Abstract
Total pyridine nucleotide concentration of root tissue for young soybean (Glycine max var. Bansei) and sunflower (Helianthus annuus L. var. Mammoth Russian) plants is the same with either ammonium or nitrate, but nitrate results in an increased proportion of total oxidized plus reduced NADP (NADP[H]) seemingly at the expense of NAD. The activity of NADH- and NADPH-dependent forms of glutamic acid dehydrogenase is correlated with the ratio of total oxidized plus reduced NAD to NADP(H). The low NAD: NADH ratio maintained in nitrate roots despite active NADH utilization via nitrate reductase and glutamic acid dehydrogenase may be the result of nitrate-stimulated glycolysis. Nitrate roots also maintain a high level of NADPH, presumably by the stimulatory effect of nitrate utilization on glucose-6-phosphate dehydrogenase activity. In the presence of nitrate rather than ammonium, the highly active nitrate-reducing leaves of soybean show a greater proportion of total pyridine nucleotide in the form of NADP(H) than do the inactive leaves of sunflower.
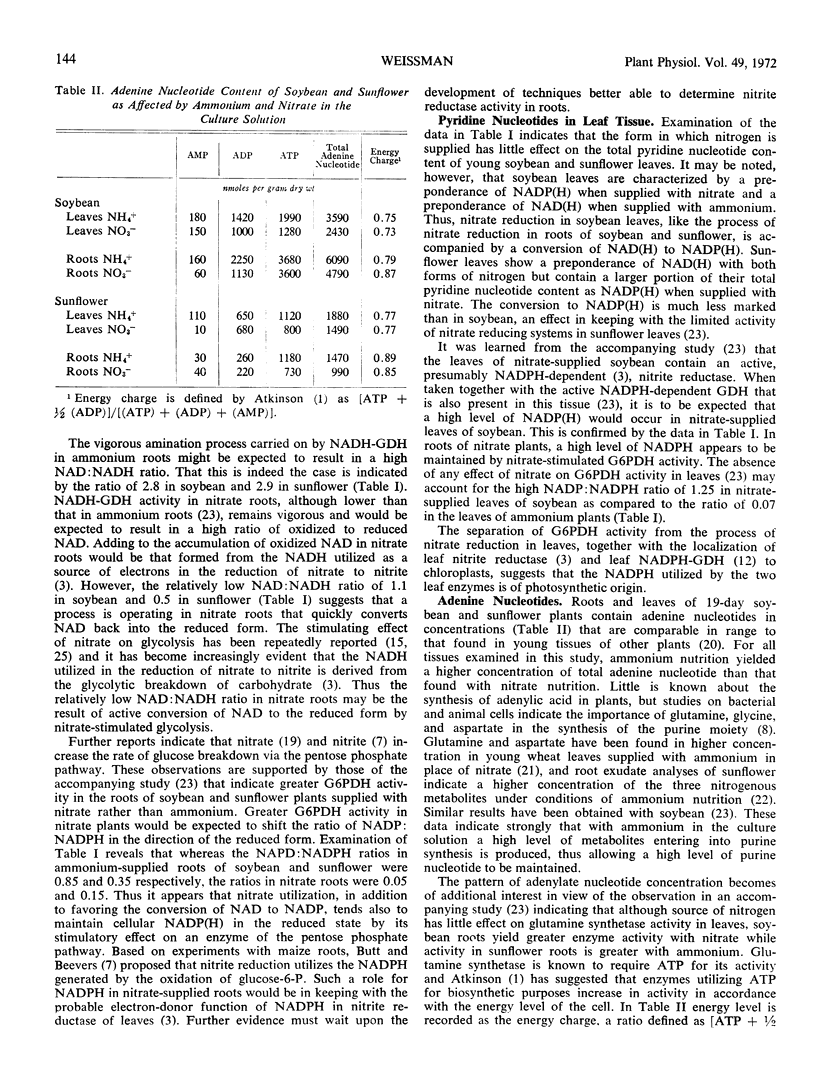
For all tissues examined, ammonium nutrition yields a higher concentration of total adenine nucleotide than is found with nitrate. The data indicate the production of a higher level of metabolites that enter into purine synthesis with ammonium than with nitrate. Glutamine synthetase activity can be correlated with the concept that enzymes utilizing ATP for biosynthetic purposes increase in activity in accordance with the energy level of the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., JAGENDORF A. T. A TPNH diaphorase from chloroplasts. Arch Biochem Biophys. 1956 Dec;65(2):475–490. doi: 10.1016/0003-9861(56)90207-7. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E. Regulation of enzyme function. Annu Rev Microbiol. 1969;23:47–68. doi: 10.1146/annurev.mi.23.100169.000403. [DOI] [PubMed] [Google Scholar]
- BUTT V. S., BEEVERS H. The regulation of pathways of glucose catabolism in maize roots. Biochem J. 1961 Jul;80:21–27. doi: 10.1042/bj0800021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hayyim G., Gromet-Elhanan Z., Avron M. A specific and sensitive method for the determination of NADPH. Anal Biochem. 1969 Apr 4;28(1):6–12. doi: 10.1016/0003-2697(69)90151-1. [DOI] [PubMed] [Google Scholar]
- Ben-Hayyim G., Hochman A., Avron M. Phosphoadenosine diphosphate ribose, a specific inhibitor of nicotinamide adenine dinucleotide phosphate enzymes. J Biol Chem. 1967 Jun 25;242(12):2837–2839. [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. The determination of oxidized and reduced diphosphopyridine nucleotide and triphosphopyridine nucleotide in animal tissues. Biochem J. 1955 Nov;61(3):381–388. doi: 10.1042/bj0610381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys A. J. The intracellular distribution of free nucleotides in the tobacco leaf. Formation of adenosine 5'-phosphate from adenosine 5'-triphosphate in the chloroplasts. Biochem J. 1968 Jun;108(1):1–8. doi: 10.1042/bj1080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby E. A., Mengel K. Ionic balance in different tissues of the tomato plant in relation to nitrate, urea, or ammonium nutrition. Plant Physiol. 1967 Jan;42(1):6–14. doi: 10.1104/pp.42.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., ROCK M. K. The stability of pyridine nucleotides. J Biol Chem. 1961 Oct;236:2756–2759. [PubMed] [Google Scholar]
- Leech R. M., Kirk P. R. An NADP-dependent L-glutamate dehydrogenase from chloroplasts of Vicia faba L. Biochem Biophys Res Commun. 1968 Aug 21;32(4):685–690. doi: 10.1016/0006-291x(68)90293-3. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. The regulation of glutamine synthesis in microorganisms. Annu Rev Microbiol. 1970;24:501–524. doi: 10.1146/annurev.mi.24.100170.002441. [DOI] [PubMed] [Google Scholar]
- Weinstein L. H., McCune D. C., Mancini J. F., van Leuken P. Acid-soluble nucleotides of pinto bean leaves at different stages of development. Plant Physiol. 1969 Nov;44(11):1499–1510. doi: 10.1104/pp.44.11.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman G. S. Effect of Ammonium and Nitrate Nutrition on Protein Level and Exudate Composition. Plant Physiol. 1964 Nov;39(6):947–952. doi: 10.1104/pp.39.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman G. S. Influence of ammonium and nitrate nutrition on enzymatic activity in soybean and sunflower. Plant Physiol. 1972 Feb;49(2):138–141. doi: 10.1104/pp.49.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. Pyridine Nucleotide Content in the Higher Plant. Effect of Age of Tissue. Plant Physiol. 1963 Jan;38(1):45–54. doi: 10.1104/pp.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


