Abstract
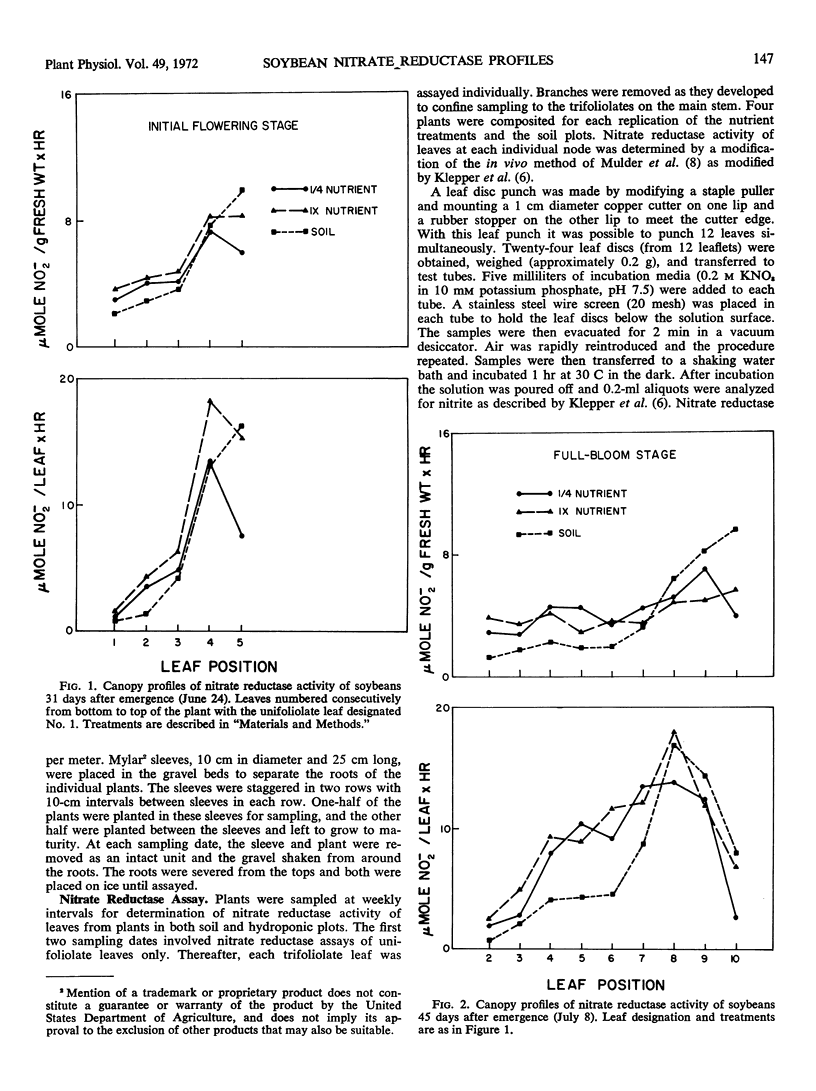
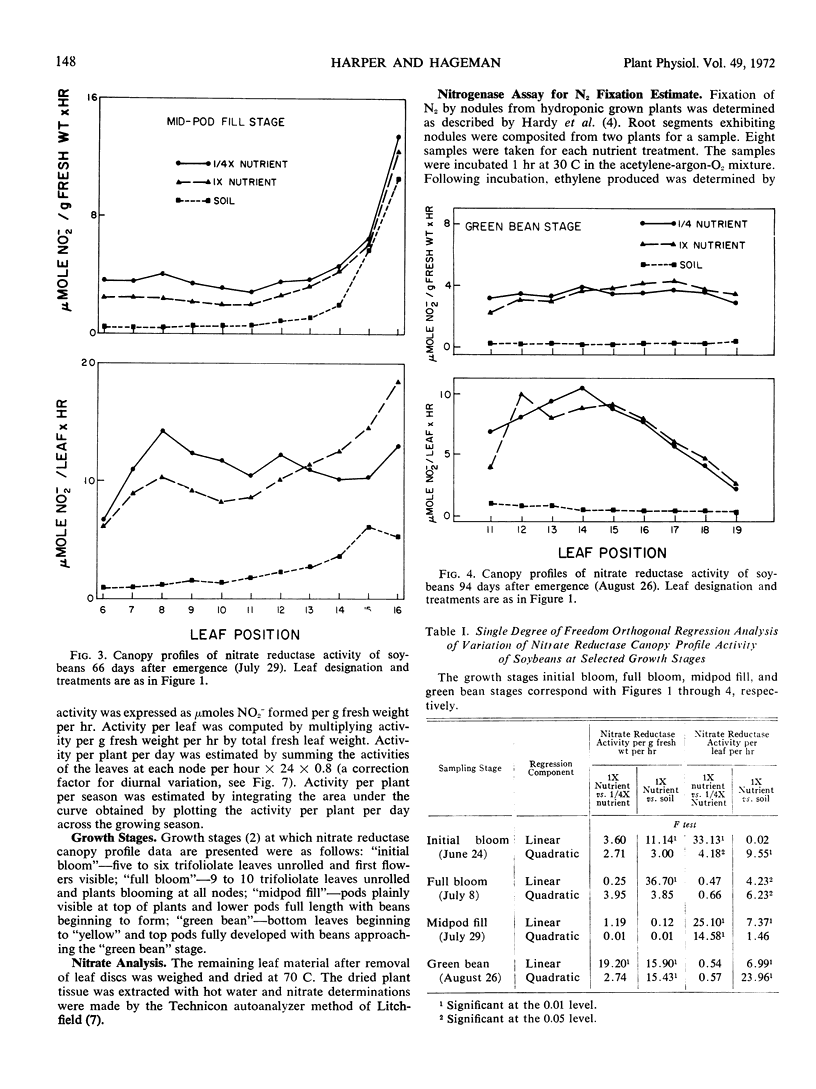
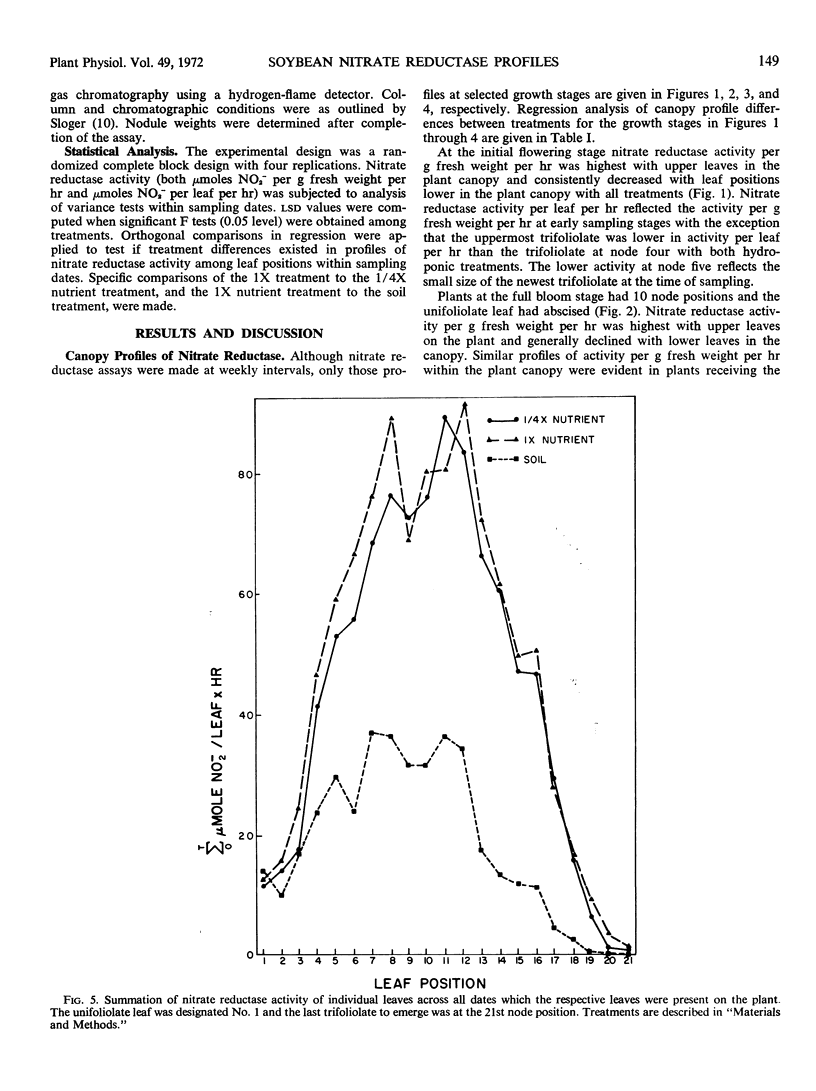
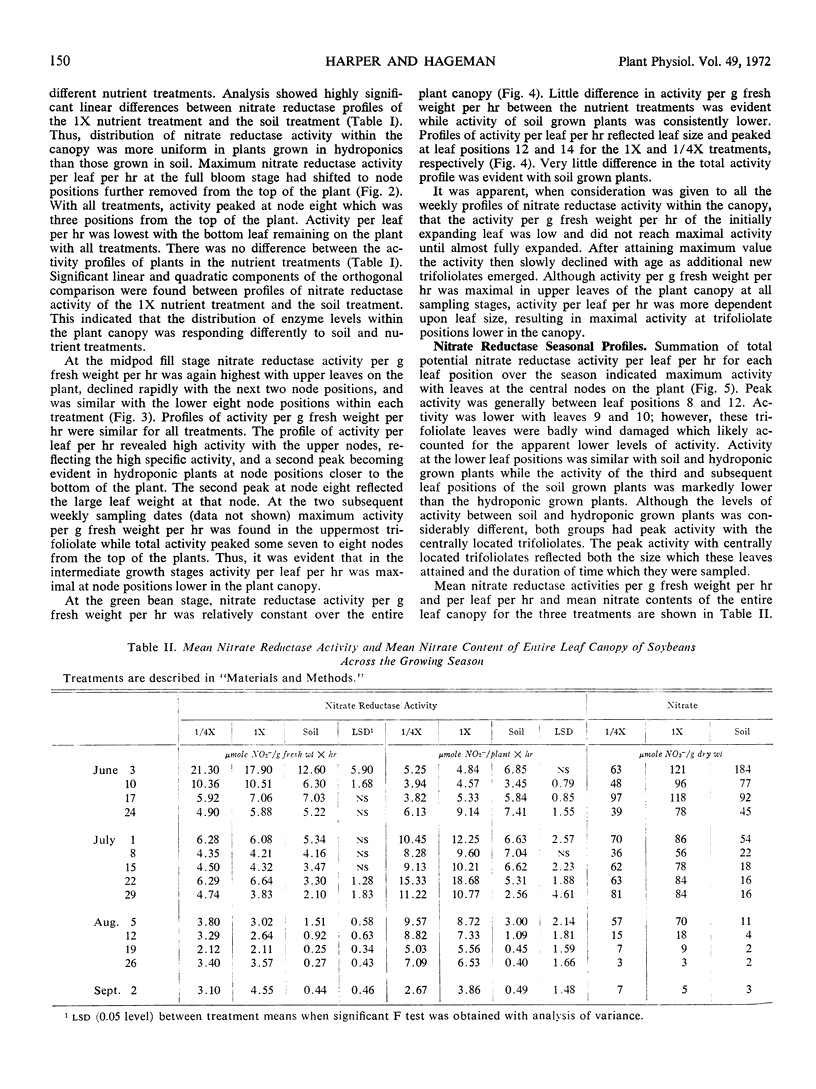
Nitrate reductase activity of soybeans (Glycine max L. Merr.) was evaluated in soil plots and outdoor hydroponic gravel culture systems throughout the growing season. Nitrate reductase profiles within the plant canopy were also established. Mean activity per gram fresh weight per hour of the entire plant canopy was highest in the seedling stage while total activity (activity per gram fresh weight per hour times the total leaf weight) reached a maximum when plants were in the full bloom to midpod fill stage. Nitrate reductase activity per gram fresh weight per hour was highest in the uppermost leaf just prior to full expansion and declined with leaf position lower in the canopy. Total nitrate reductase activity per leaf was also highest in the upper-most fully expanded leaf during early growth stages. Maximum total activity shifted to leaf positions lower in the plant canopy with later growth stages.
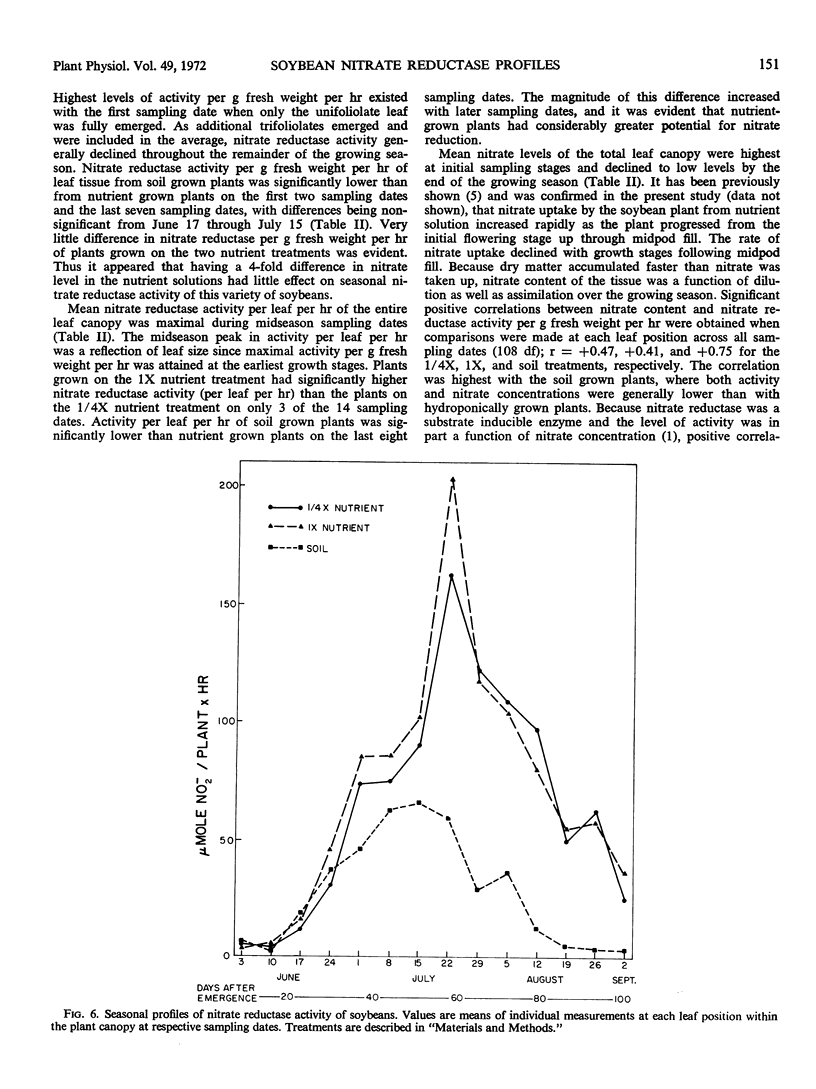
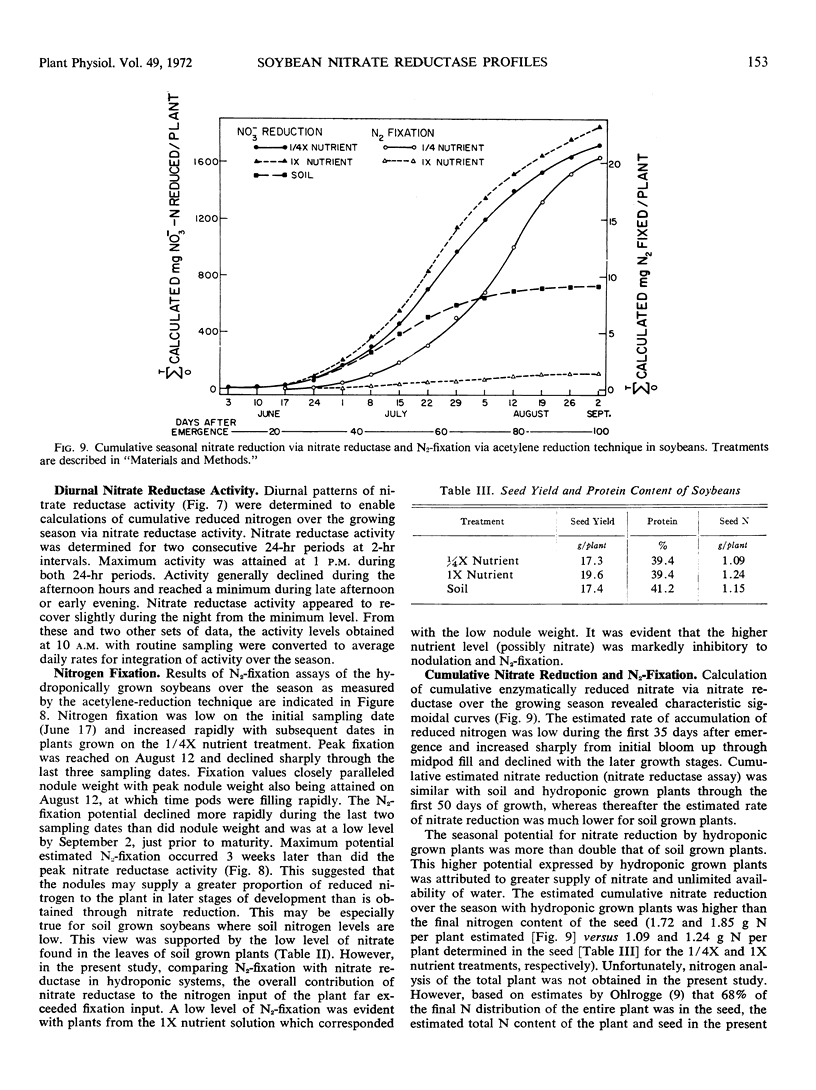
Nitrate reductase activity of soybeans grown in hydroponic systems was significantly higher than activity of adjacent soil grown plants at later growth stages, which suggested that under normal field conditions the potential for nitrate utilization may not be realized. Nitrate reductase activity per gram fresh weight per hour and nitrate content were positively correlated over the growing season with plants grown in either soil or solution culture. Computations based upon the nitrate reductase assay of plants grown in hydroponics indicated that from 1.7 to 1.8 grams N could have been supplied to the plant via the nitrate reductase process. The harvested seed contained 1.1 to 1.2 grams N per plant. Thus, based on previous estimates of approximately 32% of the final N distribution being in the vegetative plant parts, the estimated input of reduced nitrogen via the enzyme assay was in agreement with the actual N accumulation.
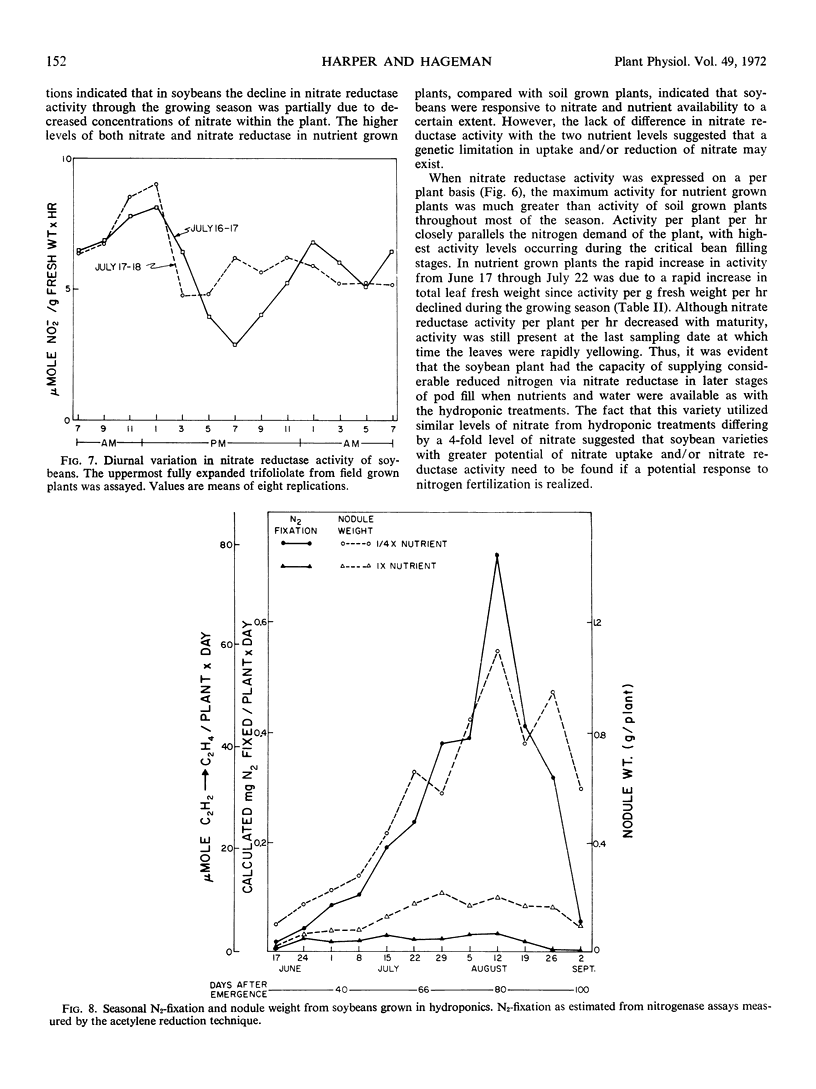
The amount of calculated N2-fixation by nodules per season with plants grown in hydroponics was less than 2% of the computed nitrate reduced via leaf nitrate reductase. Thus, the level of nitrate in the nutrient solution appeared to be quite inhibitory to N2-fixation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield M. H. The automated analysis of nitrite and nitrate in blood. Analyst. 1967 Feb;92(91):132–136. doi: 10.1039/an9679200132. [DOI] [PubMed] [Google Scholar]
- Sloger C. Symbiotic effectiveness and n(2) fixation in nodulated soybean. Plant Physiol. 1969 Dec;44(12):1666–1668. doi: 10.1104/pp.44.12.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]


