Abstract
Purpose
The Schirmer test is one of two ocular surface tests included in the current classification criteria for Sjögren’s Syndrome (SS). Tear osmolarity may also be a useful test for the diagnosis of dry eye disease. The purpose of this study was to examine the relationship between tear osmolarity, the Schirmer test I, and dry eye symptoms in SS.
Methods
Patients with a diagnosis of SS were assessed for tear osmolarity with the TearLab™ Osmolarity System, tear production with Schirmer testing, symptoms with the Ocular Surface Disease Index (OSDI), and discomfort associated with each test.
Results
Forty-nine patients with a mean age of 53.7 years and a female (92%) predominance were enrolled. The majority of patients (86%) were receiving systemic therapy for severe SS. Higher tear osmolarity was moderately associated with lower scores on the Schirmer test I (ρ = −0.39, P < 0.01) and with lower scores on the OSDI (ρ = −0.45, P < 0.01). Schirmer test I results and lower OSDI scores were not correlated significantly (ρ = 0.20, P = 0.17). Tear osmolarity testing was significantly less painful than Schirmer testing (P < 0.01).
Conclusions
Signs and symptoms of dry eye in SS patients were not strongly correlated. An unexpected finding was that higher tear osmolarity was associated with lower symptom severity. Tear osmolarity testing in the clinical setting was feasible and was associated with significantly less discomfort than Schirmer testing in patients with severe SS.
Keywords: tear osmolarity, Sjögren’s syndrome, dry eye
Introduction
Sjögren’s syndrome (SS) is a systemic autoimmune disease that affects as many as four million Americans and is nine times more common in women.1 In this syndrome, mononuclear cells infiltrate the salivary and lacrimal glands, resulting in severe dry mouth and dry eye, which significantly impair one’s quality of life.2 In addition, patients can have systemic features involving the dermis, lungs, liver, kidneys, blood, gastrointestinal tract, vasculature, and nervous system.2 Keratoconjunctivits sicca (KCS) is the main ocular manifestation of SS and thus, many SS patients first seek care from eye care professionals. Treatment initially focuses on relief of symptomatic dryness with conservative measures such as artificial tears, punctal plugs, pilocarpine, and topical cyclosporine.3 However, as the disorder progresses, systemic therapy may become necessary and typically is initiated with hydroxychloroquine.3 Refractory symptoms that are accompanied by extraglandular manifestations such as vasculitis or neurologic disease may require systemic immunosuppressive agents such as glucocorticoids, cyclosporine, methotrexate, and TNF inhibitors (infliximab).3 Unfortunately, the efficacy of these agents appears to be limited in some patients and emerging therapies including anti-CD20 agents and T-cell inhibitors are under investiagtion.3
In 2002, the American-European Consensus Group revised the European classification criteria for SS. The current consensus for SS diagnosis includes the areas of histopathology, serology, as well as ocular and oral signs and symptoms.4 The objective ocular signs are determined using the Schirmer test I (without anesthesia) and ocular surface staining with vital dyes.
However, the use of the Schirmer test I and ocular surface staining have drawbacks. The Schirmer test I is commonly used in the diagnosis and evaluation of dry eye. However, the test strip can be irritating and may induce a great deal of reflex tearing, making the test inaccurate or difficult to interpret.5 Furthermore, the test does not evaluate the evaporative features of dry eye.6 Versura et al found that the Schirmer test showed a poor diagnostic performance in SS.7 Ocular surface staining includes the use of fluorescein and lissamine green to grade changes in the cornea and conjunctiva. However, there are several different grading scales for staining that are subjective and the interpretation of which can vary widely among different graders.
In recent years, some investigators have suggested that tear film osmolarity may be a useful test for the diagnosis of dry eye.8,9,10 This was described as early as 1970 by Mishima and colleagues when they compared tear osmolarity in normal subjects and those with KCS.11 But difficulty collecting tears using hand-drawn glass capillary pipettes, excessive reflex tearing, and delayed osmolarity determination using freezing-point depression have historically made measuring tear osmolarity cumbersome to perform in clinical practice. Thus in the past, tear osmolarity measurements were rarely used as a diagnostic criterion for dry eye in the clinical setting. Recent improvements in technology now allow tear osmolarity tests to be performed rapidly in the office with minimal discomfort to patients.
The development of a more objective, reliable ocular surface test for the evaluation of KCS would aid in the diagnosis and monitoring of this disease. Previous studies support the idea that there are alterations in the tear film of SS patients and various components of the tear film have been proposed as possible aids in the diagnosis and study of SS, including lysozome, lactoferrin, and beta2-microglobulin.12,13,14,15 Tear osmolarity may serve as a useful marker of KCS in this population. The purpose of this study was to determine the correlation of tear osmolarity with the OSDI and Schirmer I test in patients with severe SS, and to evaluate the ease of testing as well as the level of patient discomfort associated with each test.
Materials and Methods
A cross-sectional study design was used. Patients were recruited from the ophthalmology and rheumatology practices at an academic center. Inclusion criteria were: age over 18 years with a diagnosis of Sjögren’s syndrome (SS) in accordance with the American-European Consensus Group criteria (Table 1).4
Table 1.
| American-European Consensus Sjögren’s Syndrome Classification Criteria4 |
For a primary Sjögren’s syndrome diagnosis:
|
|
For a secondary Sjögren’s syndrome diagnosis: In patients with another well-defined major connective tissue disease, the presence of one symptom (I or II) plus 2 of the 3 objective criteria (III, IV and V) is indicative of secondary SS. |
| I. Ocular Symptoms (at least one) |
|
| II. Oral Symptoms (at least one) |
|
| III. Ocular Signs (at least one) |
|
| IV. Histopathology |
|
| V. Oral Signs (at least one) |
|
| VI. Autoantibodies (at least one) |
|
| Exclusion Criteria |
|
Exclusion criteria for the study were: history of any significant ocular surface disease or ocular inflammation(other than KCS); history of ocular surgery within the past 1 month; history of contact lens wear in the last 1 week; use of eye medications within 2 hours prior to measuring tear osmolarity.
The study was approved by the Institutional Review Board and conducted in accordance with the guidelines for experimental investigation with human subjects. Demographic information, past medical and ocular history, current medications, and history of dry eye therapies were recorded. Dry eye symptoms were assessed using the Ocular Surface Disease Index questionnaire (OSDI; Allergan, Inc, Irvine, CA). The OSDI consists of 12 questions on symptoms within the past week and yields scores ranging from 0 (least severe) to 100 (most severe). A score of 12 is typically used as a cutoff for normal, 13–22 for mild dry eye, 23–32 for moderate dry eye, and ≥ 33 for severe dry eye.
Tear osmolarity measurements were taken from each eye using the TearLab Osmolarity System (TearLab, San Diego, CA). Tear samples were collected atraumatically from the lateral tear meniscus. At the beginning of each day of patient testing, the system was calibrated according to the manufacturer’s instructions. There have been several studies that have proposed different cut-off values for dry eye including 316 mOsm/L for moderate-severe disease.8,16
The Schirmer test I without anesthesia was performed after tear osmolarity measurements were obtained. Person-level scores for tear osmolarity and Schirmer test were calculated as the mean of the two eyes unless otherwise noted as the “worse eye” (as defined as the eye with higher tear osmolarity or lower Schirmer score, even if they came from opposite eyes). A Schirmer test I value of less than 10mm in 5 minutes is considered abnormal,17 with a value less than 5mm in 5 minutes considered one of the ocular criteria for Sjogren’s syndrome.4 After the tear osmolarity and the Schirmer test I, patients were asked to report pain on a scale of 0 to 5 reflecting discomfort associated with each test (0=no pain and 5=most pain).
Spearman rank-order correlation coefficients were used to examine the relationship between results from the same test in the two eyes of the same patient, and between results from different tests administered to the same patient. Wilcoxon’s signed rank test was used to assess differences between the pain scores associated with the clinical tests, and differences between clinical test results in medicated versus non-medicated patients. All statistical computations were performed with SAS 9.2 (SAS Institute, Inc., Cary, NC).
Results
Subject Characteristics
Forty-nine subjects (98 eyes) with severe primary or secondary SS were included in the study (45 females, 4 males, mean age: 53.7 ± 15.9 years). The majority of patients were taking at least one systemic therapy, with 59% taking hydroxychloroquine (Table 2). Subjects with a history of cataract surgery were enrolled at least two years post-operatively and one subject had a history of laser-assisted in situ keratomileusis surgery (LASIK) six years prior to participating in the study.
Table 2.
Subject Characteristics
| Characteristics | n (%) |
|---|---|
| Age (years) | |
| 18–35 | 8 (16%) |
| 36–55 | 17 (35%) |
| 56–65 | 18 (37%) |
| >65 | 6 (12%) |
| Sex | |
| Female | 45 (92%) |
| Male | 4 ( 8%) |
| Race/ethnicity | |
| White | 33 (67%) |
| African American | 14 (29%) |
| Hispanic | 1 ( 2%) |
| Unknown | 1 ( 2%) |
| OSDI* | |
| Normal (0–12) | 9 (19%) |
| Mild (12–22) | 3 ( 6%) |
| Moderate (23–32) | 9 (19%) |
| Severe (33–100) | 26 (55%) |
| Tear Osmolarity (mOsm/L) | |
| ≤305 (mild) | 16 (33%) |
| >305 – 316 (moderate) | 12 (24%) |
| >316 (moderate-severe) | 21 (43%) |
| Schirmer’s Test I (mm/5 min) | |
| 0 – 2 | 4 ( 8%) |
| 3 – 5 | 7 (14%) |
| 6 – 10 | 17 (35%) |
| >10 | 21 (43%) |
| Type of Sjögren’s syndrome | |
| Primary | 41 (84%) |
| Secondary | 8 (16%) |
| Current medications | |
| Hydroxychloroquine | 29 (59%) |
| Pilocarpine | 13 (27%) |
| Prednisone | 12 (24%) |
| Methotrexate | 5 (10%) |
| Azathioprine | 3 ( 6%) |
| Rituximab | 2 ( 4%) |
| Fish oil | 31 (63%) |
| Flaxseed oil | 7 (14%) |
| Topical cyclosporine | 12 (12%) |
| Erythromycin ointment | 4 ( 8%) |
OSDI=Ocular Surface Disease Index, score is rounded to nearest whole number
The mean ± SD for the OSDI was 37.5 ± 24.0, for tear osmolarity was 314.5 ± 18.0 mOsm/L, and for the Schirmer test I was 12.9 ± 9.7 mm. Twenty-one patients (43%) had tear osmolarity >316 mOsm/L. A Schirmer test I score of less than or equal to 5mm was found in eleven (21%) subjects, and four subjects (8%) had a score of 2mm or less. Nine subjects (19%) had moderate dry eye symptoms (OSDI 23–32) and twenty-six subjects (55%) had severe dry eye symptoms (OSDI >33). Twenty-six percent of subjects on some form of dry eye therapy had low Schirmer values (less than or equal to 5mm and seventy-four percent had an OSDI score greater than 22, compared with fifty-seven percent and seventy-five percent of untreated patients, respectively. The results from the Schirmer test I were strongly correlated between a subject’s eyes (ρ = 0.76, P < 0.01). Tear osmolarity had a moderate positive between eye correlation within subjects (ρ=0.46, P <0.01).
Correlation of tear osmolarity, Schirmer testing, and OSDI
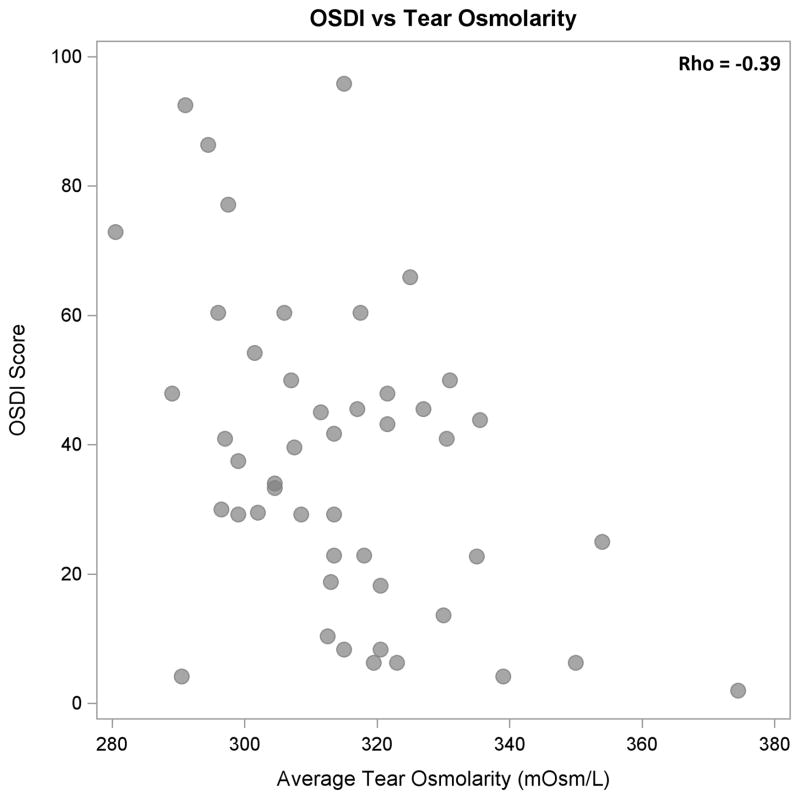
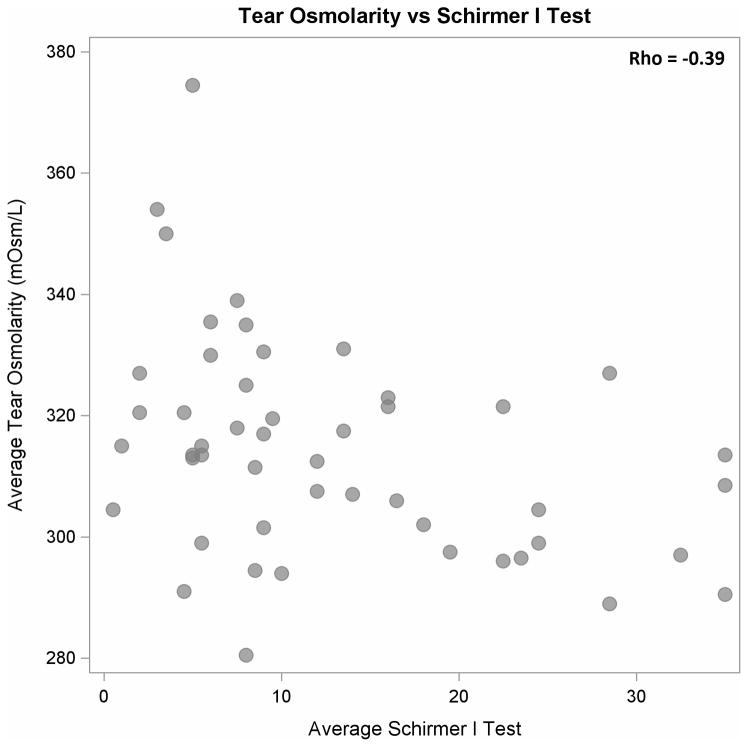
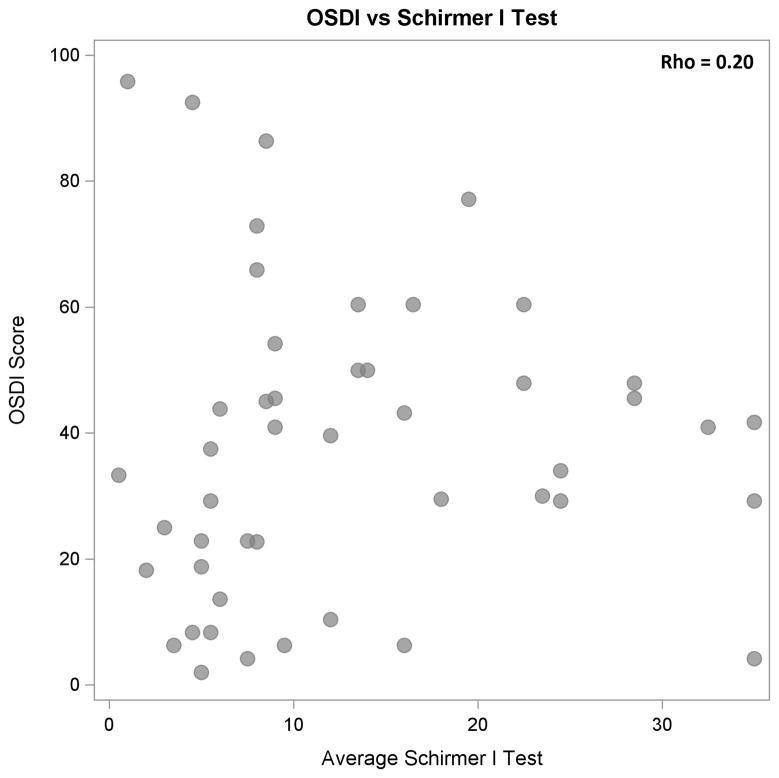
Higher tear osmolarity values were moderately and significantly associated with lower OSDI scores (ρ = −0.39, P < 0.01, Figure 1) and with lower Schirmer test results (ρ = −0.39, P < 0.01, Figure 2). Schirmer test I results and OSDI scores were not correlated significantly (ρ = 0.20, P = 0.17, Figure 3). When the results from the “worse eye” were used, similar correlations were observed between tear osmolarity and OSDI scores (ρ = −0.45, P < 0.01) , tear osmolarity and Schirmer I results (ρ = −0.35, P = 0.01), and Schirmer I results and OSDI scores (ρ = 0.13, P = 0.38). Tear osmolarity testing was significantly less painful than the Schirmer test by a median of 2.75 on a pain scale from 0–5 (Wilcoxon test, P < 0.01).
Figure 1.
Scatter plot showing statistically significant, moderate negative correlation between OSDI scores and tear osmolarity (P < 0.01).
Figure 2.
Scatter plot showing a stastically significant, moderate negative correlation between tear osmolarity and Schirmer I test results (P < 0.01).
Figure 3.
Scatter plot demonstrating no statistically significant correlation between OSDI scores and Schirmer test I scores (P = 0.17).
Association With Immunomodulatory Therapy
There was no significant difference in symptoms, tear osmolarity, or Schirmer testing among patients on hydroxychloroquine compared to those not taking the medication. Subjects on at least one anti-inflammatory medication (hydroxychloroquine, prednisone, methotrexate, azathioprine, rituximab) had higher Schirmer values (14.6 ± 9.6 mm) compared to those not on any of these medications (8.4 ± 9.0 mm; P <0.01). Subjects on anti-inflammatory medications also had higher Schirmer values in their worse eye (11.9 ± 9.3 mm) compared to the worse eye of subjects not on medication (7.1 ± 8.8 mm; P <0.03). However, there was no significant difference in OSDI or tear osmolarity between these two groups (Table 3). When the correlations between test results and with OSDI were reviewed by subgroups based on the use of systemic anti-inflammatory medications and on the use of hydroxychloroquine, the direction and magnitude of the correlations observed in the full group did not change substantially.
Table 3.
Measures of Dry Eye by Systemic Therapy
| N† | Mean ± Standard Deviation [minimum - maximum]
|
|||||
|---|---|---|---|---|---|---|
| OSDI | Tear osmolarity (worse eye) | Tear osmolarity (average) | Schirmer I (worse eye) | Schirmer I (average) | ||
|
| ||||||
| All subjects | 49 | 37.5 ± 24.0 [2.0 – 95.8] (n=47) | 324.1 ± 24.7 [284 – 397] | 314.5 ± 18.0 [280.5 – 374.5] | 10.5 ± 9.3 [0 – 35] | 12.9 ± 9.7 [0.5 – 35.0] |
|
| ||||||
| Systemic anti-inflammatory therapy* | ||||||
|
| ||||||
| Yes | 35 | 37.0 ± 23.7 [2.0 – 95.8] | 322.6 ± 24.2 [284 – 395] | 313.3 ± 18.3 [280.5 –374.5] | 11.9 ± 9.3 [1 – 35] | 14.6 ± 9.6 [1.0 – 35.0] |
|
| ||||||
| No | 14 | 38.9 ± 25.7 [13.6 – 92.5] | 327.8 ± 26.4 [294 – 397] | 317.4 ± 17.7 [291 – 355] | 7.1 ± 8.8 [0 – 35] | 8.4 ± 9.0 [0.5 – 35.0] |
| P-value | 0.83 (n=12) | 0.43 | 0.40 | 0.03 | 0.01 | |
|
| ||||||
| Hydroxychloroquine therapy | ||||||
|
| ||||||
| Yes | 29 | 39.1 ± 23.8 [2.0 – 95.8] | 322.8 ± 25.9 [284 – 395] | 313.3 ± 19.6 [280.5 -374.5] | 11.9 ± 9.7 [1 – 35] | 9.9 ± 12.0 [1.0 – 35.0] |
|
| ||||||
| No | 20 | 34.8 ± 24.7 [6.3 – 92.5] | 326.0 ± 23.4 [294 – 397] | 316.2 ± 15.8 [291 – 355] | 8.5 ± 8.5 [0 – 35] | 10.4 ± 9.1 [0.5 – 35.0] |
| P-value | 0.37 (n=18) | 0.43 | 0.37 | 0.13 | 0.07 | |
Systemic anti-inflammatory therapy: hydroxychloroquine, prednisone, methotrexate, azathioprine, rituximab.
Number of subjects (N) for each category is listed in the “N” column unless otherwise noted.
Discussion
Tear Osmolarity and Sjogren’s Syndrome
Sjogren’s syndrome (SS) can be a difficult diagnosis to make and often patients suffer with symptoms for years before a diagnosis is made. SS patients typically suffer from moderate to severe dry eye due to aqueous tear deficiency as well as possible meibomian gland dysfunction and frequently complain of discomfort related to standard diagnostic testing for dry eye. Measurement of tear osmolarity may therefore represent a more convenient and better tolerated diagnostic alternative to traditional dry eye tests.
Threshold tear osmolarity values for dry eye disease can vary depending on the instrument used, the area of the eye sampled, and the severity of dry eye in the recruited subjects. Consequently, many different cut-off values have been proposed for the diagnosis of KCS. Tomlinson et al performed a meta-analysis of all studies performed between 1978 and 2005 and proposed a cut-off of 308 mOsm/L for early KCS and 316 mOsm/L (sensitivity 69%, specificity 92%) for moderate-severe dry eye disease.8 Others have proposed cutoffs ranging from 304–323 mOsm/L.9,16,18,19 However, no specific tear osmolarity cutoff has been proposed for SS patients. Due to a lack of consensus as to a threshold tear osmolarity value, measuring and following trends in tear osmolarity in SS patients (i.e. change from an individual patient’s baseline), may be more helpful in guiding treatment in this population than comparison to standard threshold values.
Our study included subjects with severe primary or secondary Sjogren’s syndrome and found a mean tear osmolarity of 314.5 mOsm/L, which is higher than some previously reported threshold tear osmolarity values associated with dry eye syndrome.18,20 This is higher than the mean osmolarity of 301.9 mOsm/L in the study by Utine and colleagues which included ten patients with primary SS.20 Of note, all SS patients in that study were on some form of systemic treatment versus 86% of our patients, which could explain why the mean osmolarity we found was higher in our subjects. Our average tear osmolarity result is also higher than that of Szalai et al. who found an average tear osmolarity of 303.4 ± 17.2 in twenty subjects with Sjogren’s syndrome, but they did not analyze the effect of systemic medications in their study.21
The majority of our subjects (86%) were taking at least one systemic therapy, with a large percent (59%) taking hydroxychloroquine. While treatment with anti-inflammatory medication did not have a statistically significant effect on tear osmolarity or symptoms as evaluated by the OSDI in our study, we did observe significantly higher Schirmer test scores and a non-significant trend toward lower tear osmolarity in treated versus untreated subjects. Additionally, 26% of subjects on some form of dry eye therapy had low Schirmer values (< 5mm) and 74% had an OSDI >22 despite treatment, compared with 57% and 75% of untreated patients, respectively. These findings suggest that while systemic treatment for SS may improve tear production, the symptoms of dry eye may persist. While our study showed no difference in tear osmolarity in treated and untreated patients, since the majority of our subjects were on treatment at the time of the study it is possible that future studies focusing on untreated SS patients may find that untreated SS is associated with an average tear osmolarity greater than 314.5 mOsm/L. Additionally, studies with a larger subject population may reveal a significant difference in tear osmolarity between treated and untreated subjects with SS.
Another factor which may have affected the results of this study is that tear osmolarity was only checked one time per eye of each subject. A recent study found that consecutive tear osmolarity readings using the TearLab system in a given individual without dry eye varied by up to 35mOsm/L, but that an average of three consecutive readings was found to be a reliable indicator of tear osmolarity.21 Future studies involving multiple consecutive readings in each eye of SS patients would be helpful in clarifying if more variation is seen in this population than what was previously reported by Khanal and colleagues22.
Tear Osmolarity, Schrimer I Testing, and OSDI
Our study found a moderate, statistically significant negative correlation between tear osmolarity and Schirmer I testing. This is consistent with the results of Utine et al who found a significant moderate negative correlation between tear osmolarity and Schirmer testing in subjects with SS.20 This finding was expected since a low Schirmer test value is representative of decreased tear film secretion, and aqueous tear deficiency is known to result in increased tear osmolarity.17 Therefore, it is logical that tear film osmolarity would increase in patients with decreased Schirmer values.
Interestingly, we found a statistically significant negative correlation between OSDI and tear osmolarity in our population in which patients with a higher tear osmolarity reported lower symptom severity. This differs from the findings of Utine et al who found a significant moderate positive correlation between OSDI and tear osmolarity.20 One possible explanation for the negative correlation found in our study is that SS may decrease corneal sensation, dampening the patient’s perception of ocular irritation and dry eye symptoms. Therefore, as dry eye worsens due to decreased aqueous tear production, tear osmolarity would increase while perceived symptoms would decrease. Several studies have shown that decreased corneal sensitivity is a feature of severe dry eye and SS.22,23,24 These studies suggest that the longer duration or greater severity of disease, the less sensitive the cornea. Accordingly, the higher the tear osmolarity, a potential marker of dry eye disease severity, the less sensitive the cornea. However, one study found that patients with primary SS in particular have corneal mechanical hypersensitivity,25 therefore further larger studies focusing on tear osmolarity, corneal innervation, and correlation with signs of severe KCS such as hyperemia and mucous discharge are needed to elucidate this relationship.
Another possible explanation for the negative correlation between OSDI and tear osmolarity is that the OSDI may not adequately reflect the severity of SS-related dry eye symptoms. In comparing the National Eye Institute Vision Functioning Questionnaire (NEI-VFQ) and OSDI questionnaires in SS patients, Vitale et al speculated that the systemic nature and chronicity of SS-related dry eye may be more readily captured by the NEI-VFQ, which measures both frequency and intensity of vision-targeted health-related quality of life.26 Further studies using other questionnaires might be helpful in evaluating what the best questionnaire is for use with SS patients in capturing their dry eye symptoms.
Utility of Tear Osmolarity Testing
The current ocular criteria for the diagnosis of SS include unanesthetized Schirmer testing and ocular surface staining with vital dyes. However, both of these tests are imperfect and have drawbacks. We found that tear osmolarity could be assessed in the office in SS patients, despite the fact that they may have severe dry eye due to aqueous tear deficiency. In addition, subjects reported having significantly less discomfort associated with tear osmolarity testing than Schirmer I testing (P < 0.01). The current study did not include all currently available dry eye diagnostic tests such as tear break-up time, ocular surface staining, and interferometry. Further larger studies which include the use of these tests are needed in order to assess the utility of tear osmolarity testing both for clinical and research purposes. If tear osmolarity testing can be shown to be accurate, reproducible, and reliable, it may be useful for tracking patient response to therapy and even supplement or replace the current ocular criteria for the diagnosis of SS.
Acknowledgments
Source of Funding:
Giacomina Massaro-Giordano receives grant support from Research to Prevent Blindness. Vatinee Y. Bunya has received research supplies from TearLab for a separate study and receives support from a grant from the National Eye Institute (K12-EY-015398) and a grant from Research to Prevent Blindness. Max Pistilli receives support from a grant from the National Eye Institute (P30 EY001583).
Footnotes
Conflicts of Interest
For the remaining authors none were declared.
References
- 1.Miller AV. [Accessed on 27 Feb 2012];Sjogren’s syndrome. 2011 Oct 31; Available at: http://www.emedicine.com.
- 2.Hansen A, Lipsky PE, Dorner T. Immunopathogenesis of primary Sjogren’s syndrome: implications for disease management and therapy. Curr Opin Rheumatol. 2005;17:558–565. doi: 10.1097/01.bor.0000172801.56744.c3. [DOI] [PubMed] [Google Scholar]
- 3.Thanou-Stavraki A, James JA. Primary Sjögren’s syndrome: Current and prospective therapies. Semin Arthritis Rheum. 2008;37:273–292. doi: 10.1016/j.semarthrit.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucca JA, Nunez JN, Farris RL. A comparison of diagnostic tests for keratoconjunctivitis sicca: lactoplate, Schirmer, and tear osmolarity. CLAO J. 1990;16:109–12. [PubMed] [Google Scholar]
- 6.Cedarstaff TH, Tomlinson A. Human tear volume, quality, and evaporation: a comparison of Schirmer, tear break-up time, and resistance hygrometry techniques. Ophthalmic Physiol Opt. 1983;3:239–245. [PubMed] [Google Scholar]
- 7.Versura P, Frigato M, Cellini M, et al. Diagnostic performance of tear function tests in Sjogren’s syndrome patients. Eye. 2007;21:229–237. doi: 10.1038/sj.eye.6702204. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson A, Khanal S, Ramaesh K, et al. Tear Film Osmolarity: Determination of a Referent for Dry Eye Diagnosis. Invest Ophthalmol Vis Sci. 2006;47:4309–4315. doi: 10.1167/iovs.05-1504. [DOI] [PubMed] [Google Scholar]
- 9.Khanal S, Tomlinson A, McFadyen A, et al. Dry eye diagnosis. Invest Ophthalmol Vis Sci. 2008;49(4):1407–1414. doi: 10.1167/iovs.07-0635. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan BD, Whitmer D, Nichols KK, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51:6125–6130. doi: 10.1167/iovs.10-5390. [DOI] [PubMed] [Google Scholar]
- 11.Mishima S, Kubota Z, Farris RL. The tear flow dynamics in normal and in keratoconjunctivitis sicca cases. In: Solanes MP, editor. Ophthalmology; Proceedings of the XXI International Congress; Mexico, DF. 8–14 March, 1970; Amsterdam: Excerpta Medica; 1971. pp. 1801–1805. [Google Scholar]
- 12.Markusse HM, Huysen JC, Nieuwenhuys EJ, et al. Beta 2-microglobulin in tear fluid from patients with primary Sjogren’s syndrome. Ann Rheum Dis. 1992;51:503–505. doi: 10.1136/ard.51.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markusse HM, van Haeringen NJ, Swaak AJ, et al. Tear fluid analysis in primary Sjogren’s syndrome. Clin Exp Rheumatol. 1993;11:175–178. [PubMed] [Google Scholar]
- 14.Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19(3):201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 15.Tishler M, Yaron I, Geyer O, et al. Elevated Tear Interleukin-6 Levels in Patients with Sjogren Syndrome. Ophthalmology. 1998;105:2327–2329. doi: 10.1016/S0161-6420(98)91236-2. [DOI] [PubMed] [Google Scholar]
- 16.Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010;35:553–564. doi: 10.3109/02713683.2010.484557. [DOI] [PubMed] [Google Scholar]
- 17.Schirmer O. Studien zur Phisiology und Pathologie der Tranenabsonderung und Tranenab fuhr. Graefes Arch Clin Exp Ophthalmol. 1903;56:197–291. [Google Scholar]
- 18.Gilbard JP, Farris RL, Santamaria J., 2nd Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96(4):677–681. doi: 10.1001/archopht.1978.03910050373015. [DOI] [PubMed] [Google Scholar]
- 19.Lemp MA, Bron AJ, Baudouin C, et al. Tear Osmolarity in the Diagnosis and Management of Dry Eye Disease. Am J Ophthalmol. 2011;151:792–798. doi: 10.1016/j.ajo.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Utine CA, Bicakcigil M, Yavuz S, et al. Tear osmolarity measurements in dry eye related to primary Sjögrens Syndrome. Curr Eye Res. 2011;36:683–690. doi: 10.3109/02713683.2011.571357. [DOI] [PubMed] [Google Scholar]
- 21.Khanal S, Millar TJ. Barriers to clinical uptake of tear osmolarity measurements. Br J Ophthalmol. 2012;96:341–344. doi: 10.1136/bjo.2011.202754. [DOI] [PubMed] [Google Scholar]
- 22.Xu KP, Yagi Y, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea. 1996;15:235–239. doi: 10.1097/00003226-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Bourcier T, Acosta MC, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46:2341–2345. doi: 10.1167/iovs.04-1426. [DOI] [PubMed] [Google Scholar]
- 24.Adatia FA, Michaeli-Cohen A, Naor J, et al. Correlation between corneal sensitivity, subjective dry eye symptoms and corneal staining in Sjögren’s syndrome. Can J Ophthalmol. 2004;39:767–771. doi: 10.1016/s0008-4182(04)80071-1. [DOI] [PubMed] [Google Scholar]
- 25.Tuisku IS, Konttinen YT, Konttinen LM, et al. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren’s syndrome. Exp Eye Res. 2008;86:879–885. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Vitale S, Goodman LA, Reed GF, et al. Comparison of the NEI-VFQ and OSDI questionnaires in patients with Sjögren’s syndrome-related dry eye. Health Qual Life Outcomes. 2004;2:44. doi: 10.1186/1477-7525-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]