)** |
| 5 Risk Factors‡ | 1.74 (1.42-2.13)*** | 1.60 (1.40-1.80)*** | 1.42 (1.21-1.63)*** |
| Metabolic Syndrome¶ | 1.32 (1.20-1.45)*** | 1.27 (1.18-1.36)*** | 1.20 (1.10-1.29)*** |
Includes participants taking antihypertensive medication
Includes participants taking glucose lowering medication
Compared to those with 0-1 components of the metabolic syndrome
Compared to those with <3 components of the metabolic syndrome
Adjusted for age, sex, race, education, income, physical inactivity, smoking, and nonsteroidal anti-inflammatory use
Adjusted for age, sex, race, education, income, physical inactivity, smoking, nonsteroidal anti-inflammatory use, estimated glomerular filtration rate, microalbuminuria, macroalbuminuria, and left ventricular hypertrophy
p<0.05,
p<0.01,
p<0.001
The prevalence of AF defined by ECG alone was 1.4%. The prevalence of AF defined using ECG alone was higher among participants with low HDL-cholesterol and high serum glucose than those without these MS components (Supplemental Table 1). Among participants with 0, 1, 2, 3, 4, and 5 MS components, the prevalence of AF was 1.0%, 1.3%, 1.4%, 1.4%, 1.4%, and 2.1%, respectively, and the prevalence of AF was 1.5% and 1.3% for those with and without MS, respectively (p=0.188).
Low HDL cholesterol and abdominal obesity were associated with ECG-defined AF after multivariable adjustment (Supplemental Table 2). There was a trend for higher odds ratios for AF as the number of MS components increased in the unadjusted analysis (p-trend<0.001), but this association was not statistically significant after multivariable adjustment (p-trend=0.068). The crude and multivariable adjusted odds ratios for AF defined by ECG only comparing those with versus without MS were 1.16 (95% CI: 0.93 – 1.45) and 1.15 (95% CI: 0.92 – 1.39), respectively. Excluding REGARDS study participants with prevalent stroke, the multivariable adjusted odds ratio for AF by ECG alone was 1.30 (95% CI: 1.05 – 1.56).
The prevalence of elevated C-reactive protein was 40.0%. Among those with and without elevated C-reactive protein, the prevalence of AF by ECG and/or self-report was 9.4% and 7.5%, respectively (p<0.001). The multivariable adjusted odds ratios for AF by ECG and/or self-report and by ECG alone associated with elevated CRP were 1.23 (95% CI: 1.13-1.33) and 1.53 (95% CI: 1.29-1.77), respectively. Using the CRP-MS definition, the crude odds ratios for AF by ECG and/or self-report and by ECG alone were 1.36 (95% CI: 1.24 – 1.49) and 1.26 (95% CI: 1.01 – 1.57), respectively. The multivariable adjusted odds ratios for AF by ECG and/or self-report and by ECG alone associated with CRP-MS were 1.24 (95% CI: 1.14 – 1.34) and 1.30 (95% CI: 1.07 – 1.54), respectively.
The association of each MS component and number of MS components with AF was similar for whites and African-Americans (each p-value for interaction >0.10; data not shown). Among whites and African-Americans, the multivariable adjusted odds ratios for AF by ECG and/or self-report associated with MS were 1.15 (95% CI: 1.03 – 1.27) and 1.27 (95% CI: 1.11 – 1.43), respectively (p-value for interaction = 0.24). Among whites and African-Americans, the multivariable adjusted odds ratios for AF by ECG alone associated with MS were 1.12 (95% CI: 0.86 – 1.38) and 1.40 (95% CI: 0.90 – 1.90), respectively.
Discussion
In the present analysis, the prevalence of AF increased with a higher number of MS components and this association persisted after adjusting for multiple confounders. While participants with MS were also more likely to have AF defined by ECG alone, this association was not statistically significant after multivariable adjustment.
Hypertension is the most common etiological factor associated with AF.1 It is associated with left ventricular hypertrophy, which in turn may lead to diastolic dysfunction, left atrial dilatation, and eventual AF.1 One prior study indicates that up to 14% of prevalent AF is attributable to hypertension alone.2 In the current study, a higher prevalence of AF was present among REGARDS study participants with elevated blood pressure. These findings are consistent with previous studies 2,3 and suggest that elevated levels of systolic/diastolic blood pressure (≥130/85 mmHg) are associated with AF.
Previous studies have reported lower HDL cholesterol levels in individuals with AF.8,9 Annoura and colleagues suggest a causal mechanism through which low HDL cholesterol leads to abnormalities in atrial myocytes through decreased reverse cholesterol transport.9 This mechanism could partially explain the increased AF prevalence among REGARDS study participants with low HDL cholesterol. However, we were unable to fully evaluate this relationship due to lack of REGARDS data on atrial structure. An association between AF and elevated triglycerides was also present in the current study. Prior studies have shown inconsistent results with regard to this association and, to our knowledge, an explanatory mechanism has not been confirmed.8,9
Diabetes is a well-established risk factor for AF.2, 4-6 A study by Nichols, et al4 reported the odds ratio for AF associated with diabetes to be 1.16 (95% CI: 1.05 - 1.28). Another study reported an association between insulin resistance and AF.7 Consistent with prior studies, an increased prevalence of AF was present among REGARDS study participants with elevated glucose levels.
Several studies have examined the association between body mass index (BMI) and AF, but considerably fewer have considered waist circumference and AF. Zhang and colleagues10 conducted a study of obesity and AF classifying obesity by both BMI and waist circumference. They reported that prevalence of AF was higher with increased BMI and waist circumference. Additionally, the results suggested that waist circumference may be a stronger predictor of AF than BMI. In the current study, an increased prevalence of AF by ECG and/or self-report was present among REGARDS study participants with elevated waist circumference.
MS has previously been associated with increased risk for AF in select populations.19-22 In a community-based study of over 20,000 Japanese individuals, Watanabe et al. reported a 13% prevalence of MS and an increased risk of AF (HR=1.78; 95% CI: 1.07-2.96) over a mean follow-up of 4.5 years.19 In the Atherosclerosis Risk in Communities (ARIC) study, participants with MS also had an increased risk of AF (hazard ratio=1.67; 95% CI: 1.49-1.87) compared to those without MS.22 The ARIC study identified AF cases from hospital discharge codes and cases not requiring hospitalization were missed. Furthermore, most of the AF cases were not chronic and therefore may not be typical of AF cases seen in routine clinical practice.
Potential limitations to this study warrant consideration. ECGs were only performed once, making detection of paroxysmal AF difficult. Furthermore, standard 12-lead ECG has a major limitation in detecting paroxysmal AF.6 Consequently, we supplemented the ECG data with self-reported AF to increase the sensitivity of AF ascertainment. Using the definition of AF by ECG alone, the prevalence of AF was far lower than using the broader definition (i.e., including self-report of AF). Although a trend was observed toward an increased prevalence of AF with an increasing number of MS components in the current analysis, missed cases of AF due to having only a single ECG would likely attenuate the association between MS and AF detected by ECG alone. An additional limitation is the cross-sectional study design, which prevents temporal inference for MS and AF. Despite these limitations, this study included a large number of white and African-American adults from throughout the continental US. Additionally, the REGARDS study used a standardized protocol to collect data.
Supplementary Material
Acknowledgments
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. The authors thank the investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Amgen did not have any role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation or approval of the manuscript.
Dr. Muntner and Ms. Tanner had full access to all study data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krishnamoorthy S, Lip GYH. Hypertension, stroke and the impact of atrial fibrillation. Expert Rev Cardiovasc Ther. 2008;6:1287–1289. doi: 10.1586/14779072.6.10.1287. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 3.Inoue H, Nozawa T, Hirai T, Goto S, Origasa H, Shimada K, Uchiyama S, Hirabayashi T, Koretsune Y, Ono S, Hasegawa T, Sasagawa Y, Kaneko Y, Ikeda Y J-TRACE Investigators. Sex-related differences in the risk factor profile and medications of patients with atrial fibrillation recruited in J-TRACE. Circ J. 2009:1–5. doi: 10.1253/circj.CJ-09-0802. [DOI] [PubMed] [Google Scholar]
- 4.Nichols GA, Reinier K, Chugh SS. Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care. 2009;32:1851–1856. doi: 10.2337/dc09-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 6.Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM CHS Research Collaborative Group. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74:236–241. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 7.Johansen OE, Brustad E, Enger S, Tveit A. Prevalence of abnormal glucose metabolism in atrial fibrillation: a case control study in 75-year old subjects. Cardiovasc Diabetol. 2008;7:1–8. doi: 10.1186/1475-2840-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Peromingo J, Alban-Salgado A, Garcia-Suarez F, Sanchez-Leira J, Saborido-Frojan J, Iglesias-Gallego M. Lipoprotein(a) and lipid profile in patients with atrial fibrillation. Med Sci Monit. 2006;12:CR122–CR125. [PubMed] [Google Scholar]
- 9.Annoura M, Ogawa M, Kumagai K, Zhang B, Saku K, Arakawa K. Cholesterol paradox in patients with paroxysmal atrial fibrillation. Cardiology. 1999;92:21–27. doi: 10.1159/000006942. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Zhang S, Li Y, Detrano RC, Chen K, Li X, Zhao L, Benjamin EJ, Wu Y. Association of obesity and atrial fibrillation among middle-aged and elderly Chinese. Int J Obesity. 2009;33:1318–1325. doi: 10.1038/ijo.2009.157. [DOI] [PubMed] [Google Scholar]
- 11.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 12.Soliman EZ, Howard G, Prineas RJ, McClure LA, Howard VJ. Calculating Cornell voltage from nonstandard chest electrode recording site in the Reasons for Geographic And Racial Differences in Stroke study. Electrocardiol. 2010;43:209–214. doi: 10.1016/j.jelectrocard.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WPT, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Katz R, Jenny NS, Zakai NA, LeWinter MM, Barzilay JI, Cushman M. Metabolic syndrome, inflammation, and incident heart failure in the elderly: the cardiovascular health study. Circ Heart Fail. 2008;1:242–248. doi: 10.1161/CIRCHEARTFAILURE.108.785485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prineas RJ, Soliman EZ, Howard G, Howard VJ, Cushman M, Zhang ZM, Moy CS. The sensitivity of the methods of detection of atrial fibrillation in population studies affects group-specific prevalence estimates: ethnic and regional distribution of atrial fibrillation in the REGARDS study. J Epidemiol. 2009;19:177–81. doi: 10.2188/jea.JE20081032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 18.Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74:236–241. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation. Circulation. 2008;117:1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang R, Gao L, Dong J, Liu X, Liu X, Wu J, Long D, Yu R, Du X, Ma C. Metabolic syndrome in patients with atrial fibrillation in the absence of structural heart disease from a tertiary hospital in China. Chin Med J. 2009;122(22):2744–2747. [PubMed] [Google Scholar]
- 21.Yasar AS, Bilen E, Bilge M, Ipek G, Ipek E, Kirbas O. P-wave duration and dispersion in patients with metabolic syndrome. PACE. 2009;32:1168–1172. doi: 10.1111/j.1540-8159.2009.02460.x. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonzo A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2010;159:850–856. doi: 10.1016/j.ahj.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.