Abstract
Background
There have been no studies describing post-intensive care unit (ICU) alcohol use among medical-surgical ICU survivors.
Objective
To examine alcohol use and identify potentially modifiable risk factors, such as in-hospital probable acute stress disorder, for increased alcohol use following medical-surgical ICU admission.
Method
This longitudinal investigation included 150 medical-surgical ICU survivors. In-hospital interviews obtained baseline characteristics including pre-ICU alcohol use with the Alcohol Use Disorders Identification Test (AUDIT) and in-hospital probable acute stress disorder with the Posttraumatic Stress Disorder Checklist-civilian version. Clinical factors were obtained from medical records. Post-ICU alcohol use was ascertained via telephone interviews at 3 and 12 months post-discharge using the AUDIT. Mixed-model linear regression was used to examine potential risk factors for increased post-ICU alcohol use.
Results
There was a significant decline in the mean AUDIT score from baseline (3.9, 95%Confidence Interval [95%CI]: 2.9, 5.0) to 3 months post-ICU (1.5, 95%CI: 1.0, 2.1) (P < 0.001 by one-way analysis of variance [ANOVA]), with a significant increase between 3 and 12 months post-ICU (2.7, 95%CI: 1.8, 3.5) (P < 0.001 by one-way ANOVA). After adjusting for patient and clinical factors, in-hospital probable acute stress disorder (beta: 3.0, 95%CI: 0.9, 5.0) and pre-ICU unhealthy alcohol use (beta: 5.4, 95%CI: 3.4, 7.4) were independently associated with increased post-ICU alcohol use.
Conclusions
Alcohol use decreases in the early aftermath of medical-surgical ICU admission and then increases significantly by one year post-ICU. Interventions for unhealthy alcohol use among medical-surgical ICU survivors that take into account comorbid psychiatric symptoms are needed.
Keywords: critical care, alcohol use disorders, acute stress disorder, outcome assessment (health care)
INTRODUCTION
Alcohol use disorders, which encompass a spectrum from unhealthy alcohol use to alcohol abuse to frank dependence, are an important cause of morbidity, mortality and increased healthcare costs worldwide.1 In some studies nearly 40% of patients hospitalized for medical and surgical illnesses have alcohol use disorders,1 and a similar percentage of critically ill patients may have alcohol use disorders.2 Alcohol use disorders have been established as independent risk factors for the development of critical illnesses such as acute respiratory distress syndrome and sepsis,3–5 and have been associated with increased risk of mortality in the context of these illnesses.6
In an effort to minimize problem drinking among patients in hospital settings, systematic screening and brief interventions (SBI) for unhealthy alcohol use have been studied in traumatic injury survivors,7 general medical inpatients8,9 and emergency rooms.10 Across studies, the most consistently positive findings in support of SBI for decreasing unhealthy alcohol use have been in hospitalized trauma populations.11 In part, the success of these interventions in this population may be attributable to identifying the first few months post-injury as a time period where patients may have motivation to change their behavior. Observational and intervention studies have found that alcohol use decreased in the first few months post-injury but then tended to return to pre-injury drinking amounts by one year later.12–14 Yet, although the ICU may represent a “teachable moment” to affect change in drinking behaviors,15,16 there have been no studies to date describing post-ICU patterns of alcohol use among medical-surgical ICU survivors.
Prior studies have also found that alcohol use may increase in the aftermath of exposure to traumatic stressors,17,18 particularly among patients with psychiatric symptoms following the stressor.17 Critical illnesses expose patients to profound stressors such as respiratory insufficiency, pain, and delirium,19 and are by definition life-threatening experiences. To that end, three systematic reviews of 24 cohort studies of general ICU and acute respiratory distress syndrome survivors have found that 22% and 28% of patients surviving critical illnesses may have substantial posttraumatic stress disorder (PTSD) and depressive symptoms, respectively.19–21 However, no studies have examined if psychiatric morbidity in the early aftermath of medical-surgical ICU admission, such as acute stress disorder, is associated with increased alcohol use. Importantly, comorbid PTSD can complicate the treatment of alcohol use disorders,22 and interventions for comorbid PTSD and substance use disorders that decrease PTSD symptoms may also decrease substance use.23 Therefore, early post-ICU psychiatric symptoms could represent potentially modifiable risk factors for unhealthy alcohol use.
The present study is a longitudinal investigation of alcohol use, as well as potential risk factors for increased alcohol use, over the course of the year following medical-surgical ICU admission. In addition to quantifying the prevalence of post-ICU unhealthy alcohol use, we hypothesized that the development of probable acute stress disorder would be independently associated with increased alcohol use over the course of the year post-ICU even after adjusting for patient characteristics such as unhealthy alcohol use during the year pre-ICU as well as clinical factors of the ICU admission.
METHODS
Study Setting, Participants, and Procedures
The current investigation took place at Harborview Medical Center (HMC) in Seattle, WA. HMC is an urban, public medical center operated by the University of Washington (UW). Between September 2010 and August 2011, we prospectively recruited 150 patients admitted to any of the ICUs at HMC. Key exclusion criteria were: 1) admission diagnosis of traumatic injury; 2) pre-existing cognitive impairment or dementia diagnosis noted in the medical record; 3) communication/language barrier; 4) ICU length of stay (LOS) ≤ 24 hours; 5) pre-existing medical illness with life-expectancy of < 12 months; and 6) admission for a suicide attempt. The study protocol was approved by the UW Institutional Review Board (IRB), and all participants provided informed consent prior to enrollment.
Study research assistants identified potentially eligible subjects through review of HMC’s electronic admission records. Eligible subjects were approached for study consent prior to transfer from the ICU to the general medical-surgical ward. Since the UW IRB did not allow surrogate consent for study participation, if the patient was unable to provide consent due to ongoing delirium and/or cognitive impairment, consent was not obtained, and they were re-approached once their delirium and/or cognitive impairment had resolved. Cognitive impairment was assessed using a cutoff score of ≥ 3 errors on the Six-Item Cognitive Screen.24 The area under the receiver operating characteristic curve for the Six-Item Cognitive Screen using cognitive impairment as the gold standard was 0.86 for a community-based sample compared to the Community Screening Instrument for Dementia and 0.91 for a clinical sample compared to a battery including the Mini-Mental State Examination and the Consortium for Establishment of Registry for Alzheimer Disease battery.24
Enrolled patients were administered an in-person interview prior to hospital discharge and were re-interviewed via telephone at 3 and 12 months post-ICU.
Measurements and Assessments
Probable Acute Stress Disorder
Our independent variable of interest was the presence of in-hospital probable acute stress disorder. We assessed in-hospital probable acute stress disorder with the PTSD Checklist-civilian version (PCL-C).25 The PCL-C includes questions regarding 5 symptoms in the intrusive symptom cluster (e.g., intrusive thoughts, nightmares), 7 symptoms in the avoidant symptom cluster (e.g., avoidance of thoughts or activities that remind the patient of the stressor, emotional numbing), and 5 symptoms in the arousal symptom cluster (e.g., impaired sleep, hypervigilance) and symptom severity is rated on a 5 point Likert scale.25 Probable acute stress disorder can be ascertained with the PCL-C by following an algorithm that considers a score of 3 or more on at least 1 intrusive symptom, 3 avoidant symptoms, and 2 arousal symptoms as consistent with DSM diagnostic criteria.25
Unhealthy Alcohol Use
Our outcome of interest was the degree of alcohol use over the course of the year post-ICU. We assessed alcohol use during the year pre-ICU and 3 and 12-months post-ICU with the Alcohol Use Disorders Identification Test (AUDIT).26 The AUDIT is comprised of 10 questions on amount of alcohol use as well as alcohol abuse-related behaviors. Using an AUDIT cutoff score of ≥ 8 to define unhealthy alcohol use has been found to have sensitivities ranging from 50% to 90% and a specificity of approximately 80%.27
Patient and ICU-related Characteristics
Baseline patient characteristics and ICU clinical factors were obtained through medical record review and in-person interview with the patient. Medical record-obtained characteristics included demographics (i.e., age, sex, race); ICU admission diagnosis; baseline medical comorbidity information to compute a Charlson Comorbidity Score;28 illness severity measures at ICU admission to compute a Simplified Acute Physiology Score II (SAPS II);29 ICU LOS; requirements for mechanical ventilation and duration of ventilation; requirement for major surgery and number of surgeries; number of blood product transfusions; days of exposure (both in-ICU and on medical-surgical ward) to benzodiazepine, opioid, antipsychotic, and antidepressant medications; requirements for physical restraint and days restrained; presence of delirium in-ICU per nursing documented assessment using the Confusion Assessment Method-ICU (CAM-ICU);30 and presence of confusion/disorientation/difficulty following commands in nursing documentation. We defined probable delirium as a documented positive CAM-ICU assessment in the ICU or nursing documented presence of confusion/disorientation/difficulty following commands at any point during the hospitalization.
Additional patient characteristics obtained from the baseline interviews included demographics not obtained from medical records (i.e., marital/partner status, education); assessment of prior trauma exposure with the National Comorbidity Survey-Replication Trauma History Screen;31 lifetime history of major depression with the Mini International Neuropsychiatric Interview (MINI) major depressive episode module;32 and drug use in the prior year with the Drug Abuse Screening Test-10 (DAST-10), with baseline problem drug use defined as a DAST-10 score ≥ 3.33 Post-ICU cognition was assessed at 3 and 12 month follow-up with the modified Telephone Interview for Cognitive Status (TICSm).34
Statistical Analysis
We present descriptive data as means and standard deviations (SDs), medians and interquartile ranges (IQRs) or proportions. To examine if there were significant differences between patients who did and did not develop probable acute stress disorder during the course of hospitalization, we used one-way analysis of variance (ANOVA) or Mann-Whitney U tests for continuous baseline and clinical variables and χ2-tests or Fisher’s Exact tests for categorical variables. We also examined if there were significant differences between patients who completed 12-month follow-up and those that did not using one-way ANOVA for continuous baseline and clinical variables and χ2-tests or Fisher’s Exact tests for categorical variables.
We used one-way ANOVA to examine the mean changes in alcohol use from baseline to 3 months post-ICU and 3 to 12 months post-ICU, respectively.
We used mixed-model linear regression analyses in order to examine potential risk factors for increased alcohol use over the course of the year post-ICU. The dependent variable was the repeated measures of alcohol use at 3 and 12 months post-ICU. Initially, we tested the associations of in-hospital probable acute stress disorder with post-ICU alcohol use without adjustment. We then added two groups of potential confounding variables to the regression models all chosen a priori because they have been found to be important in alcohol and critical illness-related research:12–14,16,19–21,35 1) demographics and baseline clinical characteristics (age, sex, race, education, marital/partnered status, lifetime history of major depression, number of prior traumatic event exposures, presence of unhealthy alcohol use during the year pre-ICU, problem drug use during the year pre-ICU and Charlson score); and 2) clinical characteristics of the ICU admission (SAPS II score, duration of mechanical ventilation, number of surgeries, number of blood product transfusions, days physically restrained, presence of probable delirium during the hospitalization, days of exposure to benzodiazepines, opioids, antipsychotics, and antidepressants) and post-ICU TICSm scores. For continuous clinical variables that were non-normally distributed (i.e., duration of mechanical ventilation, number of surgeries, number of blood product transfusions, days physically restrained, days of exposure to benzodiazepines, opioids, antipsychotics, and antidepressants), we transformed them into quartiles for inclusion in the final regression model. We implemented our regression analyses using xtmixed in STATA 11.2 (Stata Corporation, College Station, TX).
We used two-sided significance tests for all analyses with statistical significance set at a P value of 0.05. Analyses were performed with appropriate components of the IBM SPSS Statistics 18 (SPSS Inc., Chicago, IL) and STATA 11.2 statistical software programs.
RESULTS
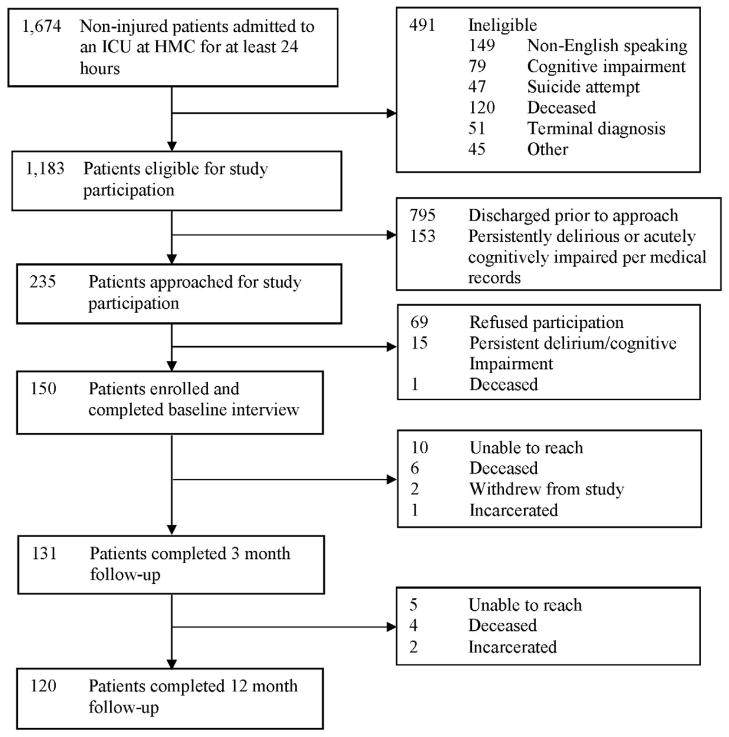
Of the 150 patients enrolled in the study, 120 (80%) completed a 12-month telephone follow-up interview (Figure 1). Table 1 presents the baseline and clinical characteristics of patients who did and did not develop probable acute stress disorder during the course of hospitalization. Patients who did not complete follow-up were more likely to develop probable acute stress disorder during their hospitalization (χ2 = 6.7, P = 0.01). Baseline interviews were completed at a mean of 11.1 days after hospital admission (SD: 9.5, median 8.0, IQR: 5.0, 15.0). Patients’ mean age at hospitalization was 48.2 years (SD: 13.7). Their median ICU length of stay was 5.0 days (IQR: 3.0, 9.0), and their median SAPS II score on admission was 23.0 (IQR: 13.0, 37.0). Nearly half required mechanical ventilation during their ICU admission with a median duration of 2.0 days (IQR: 1.0, 4.0). The prevalence of probable delirium during the course of hospitalization was 51% and nearly half of patients were physically restrained. At baseline, 15% developed probable acute stress disorder and 13% exhibited unhealthy alcohol use during the year pre-ICU.
Figure 1. Study Flow Diagram.
Abbreviations (in alphabetical order): HMC = Harborview Medical Center; ICU = intensive care unit.
Table 1.
Baseline patient characteristics and intensive care unit factors by probable acute stress disorder status
| Variables | Probable acute stress disorder (n = 23) | Without probable acute stress disorder (n = 127) | P Valuea |
|---|---|---|---|
|
Patient Baseline Characteristics
| |||
| Age | 47.1 (11.9) | 48.5 (14.1) | 0.66 |
| Female | 8 (34.8%) | 53 (41.7%) | 0.51 |
| Non-white | 10 (43.5%) | 30 (23.6%) | 0.06 |
| < High school graduate | 4 (17.4%) | 17 (13.4%) | 0.62 |
| Married/partnered | 9 (39.1%) | 66 (52.0%) | 0.24 |
| Prior history of major depression per interview | 11 (47.8%) | 32 (25.2%) | 0.03 |
| Lifetime number of traumatic event exposures | 5.7 (2.9) | 3.9 (2.7) | 0.006 |
| Unhealthy alcohol use (AUDIT ≥ 8) in the year pre-ICU | 7 (30.4%) | 13 (10.2%) | 0.009 |
| Problem drug use (DAST-10 ≥ 3) in the year pre-ICU | 6 (26.1%) | 13 (10.2%) | 0.04 |
| Charlson Comorbidity score | 1.7 (2.2) | 1.7 (2.0) | 0.95 |
|
| |||
|
Admission Clinical Characteristics
| |||
| ICU LOS (days) | 4.0, (3.0, 7.0) | 5.0 (3.0, 9.0) | 0.86 |
| SAPS II | 31.7 (16.7) | 25.4 (15.0) | 0.07 |
| Admission diagnostic category | |||
| Cardiovascular | 1 (4.3%) | 10 (7.9%) | 1.00 |
| Pulmonary | 3 (13.0%) | 16 (12.6%) | 1.00 |
| Infectious Disease | 8 (34.8%) | 34 (26.8%) | 0.45 |
| Neurologic | 5 (21.7%) | 36 (28.3%) | 0.50 |
| Vascular Surgery | 3 (13.0%) | 19 (15.0%) | 1.00 |
| Gastrointestinal | 3 (13.0%) | 15 (11.8%) | 1.00 |
| Endocrine/Renal | 2 (8.7%) | 5 (3.9%) | 0.30 |
| Orthopedic | 1 (4.3%) | 10 (7.9%) | 1.00 |
| Oncologic | 1 (4.3%) | 4 (3.1%) | 0.57 |
| Other | 1 (4.3%) | 4 (3.1%) | 0.57 |
| Mechanically ventilated | 12 (52.2%) | 56 (44.1%) | 0.49 |
| MV duration among ventilated (days) | 1.5 (1.0, 3.8) | 2.0 (1.0, 4.0) | 0.67 |
| Number of surgeries among patients requiring surgery | 2.0 (1.0, 3.0) | 1.0 (1.0, 2.0) | 0.32 |
| Number of blood product transfusions among transfused | 2.0 (2.0, 3.0) | 2.0 (1.0, 2.8) | 0.47 |
| Probable delirium | 14 (60.9%) | 62 (48.8%) | 0.30 |
| Restrained in hospital | 13 (56.5%) | 59 (46.5%) | 0.39 |
| Days restrained in hospital among patients requiring restraints | 4.0 (1.5, 9.0) | 3.0 (1.0, 5.3) | 0.54 |
| Total days received benzodiazepines | 3.0 (1.0, 10.0) | 3.0 (2.0, 7.0) | 0.69 |
| Total days received opioids | 17.0 (9.0, 32.0) | 9.0 (5.0, 17.0) | 0.001 |
| Total days received antipsychotics | 13.5 (6.8, 24.0) | 3.5 (1.0, 7.8) | 0.03 |
| Total days received antidepressants | 12.0 (7.3, 28.5) | 8.5 (3.0, 15.3) | 0.15 |
|
| |||
| Post-ICU 3 TICSm Scores | |||
| 3 Months Post-ICUb | 22.4 (3.9) | 23.8 (4.3) | 0.22 |
| 12 Months Post-ICUc | 22.2 (4.8) | 24.2 (4.6) | 0.14 |
All values are mean ± SD, median (IQR) or n (%) unless otherwise indicated.
Abbreviations (in alphabetic order): AUDIT = Alcohol Use Disorders Identification Test; DAST-10 = Drug Abuse Screening Test=10; ICU = intensive care unit; LOS = length of stay; MV = mechanical ventilation; SAPS II = Simplified Acute Physiology Score II; TICSm = Modified Telephone Interview for Cognitive Status.
Results from Pearson χ2 test with 1 degree of freedom, Fisher’s Exact test, Mann-Whitney U test, or F-statistic with 148 degrees of freedom.
131 subjects (18 with probable in-hospital acute stress disorder and 113 without) completed 3 month follow-up.
120 subjects (14 with probable in-hospital acute stress disorder and 106 without) completed 12 month follow-up.
Alcohol Use Following Medical-Surgical ICU Admission
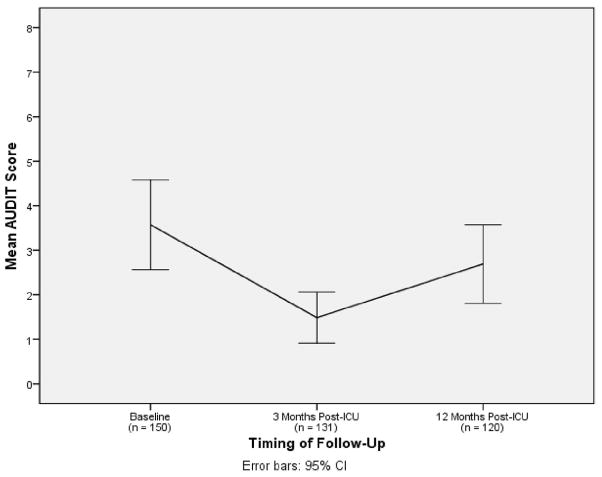
Figure 2 illustrates the change in mean AUDIT scores from baseline to 3 months (mean: 88.8 days, SD: 28.0) and 12 months (mean: 356.4 days, SD: 29.2) post-ICU. There was a significant decline in the mean AUDIT score from baseline to 3 months post-ICU (P < 0.001 by one-way ANOVA, F = 13.6), with a significant increase between 3 and 12 months post-ICU (P < 0.001 by one-way ANOVA, F = 33.7), although not returning to baseline levels. Patients with in-hospital probable acute stress disorder had significantly higher mean AUDIT scores at baseline (8.3 [SD: 12.3] vs. 3.2 [SD 4.4], P < 0.001, F = 13.3) as well as at 3-month (3.8 [SD: 7.2] vs. 1.2 [SD: 1.8], P = 0.001, F = 10.8) and 12-month follow-up (5.6 [SD: 9.0] vs. 2.3 [SD: 3.8], P = 0.01, F = 6.2) than those without substantial acute stress symptoms.
Figure 2. Alcohol use over the course of the year following medical-surgical intensive care unit admission.
Abbreviations (in alphabetical order): AUDIT = Alcohol Use Disorders Identification Test; CI = confidence interval; ICU = intensive care unit.
Interpretation: The mean AUDIT score decreased from 3.9 (95%CI: 2.9, 5.0) at baseline to 1.5 (95%CI: 1.0, 2.1) at 3 months post-ICU, and then increased to 2.7 (95%CI: 1.8, 3.5) at 12 months post-ICU.
The prevalence of unhealthy alcohol use at 3 months post-ICU was 3.8% (95% Confidence Interval [95%CI]: 1.0%, 7.1%), and increased to 7.5% by 9 months later (95%CI: 2.7%, 12.3%). Eighty percent of patients with unhealthy alcohol use at 3 months post-ICU were drinking excessively during the year prior to the ICU admission, while two-thirds of patients with unhealthy alcohol use 12 months post-ICU were exhibiting unhealthy alcohol use pre-ICU.
Patient and Clinical Characteristics and Post-ICU Alcohol Use
In unadjusted analyses, probable acute stress disorder during the hospitalization was associated with a 2.9 point higher AUDIT score (95%CI: 1.1, 4.7; P = 0.001) over the course of the year following the ICU admission. After adjusting for baseline patient characteristics, probable acute stress disorder was associated with a 2.1 point higher AUDIT score (95%CI: 0.4, 3.8; P = 0.02) (Table 2). In addition, unhealthy alcohol use during the year pre-ICU was associated with increased post-ICU alcohol use (beta: 5.5, 95%CI: 3.8, 7.3; P < 0.001).
Table 2.
Unadjusted and fully adjusted associations of patient and clinical characteristics with alcohol use following medical-surgical intensive care unit admissiona
| Adjusted for Patient Characteristics | Adjusted for Hospitalization Characteristics and Post-ICU Cognition | |
|---|---|---|
|
| ||
| β Coefficient (95% Confidence Interval) | ||
| In-hospital probable acute stress disorder | 2.1 (0.4, 3.8)* | 3.0 (1.0, 5.1)† |
| Baseline unhealthy alcohol use | 5.5 (3.8, 7.3)‡ | 5.5 (3.6, 7.5)‡ |
| Age | 0.002 (−0.04, 0.1) | 0.01 (−0.04, 0.1) |
| Male | −0.1 (−1.2, 1.0) | 0.2 (−1.0, 1.4) |
| Non-white | −0.7 (−2.0, 0.6) | −0.8 (−2.2, 0.7) |
| Education less than high school | 0.8 (−0.9, 2.4) | 0.9 (−1.1, 2.8) |
| Married | −1.0 (−2.1, 0.1) | −1.1 (−2.4, 0.1) |
| Illicit drug problem at baseline | −0.9 (−2.7, 0.9) | −0.9 (−3.1, 1.3) |
| Lifetime history of major depression | −0.04 (−1.3, 1.2) | 0.2 (−1.2, 1.6) |
| Prior traumatic event exposure | −0.02 (−0.2, 0.2) | −0.1 (−0.3, 0.1) |
| Charlson Comorbidity Score | −0.1 (−0.3, 0.2) | −0.1 (−0.4, 0.3) |
| SAPS II | −0.02 (−0.1, 0.04) | |
| MV duration | ||
| 1 – 2 days | −1.0 (−3.3, 1.4) | |
| 2 – 4 days | −1.3 (−4.5, 1.8) | |
| > 4 days | −0.9 (−4.2, 2.4) | |
| Number of major surgeries | ||
| 1 | −1.2 (−2.7, 0.3) | |
| 2 | −1.2 (−3.6, 1.1) | |
| > 2 | −2.6 (−5.1, −0.1)* | |
| Number of blood product transfusions | ||
| 1 – 2 | 0.6 (−1.2, 2.3) | |
| 2 – 3 | −1.5 (−7.5, 4.5) | |
| > 3 | −0.2 (−4.0, 3.5) | |
| Probable delirium | −0.3 (−2.0, 1.3) | |
| Days physically restrained | ||
| 1 – 3 days | 2.9 (0.8, 5.0)† | |
| 3 – 6 days | 2.7 (−0.4, 5.9) | |
| > 6 days | 1.0 (−2.4, 4.5) | |
| Days of benzodiazepines | ||
| 1 – 3 days | −0.6 (−2.2, 0.9) | |
| 3 – 7.3 days | −2.0 (−4.3, 0.2) | |
| > 7.3 days | 1.2 (−1.3, 3.6) | |
| Days of opioids | ||
| 5 – 10 days | 1.7 (−0.1, 3.4) | |
| 10 – 19 days | 0.6 (−1.5, 2.6) | |
| > 19 days | 1.5 (−1.3, 4.3) | |
| Days of antipsychotics | ||
| 1.3 – 4.5 days | 0.6 (−2.2, 3.4) | |
| 4.5 – 9 days | −2.1 (−5.1, 1.0) | |
| > 9 days | −0.4 (−3.5, 2.6) | |
| Days of antidepressants | ||
| 3.8 – 9 days | −1.3 (−3.8, 1.2) | |
| 9 –16 days | 0.03 (−2.4, 2.5) | |
| > 16 days | −1.9 (−5.0, 1.3) | |
| Post-ICU TICSm scores | 0.1 (−0.04, 0.2) | |
Abbreviations (in alphabetic order): MV = mechanical ventilation; ICU = intensive care unit; SAPS II = Simplified Acute Physiology Score II; TICSm = Modified Telephone Interview for Cognitive Status.
The dependent variable of the mixed-model regression analyses above are the repeated measures of alcohol use at 3 and 12 months following medical-surgical ICU admission. The β coefficients can be interpreted as mean change in AUDIT scores.
P < 0.05
P < 0.01
P < 0.001
In the final model that adjusted for both patient and in-hospital clinical characteristics of the ICU admission as well as post-ICU cognition, in-hospital probable acute stress disorder remained independently associated with higher levels of alcohol use over the course of the year post-ICU (beta: 3.0, 95%CI: 1.0, 5.1; P = 0.004). Unhealthy alcohol use during the year pre-ICU (beta: 5.5, 95%CI: 3.6, 7.5; P < 0.001) was the only additional factor consistently associated with higher levels of post-ICU alcohol use.
DISCUSSION
To our knowledge, the present study is the first longitudinal investigation of alcohol use over the course of the year following medical-surgical ICU admission. Alcohol use among medical-surgical ICU survivors decreased in the first 3 months after hospitalization and then increased significantly by one year after hospitalization, a pattern of drinking similar to that found in traumatic injury survivors,12–14 a patient population in which SBI has been found to be effective.11 Prior work has identified that the severity of acute illness at the time of ICU admission may be associated with greater readiness to change drinking behavior in critically ill patients with unhealthy alcohol use.16 These findings along with the decline in drinking during the early aftermath of a medical-surgical ICU admission found in our study suggest that surviving a critical medical illness may indeed represent a “teachable moment” for some patients that could warrant screening and intervention. Although the evidence for the effectiveness of SBI for unhealthy alcohol use in general medical inpatients has been mixed,11,36–39 these interventions have not been studied in medical-surgical ICU patients,15 and our results argue that further studies in this population are warranted.
Importantly, we found that in-hospital probable acute stress disorder was associated with increased alcohol use over the course of the year post-ICU even after adjusting for unhealthy alcohol use during the year before ICU admission. Since in-hospital probable acute stress disorder was associated with loss to follow-up at 12 months, the association we found here is likely an underestimate. Our findings suggest that high levels of patient distress prior to hospital discharge may be a potential risk factor for increased alcohol use in addition to prior studies identifying their role as a risk factor for mental disorders such as PTSD.40 Acute stress disorder in patients surviving admission to medical-surgical ICUs may be amenable to treatment if distressed patients are identified and receive evidence-based treatments early, potentially preventing longer-term adverse outcomes.41 Studies that test novel interventions for unhealthy alcohol use and acute stress disorder in patients admitted to medical-surgical ICUs with the goal of preventing the development of PTSD as well as reducing alcohol consumption are needed.
We identified that patients in our cohort who reported a pattern of unhealthy alcohol use during the year prior to ICU admission and/or who had a lifetime history of major depression were more likely to develop probable acute stress disorder during the hospitalization. This finding has important clinical implications because unrecognized comorbid psychiatric symptoms may impede the effectiveness of SBI for unhealthy alcohol use.42 In addition to amount of pre-ICU alcohol use and severity of the acute illness,16 psychiatric comorbidity should also be examined as a moderator of treatment response in studies of SBI in patients hospitalized for critical medical illnesses.
Our study has several limitations. Since we were unable to obtain surrogate consent from patients who were persistently delirious and therefore could not include them, our study may be subject to selection bias, necessitating caution when interpreting our findings. Information on pre-ICU substance use, prior traumatic event exposure, and lifetime major depression history may be subject to recall bias since this data was obtained from patients while they were still in the hospital, and is therefore retrospective in nature. Although it is possible that our assessment of in-hospital probable acute stress disorder could be confounded by residual hyperactive delirium, this prospect is minimized by patients having to pass a validated cognitive screen prior to providing consent for study participation, which likely would exclude the majority of persistently delirious patients. Further, while we cannot exclude the possibility that our assessment of in-hospital probable acute stress disorder was confounded by alcohol withdrawal symptoms, the majority of patients in our cohort were assessed for in-hospital acute stress disorder beyond the window typical for alcohol withdrawal.
Since we assessed in-hospital probable acute stress disorder as well as alcohol use pre- and post-ICU with questionnaires, we could not make clinical diagnoses of acute stress disorder or alcohol abuse or dependence. Also, although it is possible that alcohol use over the year following a medical-surgical ICU admission may not change in a linear fashion, our study lacked sufficient statistical power to model post-ICU alcohol use differently. We did not examine specific post-ICU physical impairments in our study, and therefore could not ascertain their potential correlation with post-ICU alcohol use. Further, our study was conducted in a single center serving a safety net population and our sample size was relatively small. Additionally, we lack data on the representativeness of our sample relative to the entire population of critically ill patients admitted to medical-surgical ICUs at HMC. Therefore our results may not be generalizable to the entire population of medical-surgical ICU survivors. Finally, the possibility of residual confounding remains as in any observational study.
In conclusion, in the first investigation of alcohol use following medical-surgical ICU admission, we found that alcohol use decreased in the early aftermath post-ICU and then increased significantly by one year post-ICU. In addition to unhealthy alcohol use during the year prior to ICU admission, we identified that a potentially modifiable factor, in-hospital probable acute stress disorder, was independently associated with increased alcohol use over the course of the year post-ICU. Further study of SBI in patients admitted to medical-surgical ICUs as well as into interventions targeting both unhealthy alcohol use and psychiatric comorbidity is crucially important in light of the enormous toll that alcohol use disorders, mental disorders such as PTSD, and critical illnesses take on patients, their families and society.
Acknowledgments
Funding: This work was supported by grants KL2 TR000421, NRSA-T32/MH20021-12, R03 AA020146-02, R01 AA01602 and K24 MH086814-03 from the National Institutes of Health and grant ADAI-1009-2 from the University of Washington Alcohol and Drug Abuse Institute.
The authors thank Collin McFadden, B.A., and Jeffrey Love, B.A., for assistance with patient recruitment and data collection, and Jin Wang, Ph.D., for assistance with data cleaning.
Footnotes
All authors have had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: Drs. Davydow, Zatzick, and Hough have no relevant conflicts of interest to disclose. Dr. Katon discloses that he has received honoraria in the last 12 months for CME lectures funded indirectly by Lilly, Forest and Pfizer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol use disorders in the critically ill patient. Chest. 2010;138:994–1003. doi: 10.1378/chest.09-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marik P, Mohedin B. Alcohol-related admissions to an inner city hospital intensive care unit. Alcohol Alcohol. 1996;31:393–396. doi: 10.1093/oxfordjournals.alcalc.a008168. [DOI] [PubMed] [Google Scholar]
- 3.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- 4.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 5.Moss M. Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clin Infect Dis. 2005;15(Suppl 7):S490–497. doi: 10.1086/432003. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien JM, Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35:345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- 7.Gentilello LM, Rivara FP, Donovan DM, Jurkovich GJ, Daranciang E, Dunn CW, et al. Alcohol interventions in a trauma center as a means of as a means of reducing the risk of injury recurrence. Ann Surg. 1999;230:473–483. doi: 10.1097/00000658-199910000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McManus S, Hipkins J, Haddad P, Guthrie E, Creed F. Implementing an effective intervention for problem drinkers on medical wards. Gen Hosp Psychiatry. 2003;25:332–337. doi: 10.1016/s0163-8343(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 9.Saitz R, Palfai TP, Cheng DM, Horton NJ, Dukes K, Kraemer KL, et al. Some medical inpatients with unhealthy alcohol use may benefit from brief intervention. J Stud Alcohol Drugs. 2009;70:426–435. doi: 10.15288/jsad.2009.70.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Academic ED SBIRT Research Collaborative. The impact of screening, brief intervention, and referral for treatment on emergency department patients’ alcohol use. Ann Emerg Med. 2007;50:699–710. doi: 10.1016/j.annemergmed.2007.06.486. [DOI] [PubMed] [Google Scholar]
- 11.McQueen J, Howe TE, Allan L, Mains D. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2009;3:CD005191. doi: 10.1002/14651858.CD005191.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Dunn C, Zatzick D, Russo J, Rivara F, Roy-Byrne P, Ries R, et al. Hazardous drinking in trauma patients during the year after injury. J Trauma. 2003;54:707–712. doi: 10.1097/01.TA.0000030625.63338.B2. [DOI] [PubMed] [Google Scholar]
- 13.Dunn C, Rivara FP, Donovan D, Fan MY, Russo J, Jurkovich G, et al. Predicting adolescent alcohol drinking patterns after major injury. J Trauma. 2008;65:736–740. doi: 10.1097/TA.0b013e31817de40f. [DOI] [PubMed] [Google Scholar]
- 14.Zatzick D, Roy-Byrne P, Russo J, Rivara F, Droesch R, Wagner A, et al. A randomized effectiveness trial of stepped collaborative care for acutely injured trauma survivors. Arch Gen Psychiatry. 2004;61:498–506. doi: 10.1001/archpsyc.61.5.498. [DOI] [PubMed] [Google Scholar]
- 15.Clark BJ, Moss M. Secondary prevention in the intensive care unit: does intensive care unit admission represent a “teachable moment? Crit Care Med. 2011;39:1500–1506. doi: 10.1097/CCM.0b013e31821858bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark BJ, Smart A, House R, Douglas I, Burnham EL, Moss M. Severity of acute illness is associated with baseline readiness to change in medical intensive care unit patients with unhealthy alcohol use. Alcohol Clin Exp Res. 2012;36:544–551. doi: 10.1111/j.1530-0277.2011.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlahov D, Galea S, Resnick H, Ahern J, Boscarino JA, Bucuvalas M, et al. Increased use of cigarettes, alcohol and marijuana among Manhattan, New York, residents after the September 11 terrorist attacks. Am J Epidemiol. 2002;155:988–996. doi: 10.1093/aje/155.11.988. [DOI] [PubMed] [Google Scholar]
- 18.North CS, Ringwaldt CL, Downs D, Derzon J, Galvin D. Postdisaster course of alcohol use disorders in systematically studied survivors of 10 disasters. Arch Gen Psychiatry. 2011;168:173–180. doi: 10.1001/archgenpsychiatry.2010.131. [DOI] [PubMed] [Google Scholar]
- 19.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30:421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70:512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaysen D, Simpson T, Dillworth T, Larimer ME, Gutner C, Resick PA. Alcohol problems and posttraumatic stress disorder in female crime victims. J Trauma Stress. 2006;19:399–403. doi: 10.1002/jts.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hien DA, Jiang H, Campbell AN, Hu MC, Miele GM, Cohen LR, et al. Do treatment improvements in PTSD severity affect substance use outcomes? A secondary analysis from a randomized clinical trial in NIDA’s Clinical Trials Network. Am J Psychiatry. 2010;167:95–101. doi: 10.1176/appi.ajp.2009.09091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Weathers FW, Huska JA, Keane TM. The PTSD Checklist – civilian version. Boston, MA: TheNational Center for PTSD; Boston VA: Medical Center; 1991. [Google Scholar]
- 26.Babor TF, de la Fuente JR, Saunders J, Grant M. Guidelines for use in primary health care. Geneva, CH: World Health Organization; 1992. The Alcohol Use Disorders Identification Test. [Google Scholar]
- 27.Schuckit MA. Alcohol-use disorders. Lancet. 2009;373:492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American Multicenter Study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 30.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 31.Ramstad SM, Russo J, Zatzick DF. Is it an accident? Recurrent traumatic life events in level I trauma center patients compared to the general population. J Trauma Stress. 2004;17:529–534. doi: 10.1007/s10960-004-5802-z. [DOI] [PubMed] [Google Scholar]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:S22–S33. [PubMed] [Google Scholar]
- 33.Skinner HA. The Drug Abuse Screening Test. Addict Behav. 1982;7:363–367. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 34.Welsh KA, Breitner JC, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1993;6:103–110. [Google Scholar]
- 35.Davydow DS, Zatzick DF, Rivara FP, Jurkovich GJ, Roy-Byrne PP, Katon WJ, et al. Predictors of posttraumatic stress disorder and return to usual major activity in traumatically injured intensive care unit survivors. Gen Hosp Psychiatry. 2009;31:428–435. doi: 10.1016/j.genhosppsych.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saitz R, Palfai TP, Cheng TM, Horton NJ, Freedner N, Dukes K, et al. Brief intervention for medical inpatients with unhealthy alcohol use: a randomized, controlled trial. Ann Intern Med. 2007;146:167–176. doi: 10.7326/0003-4819-146-3-200702060-00005. [DOI] [PubMed] [Google Scholar]
- 37.Field CA, Baird J, Saitz R, Caetano R, Monti PM. The mixed evidence for brief intervention in emergency departments, trauma care centers, and inpatient hospital settings: What should we do? Alcohol Clin Exp Res. 2010;34:2004–2010. doi: 10.1111/j.1530-0277.2010.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saitz R. Candidate performance measures for screening for, assessing, and treating unhealthy substance use in hospitals: Advocacy or evidence-based practice? Ann Intern Med. 2010;153:40–43. doi: 10.7326/0003-4819-153-1-201007060-00008. [DOI] [PubMed] [Google Scholar]
- 39.Saitz R. Candidate performance measures for screening for, assessing, and treating unhealthy substance use in hospitals. Ann Intern Med. 2011;154:73–74. doi: 10.7326/0003-4819-154-1-201101040-00017. [DOI] [PubMed] [Google Scholar]
- 40.Zatzick DF, Kang SM, Müller HG, Russo JE, Rivara FP, Katon W, et al. Predicting posttraumatic distress in hospitalized trauma survivors with acute injuries. Am J Psychiatry. 2002;159:941–946. doi: 10.1176/appi.ajp.159.6.941. [DOI] [PubMed] [Google Scholar]
- 41.Shalev AY, Ankri Y, Israeli-Shalev Y, Peleg T, Adessky R, Freedman S. Prevention of posttraumatic stress disorder by early treatment: results from the Jerusalem Trauma Outreach and Prevention Study. Arch Gen Psychiatry. 2012;69:166–176. doi: 10.1001/archgenpsychiatry.2011.127. [DOI] [PubMed] [Google Scholar]
- 42.Zatzick D, Donovan D, Dunn C, Russo J, Wagner A, Wang J, et al. Substance use and posttraumatic stress disorder symptoms in trauma center patients receiving mandate alcohol screening and brief intervention. J Subst Abuse Treat. 2012;43:410–417. doi: 10.1016/j.jsat.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]