Abstract
Almost half of the 48 human ATP binding cassette (ABC) transporter proteins are thought to facilitate the ATP-dependent translocation of lipids or lipid-related compounds. Such substrates include cholesterol, plant sterols, bile acids, phospholipids and sphingolipids. Mutations in a substantial number of the 48 human ABC transporters have been linked to human disease. Indeed the finding that 12 diseases have been associated with abnormal lipid transport and/or homeostasis, demonstrates the importance of this family of transporters in cell physiology. This review highlights the role of ABC transporters in lipid transport and movement, in addition to discussing their roles in cellular homeostasis and inherited disorders.
Keywords: Lipid transport, intracellular, ABC transporter
The ABC’s of lipid transport
ATP binding cassette (ABC) transporters (see Glossary) are transmembrane proteins that utilize the hydrolysis of ATP to facilitate the movement of a wide variety of substrates across membrane bilayers. There are more than 250 members in the ABC superfamily that simplistically can be thought of as importers or exporters [1]. Importers are typically associated with the uptake of hydrophilic nutrients, such as peptides, ions, and sugars, into the cell, and are common in bacteria and absent from eukaryotes. Both eukaryotes and prokaryotes possess ABC transporters that function to export substrates out of the cytosol and either into an organelle, or across the plasma membrane and out of the cell.
Proteins are classified as ABC transporters based on the organization of their ATP binding cassette, a region that spans ~180 amino acids, and contains three highly conserved motifs: the Walker A/P-loop (12 amino acids), a Signature motif/C-loop (5 amino acids) and the Walker B motif (5 amino acids). Functional ABC transporters contain two transmembrane domains (TMD), each generally thought to consist of six transmembrane α-helices, and two ABCs [1]. ABC transporters can be further divided into either “full” or “half” transporters. In full transporters, the two TMD and two ABCs are encoded by a single polypeptide, where the two ABCs interact and hydrolyze ATP thereby generating energy for substrate transport. Half-transporters, in which the polypeptide chain encodes just one TMD and one ABC are thought to homo- or hetero-dimerize to form a functional transporter. Based on hydrophobicity plots a number of “full” transporters have additional TM α-helices whose function is poorly understood. There are 11 complete crystal structures of ABC transporters (9 prokaryote, 2 eukaryote) [2-11] and only two structures have been solved bound to a substrate (the maltose permease [4]) or an inhibitor (mouse ABCB1 [2]). From these medium-resolution (3.8Å and 3.4Å) structures a model has been proposed where a pore is formed by the traditional 12 TM α-helices [2, 3].
ABC transporters, also known as primary active transporters, drive substrate flow against a concentration gradient by coupling movement to energy released by ATP hydrolysis. There are other families of membrane transport proteins, such as the solute carrier (SLC) group of transporters [12]. SLC proteins differ from ABC transporters and can be categorized as either facilitative transporters, or secondary active transporters. Facilitative transporters allow substrates to move “downhill” along their concentration gradient, whereas secondary active transporters allow substrates to move “uphill” or against their concentration gradients, by coupling transport to a secondary molecule moving “downhill”, thus maintaining economical energy expenditure [12]. There are over 300 SLC proteins, and many have been implicated in lipid transport. These include the apical sodium dependent bile acid transporter (ASBT) bile salt transporters, sodium-taurocholate co-transporting peptide (NTCP), organic anion-transporting polypeptide (OATP), organic solute transporter alpha and beta (OSTα and OSTβ) [13]. Finally, there are P-type ATPase transporters that are also classified as primary active transporters [14]. These proteins are both transporters and enzymes that also utilize the energy released during ATP hydrolysis to transport ions and lipid molecules across cell membranes. ATPases catalyze auto/self-phosphorylation of a specific aspartate residue, which results in a conformational change in the transporter [14]. ATPases also possess Walker A and Walker B motifs that form the ATP binding site on the ATPase domain, yet they do not have the LSGGQ signature motif that is unique to ABC transporters. In particular, type IV (P4) ATPases have been implicated in the translocation of phospholipids [15].
Why do we need lipid transporters?
Lipids are broadly classified as hydrophobic molecules that are soluble in organic solvents and insoluble in aqueous solution. However a number of lipids, including phospholipids, are amphipathic as they have distinct surfaces or domains that are charged and uncharged. This allows them to form micelles or liposome structures in aqueous solutions. Lipids such as sterols, sterol esters, phospholipids, triglycerides, fat-soluble vitamins, and waxes are insoluble in water, and thus require specific transport mechanisms or carriers (e.g. plasma lipoproteins, fatty acid binding proteins) to move them through the blood or cytoplasm. Lipophilic molecules however, can passively diffuse across membranes, driven by concentration gradients, the availability of acceptors that facilitate desorption from the membrane bilayer, and by cellular metabolism.
Phospholipids diffuse (passive “flip-flop”) between artificial membrane leaflets at a slow rate (diffusion between leaflet layers at a rate of 10−15 cm2 s−1), with half times of hours, whereas cholesterol moves at a much faster rate with half times of 10 ms to 1 min [16]. Indeed, it was originally thought that hydrophobic molecules, such as phospholipids, could be readily inserted into membrane bilayers and diffuse across bilayer leaflets. However, mice with a deletion in the ABCB4 (MDR2) transporter lack the ability to secrete phospholipids from hepatocytes into the bile canaliculi [13], suggesting the requirement for an active transporter. The cholesterol content of bile is also much reduced in Mdr2−/− mice despite normal expression of the heterodimeric cholesterol transporter ABCG5/ABCG8 [13]. These data are consistent with the model that ABCG5/ABCG8 flips cholesterol across the apical membrane, but that desorption from the outer leaflet into the lumen of the bile cannaliculi requires phospholipid micelles that function as a lipid/sterol sink.
Membrane phospholipids are organized in a structured, asymmetrical fashion. In general, the outer leaflet of membranes is rich in phosphatidylcholine (PC) and sphingolipids, whilst the inner leaflet is enriched with the aminophospholipids phosphatidylethanolamine (PE) and phosphatidylserine (PS). The importance of P-type ATPases, or P-type flippases, was discovered over 30 years ago, in erythrocyte membranes. The erythrocyte aminophospholipid flippase, specific for PE and PS, facilitates the movement of these phospholipids from the outer membrane leaflet to the inner leaflet, in an ATP-dependent manner [17]. PC and sphingolipids are not translocated, thereby resulting in the asymmetric distribution of membrane phospholipids. In contrast to P-type flippases, ABC transporters are thought to be responsible for the opposing movement of phospholipids, and other lipid substrates, in an “outward” fashion from the inner leaflet to the outer leaflet of the membrane bilayer. This “flipped” lipid must then desorb, or diffuse to lipid acceptors (“sinks”) in the extracellular fluid.
Membrane lipid asymmetry is important in numerous cellular functions, including endocytosis of clathrin coated vesicles or caveolae, signaling via phosphatidylinositides, and the generation and fusion of secretory/exocytic vesicles with other membranes [17]. Loss of asymmetry also has functional biological consequences, that include platelet aggregation, or the recognition by macrophages of PS on the surface of apoptotic cells [18]. Asymmetry is also critical for membrane biogenesis. Phospholipid synthesis occurs in the endoplasmic reticulum (ER), where the catalytic sites of synthetic enzymes face the cytosol. In contrast, sphingolipids are primarily synthesized on the non-cytosolic surface of membranes [17]. Newly synthesized lipids must then be transported from the site of synthesis to the opposing membrane leaflet, at a rate sufficient to balance the rate of synthesis. Membrane curvature can be driven by ATP-dependent flippases that actively transport membrane lipids from one leaflet to the other. For example, endo- and exocytosis require membrane curvature [19]. Although budding vesicles are stabilized by specialized coat proteins (such as COPI, COPII), local accumulation of phospholipids in one membrane leaflet over the other is likely required to initiate outward vesicle budding and signaling [19].
Mechanisms of trans-bilayer lipid movement
The actual rate of passive/spontaneous lipid movement (flip-flop) across a membrane depends on multiple factors including lipid structure (backbone and headgroup), charge, and environment (local changes in membrane physical properties). Small, uncharged lipids, such as cholesterol, can flip across pure lipid bilayers in a matter of seconds or minutes [16]. In contrast, lipids with very polar headgroups move more slowly from one leaflet of the bilayer to the other, a process that can be regulated by the membrane cholesterol content and membrane fluidity/stiffness [16].
Energy-independent flippases have been shown to facilitate the rapid, bidirectional “flip-flop” of phospholipids, required for the balanced growth of mammalian membranes such as the ER [20]. In addition, the same rapid movement has been demonstrated for phospholipids and glucosylceramides in the Golgi apparatus [20]. Indeed, as early as the 1980’s, the presence of proteins within the membrane bilayer was shown to enhance energy-independent lipid “flip-flop” (Figure 1a) [21], which allows lipids (e.g. phosphatidylcholine) to rapidly equilibrate between the bilayer leaflets. However, energy-dependent flippases are responsible for a net movement of specific lipids (e.g. phosphatidylserine) to one leaflet in particular (Figure 1a, right two panels). In the plasma membranes of eukaryotic cells, the spontaneous “flip-flop” of phospholipids is limited due to the high cholesterol content of the membrane, therefore movement of both cholesterol and phospholipids requires energy, such as that generated by ABC transporters [16]. Any changes in membrane lipid composition in either leaflet can affect membrane curvature, vesicle membrane fusion events and protein activity and signaling pathways [22, 23].
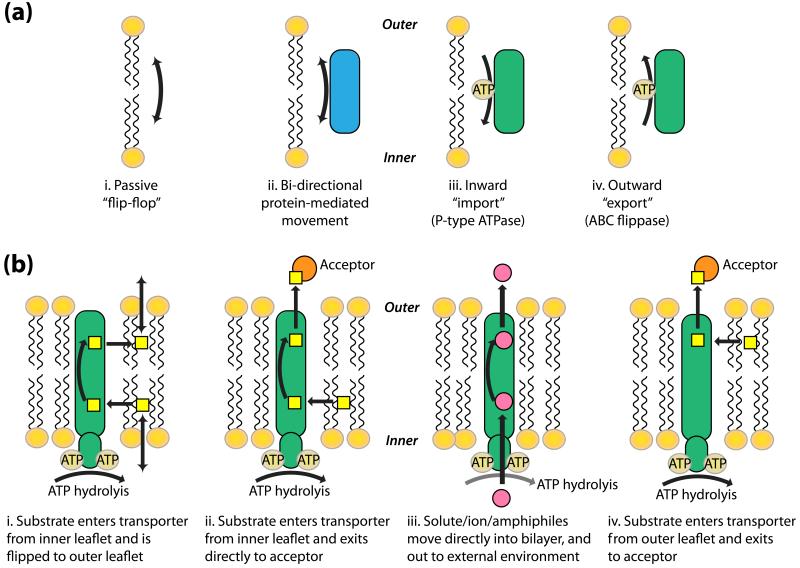
Figure 1. Mechanisms of trans-bilayer lipid transport/movement.
The models presented are based on mechanisms reviewed in [24] and [66]. (a) Lipids can move across the membrane bilayer by multiple mechanisms. Four mechanisms are proposed here: (i) membrane lipids passively diffuse or “flip-flop” from one leaflet of the bilayer to another, (ii) bi-directional movement of lipids from one membrane leaflet to another is enhanced by proteins present in the membrane bilayer, (iii) P-type ATPases mediate the movement of specific lipids (phospholipids) from the outer leaflet of the membrane bilayer, and (iv) ABC transporters/flippases mediate the “outward” movement of specific lipids (phospholipids/cholesterol) from the inner leaflet to the outer leaflet of the membrane bilayer. (b) Mechanisms of substrate recognition and transport by ABC transport proteins: (i) substrates enter the transporter from the inner leaflet and are flipped to the outer leaflet where they can exit the membrane bilayer, (ii) as in (i) but the substrate exits the transporter directly to an exogenous acceptor, (iii) solute/ions/amphiphiles move directly into the bilayer, through the transporter protein and out to the external environment, and (iv) substrates enter the transporter from the outer leaflet and exits to an acceptor molecule.
Several mechanisms have been proposed for substrate recognition and transport by ABC proteins [22, 23]. Figure 1b illustrates four possible mechanisms that have been proposed for outward export (reviewed in [24]). In the first model (i) the substrate enters the transporter from the inner leaflet, possibly by diffusing from the cytosol into the inner leaflet, and exits from the outer leaflet into the exogenous environment, having been moved in a “flip-flop” manner by the transporter. In the second model (ii), substrates enter the transporter from the inner leaflet, as previously described, but exit directly to an exogenous acceptor. Acceptors are required so that aqueous-insoluble lipid substrates may be translocated directly from the bilayer to the external aqueous environment. In contrast, there are numerous examples of ABC transporters effluxing non-lipid substrates directly to the aqueous environment, including the multi-drug resistant (MDR) transporters [25]. The third example (iii) is typical for the transport of ions and solutes. These amphiphilic molecules directly enter the transporter from the cytosol, and exit into the external environment. In the final model in Figure 1b (iv), substrates enter the transporter from the outer membrane leaflet, and exit directly to an exogenous acceptor.
Cellular Location, Location, Location
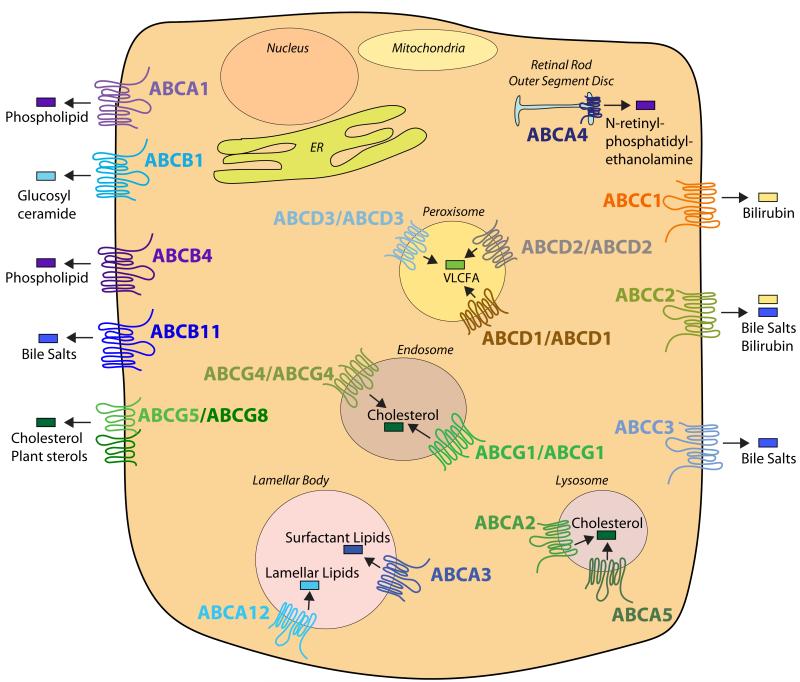
Twenty of the 48 human ABC transporters are thought to transport lipids, or lipid-related compounds (Figure 2). These transporters are not exclusively expressed at the plasma membrane, like the lipid flippases discussed in the previous section. Approximately half of them are localized to intracellular organelles, such as peroxisomes (ABCD1-3) [26], lamellar bodies (ABCA12, ABCA3) [27, 28], lysosomes (ABCA2, ABCA5) [29, 30] and endosomes (ABCG1, ABCG4) [31, 32]. Despite contrasting reports about whether ABCG1 and/or ABCG4 are present at the plasma membrane [33-35] it should be noted that loss of ABCG1 results in many specific cell intrinsic defects suggesting maintenance of intracellular lipid distribution by ABCG1 is critical (reviewed in [36]). This widespread intracellular localization of different ABC transporters suggests that the precise distribution of lipids within membranes is essential not only for membrane structure but also cell and organelle function. For example, loss of ABCD1 function results in the accumulation of saturated very long chain fatty acids due to impaired import into the peroxisome where they would normally undergo β-oxidation [26]. Additionally, functional loss of ABCA3 or ABCA12 from lamellar bodies results in impaired import of lipids into this organelle leading to defects in pulmonary surfactant secretion (ABCA3) or maintenance of skin barrier function (ABCA12) [37, 38].
Figure 2. Localization of ABC Lipid Transporters.
The cellular membrane localization of 20 out of the 48 human ABC transporters is depicted in the cartoon. ABCA1, ABCB1 (MDR1), ABCB4 (MDR2/3), ABCB11 (BSEP), ABCG5/ABCG8, ABCC1 (MRP1), ABCC2 (MRP2), and ABCC3 (MRP3) localize to the plasma membrane. Whereas, ABCA2, ABCA3, ABCA4, ABCA5, ABCA12, ABCD1-3, ABCG1, and ABCG4 localize to specific intracellular organelles. This cartoon does not exclude the possibility that ABC transporters may move between different membrane compartments under certain conditions. For example, in the absence of extracellular Apo-AI, ABCA1 can be sequestered on intracellular membranes or be degraded (reviewed in [67]), whilst ABCG1 is present on different classes of endosomal vesicles [31].
Lipid transporters and disease
Mutations in 24 of the 48 human ABC transporter genes have been linked to human diseases and 12 of these diseases are associated with abnormal lipid transport and/or homeostasis (Table 1). Below we discuss these 12 ABC transporters.
Table 1. ABC lipid transporters mutated in human disease.
| Transporter (Common Name) |
Gene Symbol |
Major Tissue Distribution |
Substrate | Disease (Loss of Function) | Reference |
|---|---|---|---|---|---|
| ABCA1 | ABCA1 | Ubiquitous | Phospholipid Cholesterol |
Tangier Disease | [40] |
| ABCA2 | ABCA2 | Brain, kidney, lung, heart |
Cholesterol | Alzheimer’s Disease | [47] |
| ABCA3 | ABCA3 | Lung | Surfactant lipids | Surfactant Metabolism Dysfunction 3 | [28] |
| ABCA4 | ABCA4 | Retina | N-retinyl- phosphatidylethanolamine |
Stargardt Disease | [49] |
| ABCA12 | ABCA12 | Lung, skin | Lipids | Harlequin Ichthyosis | [51] |
| MDR1 | ABCB1 | Many epithelia, blood-brain barrier |
Glucosylceramides | Inflammatory Bowel Disease | [68] |
| MDR3 | ABCB4 | Hepatocytes | Long chain phosphatidylcholines |
Progressive Familial Intrahepatic Cholestasis 3 (PFIC3) |
[13] |
| BSEP | ABCB11 | Hepatocytes | Bile salts | PFIC2 | [13] |
| MRP2 | ABCC2 | Liver, kidney, intestine |
Bilirubin/bile salts | Dubin-Johnson Syndrome | [13] |
| ALD | ABCD1 | Many | Very long chain fatty acids (VLCFA) |
Adrenoleukodystrophy | [58] |
| ABCG5 | ABCG5 | Enterocytes, hepatocytes |
Cholesterol, plant sterols |
Sitosterolemia | [61] |
| ABCG8 | ABCG8 | Enterocytes, hepatocytes |
Cholesterol, plant sterols |
Sitosterolemia, Gallstones | [61] |
ABCA1
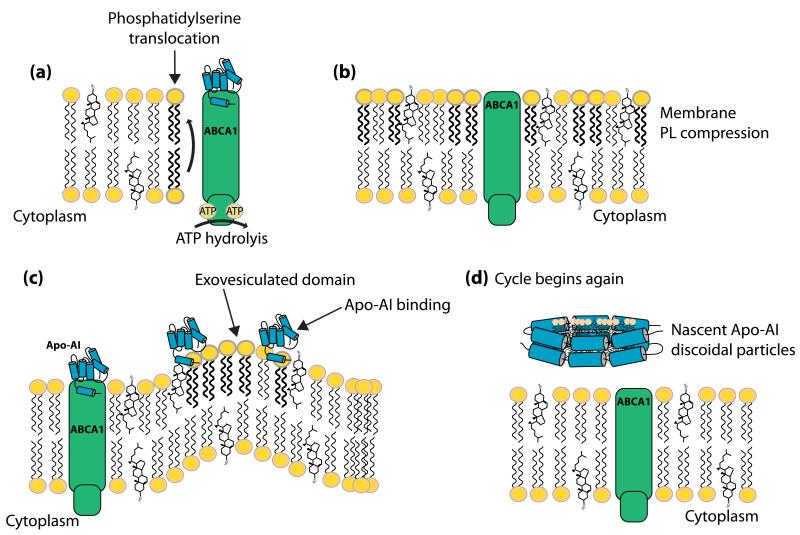
ABCA1 is ubiquitously expressed, but its physiologic function depends on cell and tissue type. For example, ABCA1 expressed in hepatocytes, intestinal enterocytes and adipocytes, is involved in the generation of high density lipoprotein (HDL) particles, whereas macrophage ABCA1 is involved in the reverse cholesterol transport pathway [39]. Patients with Tangier disease (Table 1) have plasma HDL levels in the 5th percentile (generally <5-10 mg/dl) [40]. Heterozygote patients with one functioning copy of ABCA1 exhibit hypoalphalipoproteinemia, with HDL levels in the 10th percentile. Over 50 mutations (including 23 missense and 17 insertions/deletions) have been identified in the ABCA1 gene locus, leading to the low levels of circulating HDL, and severe lipid-laden “foam” cells in multiple tissues [40]. ABCA1 transfers membrane phospholipids and cholesterol to lipid-poor apolipoproteins (Figure 2) [41]. The mechanism involved in this transfer has yet to be clearly defined. However, one attractive model suggests that ATP hydrolysis causes a conformational change within ABCA1, allowing lipid-poor Apo-AI to bind ABCA1 and stabilize the transporter at the plasma membrane [41]. There is evidence that ABCA1 subsequently flips PS from the inner leaflet to the outer leaflet of the plasma membrane (Figure 3). Vedhachalam et al. [41], have proposed that the resulting disruption in membrane asymmetry creates regions of increased membrane curvature, termed exovesiculated domains (Figure 3). These domains are more accessible to lipid-poor Apo-AI particles. Apo-AI “solubilizes” lipids from the membrane, including PS and cholesterol, to generate nascent HDL particles (Figure 3). The change in Apo-AI structure due to lipid-loading likely facilitates dissociation of the pre-beta HDL lipoprotein particles from the membrane. ABCA1, and other ABCA sub-family members, are distinguished from most other ABC transporters by the presence of large extracellular loops/domains linking the transmembrane α-helices. Hozoji et al., identified two intramolecular disulfide bonds between the two extracellular domains of ABCA1, and subsequently demonstrated that these bonds are essential for Apo-AI binding to ABCA1 and the subsequent formation of HDL [42].
Figure 3. ABCA1-mediated phospholipid and cholesterol transport.
The model illustrated is based on studies by Vedhachalam et al. [41]. (a) ABCA1 mediates the translocation of phosphatidylserine from the inner leaflet to the outer leaflet of the plasma membrane, in an ATP-dependent manner. (b) The increased concentration of phosphatidylserine in the outer leaflet of the membrane causes membrane phospholipid compression. (c) To relieve the membrane strain, these compressed regions bulge out and form exovesiculated domains. Apo-AI binds to ABCA1 and stabilizes ABCA1 within the plasma membrane. Apo-AI also binds to the exovesiculated membrane lipid domains. (d) Apo-AI “solubilizes” the phospholipids and cholesterol from the exovesiculated membrane domains, and forms nascent Apo-AI discoidal particles, where the hydrophobic face of the Apo-AI molecule interacts with the phospholipids and cholesterol forming a cage around the hydrophobic lipids. Once the membrane strain has been relieved the cycle can begin again.
ABCA2
ABCA2 is highly expressed in the brain with lower levels found in the heart, kidney and lung. The finding that ABCA2 mRNA levels are induced when macrophages are loaded with cholesterol [43] and that overexpression of ABCA2 in neuroblastoma cells results in decreased cellular cholesterol levels [44] suggests that this transporter may efflux cholesterol from cells. Accumulation of extracellular amyloid β protein (Aβ) is the leading cause of Alzheimer’s disease, and high cellular cholesterol levels have been shown to promote the release of Aβ [45]. ABCA2 has also been linked to Alzheimer’s disease (Table 1), as overexpression of ABCA2 has been shown to result in increased expression of amyloid precursor protein (APP), increased cleavage of APP at the secondary (β) cleavage site, glutamine 111, and increased levels of C89 carboxy terminal fragment (β-CTF/C89) levels [46]. In 2005, Mace et al. identified a mutation within exon 14 of the ABCA2 gene that was significantly associated with Alzheimer’s disease [47]. Further analysis of this mutation revealed that carriers of the T allele had an odds ratio of 3.82 for developing early onset Alzheimer’s disease [47].
ABCA3
ABCA3 expression is limited to alveolar type II pneumocytes in the lung [28]. Immunofluorescence studies indicate that ABCA3 localizes to the limiting membrane of lamellar bodies [28]. Lamellar bodies in type II cells are specialized secretory organelles that are responsible for the concentrated storage and subsequent secretion of pulmonary surfactant (a complex mixture of cholesterol, phospholipids and proteins). ABCA3 expression peaks before birth, and is hormonally induced at the same time as the surfactant proteins A-D [28], suggesting developmental regulation. Indeed, genetic analysis of a cohort of over 100 patients with severe neonatal respiratory distress and symptoms of surfactant deficiency and in which other hereditary mutations had been excluded, identified deletion and frameshift mutations in ABCA3 (Table 1) [28, 37]. Further analysis demonstrated that loss of ABCA3 function results in small, empty lamellar bodies consistent with a role for ABCA3 in transporting phospholipids into this organelle. Abca3−/− mice have almost a complete absence of pulmonary surfactant in the alveolar space, accompanied by a profound loss of mature lamellar bodies, and a reduction in lung phospholipid content [37]. Loss of functional ABCA3 in humans or mice is fatal, with death occurring shortly after birth [28, 37].
ABCA4
During phototransduction in the eye, when the photoreceptor pigment rhodopsin is stimulated by light, 11-cis-retinal is converted to all-trans-retinal. A by-product of this cycle is the formation of N-retinyl-phosphatidylethanolamine (NRPE), a phospholipid-all-trans-retinal adduct. ABCA4 transports NRPE out of the rod outer segment discs into the cytoplasm, where all-trans-retinal is reduced to vitamin A, which is then transported to the retinal pigment epithelium (RPE) and converted back to 11-cis-retinal, thus completing the photo-cycle [48]. Mutations in ABCA4 are causal for the autosomal recessive inheritance of Stargardt’s disease (Table 1) [49], and are associated with multiple retinal degenerative conditions. The result is accumulation of autofluorescent retinal pigment epithelium (RPE) lipofuscin, a characteristic of Stargardt’s macular degeneration, along with loss of peripheral vision and delayed dark adaptation [48]. Most recently, nanoparticle delivery of ABCA4 to Abca4−/− mice was shown to rescue the delayed dark adaptation and lipofuscin accumulation observed in the knockout mice [50].
ABCA12
Harlequin ichthyosis, the most severe lethal form of congenital ichthyosis, is characterized by defects in lipid transport, protein phosphatase activity, and differentiation, accompanied by the development of abnormal lamellar body formation in the epidermis [51]. The abnormal development of keratinocyte lamellar bodies is reminiscent of ABCA3 (see above). Neonates suffering from this form of ichthyosis have a severely compromised skin barrier resulting in predisposition to sepsis, dehydration and thermal dysregulation [51]. Mutations in ABCA12 were identified in patients with harlequin ichthyosis (Table 1), who had an abnormal and congested stratum corneum filled with vacuole-like immature lamellar granules. ABCA12 was shown to localize to the lamellar granules in the upper epidermis of keratinocytes of these patients [27], and to be involved in the transport of glucosylceramides from keratinocytes. Abnormal glucosylceramide transport impacts skin barrier function [27, 38].
P-glycoprotein family of multidrug resistant transporters
ABCB1/MDR1
MDR1, also known as multidrug resistance protein or P-glycoprotein, is a glycoprotein whose expression is commonly increased in the membranes of multidrug-resistant cells from cancer patients undergoing chemotherapeutic treatments (Table 1) [52]. MDR1 is widely expressed, and is found in the intestinal epithelium, hepatocytes, and the epithelial cells of the blood-brain barrier. MDR1 also transports a diverse array of compounds including lipids, xenobiotics, drugs (colchicine), chemotherapeutic agents (doxorubicin, etoposide) and bilirubin. In 2009, Aller et al., resolved the crystal structure of MDR1 to 3.8 Å, revealing an internal cavity of approximately 6,000 Å that can presumably accommodate the diverse range of substrates, and a 30 Å separation of the two ATP binding cassette domains [2]. Distinct drug binding sites, capable of stereo selectivity, have been further identified within the cavity [2]. Abcb1−/− mice develop a severe, spontaneous intestinal inflammation with pathology similar to that of human inflammatory bowel disease, and this inflammation has recently been suggested to be the result of changes in the caecal microbial environment [53].
ABCB4/MDR3
ABCB4, another member of the family of multidrug resistant transporters, is expressed on the apical membrane of hepatocytes, and functions to transport phosphatidylcholine (PC) from hepatocytes into the bile canaliculus [13]. Within the bile canaliculi, PC normally chaperones bile salts to prevent damage to the biliary epithelium and functions as an acceptor of cholesterol or phytosterols that have been flipped across the apical membrane by ABCG5/ABCG8 (reviewed in [54]). Thus, Abcb4−/− mice are unable to transport phospholipid into the bile [55]. Heterozygous mice do not display any pathological alterations but they have a 50% reduction in phospholipid content in the bile, when compared to wild-type mice [55]. The bile of Abcb4−/− mice is devoid of phospholipids and cholesterol, but not bile acids, consistent with the hypothesis that phospholipid vesicles in the bile cannaliculi function as cholesterol acceptors at the outer leaflet of hepatocyte apical membranes. Progressive familial intrahepatic cholestasis (PFIC), a group of inherited disorders leading to severe cholestatic liver disease appear in early infancy and result from defective biliary epithelial transporters. PFIC type 3 (PFIC-3) is caused by mutations in ABCB4 that leads to a lack of PC (and cholesterol) in the bile (Table 1). Patients are treated with bile acids (ursodeoxycholic acid) that act as acceptors for cholesterol, unless liver dysfunction is significant, in which case organ transplantation is required [13].
ABCC2/MRP3
ABCC2, also known as multidrug resistance-associated protein-3 (MRP3) or canalicular multi-specific organic anion transporter (cMOAT), is expressed both on the canalicular membrane of hepatocytes and the apical membrane of proximal renal tubule endothelial cells, where it is thought to secrete morphine-3-glucoronide (M3G) and M6G [13]. Dubin-Johnson syndrome, caused by mutations in ABCC2, is associated with a failure to secrete conjugated bilirubin glucuronides into the bile, resulting in elevated levels of conjugated bilirubin in the plasma (Table 1) [13].
ABCB11/BSEP
ABCB11 or bile salt export pump (BSEP) is the major determinant of bile formation and bile flow in humans. BSEP is expressed exclusively on the apical membrane of hepatocytes and is responsible for the transport of bile acid conjugates from the hepatocyte into the bile canaliculus (reviewed in [54]). Secretion of bile salts is reduced 94% in Bsep−/− mice, yet total bile salt output was just 30% of wild-type levels [56]. Tetra-hydroxylated bile acid products were detected in Bsep−/− but not wild-type mice, suggesting alternative mechanisms that prevent Bsep−/− mice from developing cholestasis [56]. In contrast, tetra-hydroxylated bile acids are not identified in patients with PFIC type 2, which results from inactivating mutations in BSEP. Patients with PFIC-2 often develop hepatocellular carcinoma due to liver damage caused by the accumulation of bile salts within hepatocytes (Table 1) [13].
ABCD1/ALD
ABCD1 functions to transport very long chain fatty acids into peroxisomes [26]. There are two other members of the ALD family of ABC transporters (ABCD2-3) that also localize to the limiting membranes of peroxisomes. The last member of the ALD family, ABCD4, has recently been shown to localize to the lysosomal membrane, and is mutated in an inborn error of vitamin B(12) metabolism [57]. The ABCD family of transporters are half transporters, that homo- and heterodimerize with other family members [26]. Adrenoleukodystrophy (ALD) is caused by mutations in ABCD1 and results in the accumulation of very long chain fatty acids in the cytoplasm (Table 1) [58]. The most affected organs are the myelin of the central nervous system, the adrenal cortex, and the Leydig cells in the testes. Over 600 mutations in ABCD1 have been reported, and most are familial [58]. Adrenal dysfunction in ALD can be treated with steroids, but there is no specific treatment for ALD. Hematopoietic cells offer one potential treatment, and in at least two X-linked ALD patients, hematopoietic stem cell (HSC) delivery of wild-type ABCD1 was successful in attenuating cerebral demyelination [59]. More studies are required to determine whether this type of therapy will be useful for other patients with cerebral forms of X-ALD.
ABCG5/ABCG8
This obligate heterodimer pairing of two ABC transporters represents the major route for cholesterol and plant sterol secretion into bile, and from ileal enterocytes back into the intestinal lumen (reviewed in [60]). ABCG5 and ABCG8, two adjacent yet oppositely orientated genes encoded by a locus on human chromosome 2, are expressed in the liver, and small intestine, where they function to excrete sterols into bile and limit intestinal absorption of sterols. Mutations that inactivate either half transporter result in sitosterolemia (Table 1), a disease characterized by the accumulation of plant sterols and cholesterol in numerous tissues and the blood, eventually leading to premature development of atherosclerosis [61]. Disruption of both transporters in mice (Abcg5/8−/− mice) results in a 3-fold increase in the fractional absorption of plant sterols from the intestine, and a concomitant 30% increase in plasma sitosterol, accompanied by reduced biliary cholesterol content [62]. ABCG5/ABCG8 selectively transport cholesterol, as studies with Abcg5/8−/− mice demonstrated that cholesterol levels, but not phospholipid or bile acid levels, were significantly reduced in the bile of these mice [62]. Sitosterolemia shares many characteristics with the well-characterized disease familial hypercholesterolemia (FH), including the development of premature coronary atherosclerosis [40]. However, in contrast to FH patients, individuals with sitosterolemia typically have normal to moderately elevated plasma cholesterol levels, but abnormally high levels of plant sterols (sitosterol, campesterol, stigmasterol). It is possible that modulating the activity of ABCG5/ABCG8 might be used as a therapeutic treatment for hypercholesterolemias. Indeed, overexpression of ABCG5/ABCG8 in hypercholesterolemic Ldlr−/− mice caused a significant reduction in plasma cholesterol and atherosclerotic lesions compared to control Ldlr−/− mice [63]. Whether targeting these transporters will be translated to human patients requires further study.
Concluding remarks
Membrane distribution of intracellular lipids is essential for cellular function and signaling. ABC transporters, such as ABCA1, ABCB4, ABCB11, ABCD1, ABCG1, and ABCG5/8 play critical roles in maintaining this organization. Indeed, mutations in many of these ABC lipid transporters results in human diseases, such as sitosterolemia, Tangier disease, and familial intrahepatic cholestasis. The ABC transporter superfamily is a well-characterized protein family, yet the substrates and roles of many of these proteins in human disease are unknown. Substrate specificity has mostly been inferred from the identification of metabolites/compounds that accumulate in humans with specific diseases or knockout mouse models. In the case of ABC proteins that transport drugs or small molecules, direct substrate transport can be demonstrated by the well-established in vitro vesicle transport assay [64]. However, for ABC lipid transporters, those transport assays are inherently more difficult due to the hydrophobic nature of the lipid substrate. Substrate transfer, rather than active transport, has been elegantly demonstrated for ABCG5/ABCG8 and cholesterol [65], where Hobbs, Cohen and colleagues utilized radiolabeled cholesterol in donor liposomes, and recipient proteoliposomes reconstituted with pure ABCG5/G8 protein. Nonetheless identification of the molecular mechanism of lipid transport/movement by ABC transporters will require the development of novel assays to overcome the problems associated with the insolubility of the substrates.
Our understanding of the role of ABC transporters in human disease is still cursory. The main challenge remaining, with the exception of substrates, is the identification of clinical modulators/inhibitors. The expansion of genome-wide association studies will greatly aid the discovery of novel sequence mutations and how these variations predispose patients to disease. Finally, there is now the probability that individual ABC transporters have multiple functions depending on their cellular context, as has been demonstrated for ABCG1.
Highlights.
-
–
ATP binding cassette (ABC) transporters utilize the energy derived from ATP hydrolysis to transport substrates across membrane bilayers.
-
–
There are 48 human ABC transporters and approximately half are thought to be involved in the transport of lipids and lipid-related compounds.
-
–
ABC transporters are found in almost every type of intracellular organelle, and individual transporters have multiple functions depending on their cellular context.
Acknowledgements
This work was supported in part by United States Public Health Service grant HL30568 (to P.A.E.), Laubisch Fund at UCLA (to P.A.E.) and post-doctoral fellowships from the American Heart Association Western States Affiliate to E.J.T. (11POST7300060) and T.Q. de A.V. (11POST7240070).
Glossary
- ATP binding cassette (ABC) transporter
Membrane bound proteins that utilize the energy derived from ATP hydrolysis to drive substrate transport across a membrane bilayer.
- Adrenoleukodystrophy (ALD)
a disorder of peroxisomal fatty acid β-oxidation that results in the accumulation of very long chain fatty acids within cells and tissues.
- Bile salt export pump (BSEP)
a member of the ABC transporter superfamily that transports tauro- and glycine-conjugated bile acids across the canalicular membrane from the hepatocyte into the bile canaliculus.
- Canalicular multispecific organic anion transporter (cMOAT)
also expressed at the canalicular membrane. This ABC transporter functions to export bilirubin from the hepatocyte into the bile.
- Dubin-Johnson syndrome
a very rare autosomal recessive disorder associated with a defect in the ability of hepatocytes to secrete conjugated bilirubin into the bile. The mild jaundice is usually asymptomatic but may be diagnosed in early infancy.
- Harlequin-type ichthyosis
skin disease associated with a mutation in ABCA12, and characterized by a thickening of the keratin layer in fetal human skin. The skin contains massive, diamond-shaped scales, which are caused by severe hyperkeratosis, limiting movement.
- Low density lipoprotein receptor (LDLR)
a cell surface receptor that is involved in the uptake of cholesterol-containing plasma lipoproteins via receptor-mediated endocytosis.
- Multidrug resistance protein (MDR/MRP)
two sub-families of ABC transporters that are involved in resistance to drugs.
- Progressive familial intrahepatic cholestasis (PFIC)
diseases caused by defects in biliary epithelial transporters, such as ATP8B1 (a Type I P-ATPase), ABCB4, and ABCB11, resulting in the toxic build up of bile acids in the liver. There are three known types of PFIC: PFIC1, PFIC2, and PFIC3.
- P-type ATPase
a group of evolutionarily conserved α-helical bundle primary transporters that are called P-type ATPases because they catalyze auto-/self-phosphorylation of a key aspartate residue. There are multiple groups of P-type ATPases, with Type IV ATPases being involved in the transport of phospholipids.
- Sitosterolemia
rare autosomal recessive plant sterol storage disease characterized by hyperabsorption and decreased biliary excretion of dietary sterols. It leads to hypercholesterolemia, tendon and tuberous xanthomas, and a strong propensity toward premature coronary atherosclerosis.
- Solute carrier (SLC) transporters
a group of over 300 transmembrane proteins organized into 51 families. Most members of the SLC group are located in the cell membrane. SLC transporters function as either monomers or obligate homo- or hetero-oligomers and transport both charged and uncharged organic molecules and inorganic ions.
- Stargardt disease
also known as fundus flavimaculatus, is the most common form of inherited juvenile macular degeneration. It causes progressive vision loss usually starting between the ages of six and twelve years old. It is characterized by a reduction of central vision with a preservation of peripheral vision.
- Tangier disease
also known as familial alpha-lipoprotein deficiency or hypoalphalipoproteinemia, is a rare autosomal recessive disorder resulting from defects in the ABCA1 gene. It is characterized by a severe reduction in the amount of HDL in the blood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None
REFERENCES
- 1.George AM, Jones PM. Perspectives on the structure-function of ABC transporters: The Switch and Constant Contact Models. Prog. Biophys. Mol. Biol. 2012;109:95–107. doi: 10.1016/j.pbiomolbio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Aller SG, et al. Structure of P-Glycoprotein Reveals a Molecular Basis for Poly-Specific Drug Binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 4.Oldham ML, et al. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450:515–521. doi: 10.1038/nature06264. [DOI] [PubMed] [Google Scholar]
- 5.Hohl M, et al. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. Nat. Struct. Mol. Biol. 2012;19:395–402. doi: 10.1038/nsmb.2267. [DOI] [PubMed] [Google Scholar]
- 6.Hollenstein K, et al. Structure of an ABC transporter in complex with its binding protein. Nature. 2007;446:213–216. doi: 10.1038/nature05626. [DOI] [PubMed] [Google Scholar]
- 7.Locher KP, et al. The E. coli BtuCD Structure: A Framework for ABC Transporter Architecture and Mechanism. Science. 2002;296:1091–1098. doi: 10.1126/science.1071142. [DOI] [PubMed] [Google Scholar]
- 8.Kadaba NS, et al. The High-Affinity E. coli Methionine ABC Transporter: Structure and Allosteric Regulation. Science. 2008;321:250–253. doi: 10.1126/science.1157987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinkett HW, et al. An Inward-Facing Conformation of a Putative Metal-Chelate-Type ABC Transporter. Science. 2007;315:373–377. doi: 10.1126/science.1133488. [DOI] [PubMed] [Google Scholar]
- 10.Ward A, et al. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber S, et al. Structural Basis of Trans-Inhibition in a Molybdate/Tungstate ABC Transporter. Science. 2008;321:246–250. doi: 10.1126/science.1156213. [DOI] [PubMed] [Google Scholar]
- 12.Roth M, et al. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolaou M, et al. Canalicular ABC transporters and liver disease. J Pathol. 2012;226:300–315. doi: 10.1002/path.3019. [DOI] [PubMed] [Google Scholar]
- 14.Palmgren MG, Nissen P. P-type ATPases. Annu. Rev. Biophys. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 15.Sebastian TT, et al. Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim. Biophys. Acta. 2012;1821:1068–1077. doi: 10.1016/j.bbalip.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras FX, et al. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett. 2010;584:1779–1786. doi: 10.1016/j.febslet.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 17.van Meer G. Dynamic Transbilayer Lipid Asymmetry. Cold Spring Harbor Perspectives in Biology. 2011:3. doi: 10.1101/cshperspect.a004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 19.Muthusamy B-P, et al. Linking phospholipid flippases to vesicle-mediated protein transport. Biochim. Biophys. Acta. 2009;1791:612–619. doi: 10.1016/j.bbalip.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalat M, et al. Reconstitution of Glucosylceramide Flip-Flop across Endoplasmic Reticulum: Implications for mechanism of glycosphingolipid biosynthesis. J. Biol. Chem. 2012;287:15523–15532. doi: 10.1074/jbc.M112.343038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerritsen WJ, et al. The transbilayer movement of phosphatidylcholine in vesicles reconstituted with intrinsic proteins from the human erythrocyte membrane. Biochim. Biophys. Acta. 1980;600:607–619. doi: 10.1016/0005-2736(80)90464-2. [DOI] [PubMed] [Google Scholar]
- 22.Ecker GF, et al. Computational models for prediction of interactions with ABC-transporters. Drug Discov. 2008;13:311–317. doi: 10.1016/j.drudis.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Jones PM, George AM. Mechanism of the ABC transporter ATPase domains: catalytic models and the biochemical and biophysical record. Crit. Rev. Biochem. Mol. Biol. 2012 doi: 10.3109/10409238.2012.735644. [DOI] [PubMed] [Google Scholar]
- 24.Pohl A, et al. Function of prokaryotic and eukaryotic ABC proteins in lipid transport. Biochim. Biophys. Acta. 2005;1733:29–52. doi: 10.1016/j.bbalip.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Marquez B, Van Bambeke F. ABC multidug transporters: target for modulation of drug pharmacokinetics and drug-drug interactions. Curr Drug Targets. 2011;12:600–620. doi: 10.2174/138945011795378504. [DOI] [PubMed] [Google Scholar]
- 26.Kemp S, et al. Mammalian peroxisomal ABC transporters: from endogenous substrates to pathology and clinical significance. Br. J. Pharmacol. 2011;164:1753–1766. doi: 10.1111/j.1476-5381.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakai K, et al. Localization of ABCA12 from Golgi apparatus to lamellar granules in human upper epidermal keratinocytes. Exp. Dermatol. 2007;16:920–926. doi: 10.1111/j.1600-0625.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 28.Whitsett JA, et al. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu. Rev. Med. 2010;61:105–119. doi: 10.1146/annurev.med.60.041807.123500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ile KE, et al. Identification of a novel first exon of the human ABCA2 transporter gene encoding a unique N-terminus. Biochim. Biophys. Acta. 2004;1678:22–32. doi: 10.1016/j.bbaexp.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Kubo Y, et al. ABCA5 Resides in Lysosomes, and ABCA5 Knockout Mice Develop Lysosomal Disease-Like Symptoms. Mol. Cell. Biol. 2005;25:4138–4149. doi: 10.1128/MCB.25.10.4138-4149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarling EJ, Edwards PA. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc. Natl. Acad. Sci. U.S.A. 2011;108:19719–19724. doi: 10.1073/pnas.1113021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarr PT, Edwards PA. ABCG1 and ABCG4 are coexpressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2. J. Lipid Res. 2008;49:169–182. doi: 10.1194/jlr.M700364-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi A, et al. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 2006;47:1791–1802. doi: 10.1194/jlr.M500546-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Wang N, et al. LXR-Induced Redistribution of ABCG1 to Plasma Membrane in Macrophages Enhances Cholesterol Mass Efflux to HDL. Arterioscler. Thromb. Vasc. Biol. 2006;26:1310–1316. doi: 10.1161/01.ATV.0000218998.75963.02. [DOI] [PubMed] [Google Scholar]
- 35.Xie Q, et al. Cell Surface Localization of ABCG1 Does Not Require LXR Activation. Arterioscler. Thromb. Vasc. Biol. 2006;26:e143–144. doi: 10.1161/01.ATV.0000245790.47112.b2. [DOI] [PubMed] [Google Scholar]
- 36.Tarling EJ. Expanding the roles of ABCG1 and sterol transport. Curr. Opin. Lipidol. 2013 doi: 10.1097/MOL.0b013e32835da122. In Press. [DOI] [PubMed] [Google Scholar]
- 37.Hayes DJ, et al. ABCA3 transporter deficiency. Am. J. Respir. Crit. Care Med. 2012;186:807. doi: 10.1164/ajrccm.186.8.807a. [DOI] [PubMed] [Google Scholar]
- 38.Scott CA, et al. Harlequin ichthyosis: ABCA12 mutations underlie defective lipid transport, reduced protease regulation and skin-barrier dysfunction. Cell Tissue Res. 2012 doi: 10.1007/s00441-012-1474-9. [DOI] [PubMed] [Google Scholar]
- 39.Cuchel M, et al. Pathways by which reconstituted high-density lipoprotein mobilizes free cholesterol from whole body and from macrophages. Arterioscler. Thromb. Vasc. Biol. 2010;30:526–532. doi: 10.1161/ATVBAHA.109.196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitzgerald ML, et al. ABC transporters, atherosclerosis and inflammation. Atherosclerosis. 2010;211:361–370. doi: 10.1016/j.atherosclerosis.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vedhachalam C, et al. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-1 and formation of high density lipoprotein particles. J. Biol. Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 42.Hozoji M, et al. Formation of Two Intramolecular Disulfide Bonds Is Necessary for ApoA-I-dependent Cholesterol Efflux Mediated by ABCA1. J. Biol. Chem. 2009;284:11293–11300. doi: 10.1074/jbc.M900580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaminski WE, et al. Complete Coding Sequence, Promoter Region, and Genomic Structure of the Human ABCA2 Gene and Evidence for Sterol-Dependent Regulation in Macrophages. Biochem. Biophys. Res. Commun. 2001;281:249–258. doi: 10.1006/bbrc.2001.4305. [DOI] [PubMed] [Google Scholar]
- 44.Davis W., Jr The ATP-binding cassette transporter-2 (ABCA2) regulates cholesterol homeostasis and low-density lipoprotein receptor metabolism in N2a neuroblastoma cells. Biochim. Biophys. Acta. 2011;1811:1152–1164. doi: 10.1016/j.bbalip.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ubhi K, Masliah E. Alzheimer’s disease: recent advances and future perspectives. J. Alzheimers Dis. 2013;33:S185–S194. doi: 10.3233/JAD-2012-129028. [DOI] [PubMed] [Google Scholar]
- 46.Davis W., Jr The ATP-binding cassette transporter-2 (ABCA2) increases endogenous amyloid precursor protein expression and A-beta fragment generation. Curr. Alzeihmer Res. 2010;7:566–577. doi: 10.2174/156720510793499002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mace S, et al. ABCA2 is a strong genetic risk factor for early-onset Alzheimer’s disease. Neurobiol. Dis. 2005;18:119–125. doi: 10.1016/j.nbd.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Biswas-Fiss EE, et al. Retinoid Binding Properties of Nucleotide Binding Domain 1 of the Stargardt Disease Associated ABC Transporter, ABCA4. J. Biol. Chem. 2012 doi: 10.1074/jbc.M112.409623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsybovsky Y, et al. The ATP-binding cassette transporter ABCA4: structural and functional properties and role in retinal disease. Adv. Exp. Med. Biol. 2010;703:105–125. doi: 10.1007/978-1-4419-5635-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han Z, et al. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J. Clin. Invest. 2012;122:3221–3226. doi: 10.1172/JCI64833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajpopat S, et al. Harlequin ichthyosis: A review of clinical and molecular findings in 45 cases. Arch. Dermatol. 2011;147:681–686. doi: 10.1001/archdermatol.2011.9. [DOI] [PubMed] [Google Scholar]
- 52.Chen KG, Sikic BI. Molecular Pathways: Regulation and Therapeutic Implications of Multidrug Resistance. Clin. Can. Res. 2012;18:1863–1869. doi: 10.1158/1078-0432.CCR-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nones K, et al. Multidrug resistance gene deficient (mdr1a−/−) mice have an altered caecal microbiota that precedes the onset of intestinal inflammation. J. Appl. Microbiol. 2009;107:557–566. doi: 10.1111/j.1365-2672.2009.04225.x. [DOI] [PubMed] [Google Scholar]
- 54.Vallim T.Q.d.A., et al. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013 doi: 10.1016/j.cmet.2013.03.013. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lammert F, et al. Spontaneous cholecysto- and hepatolithiasis in Mdr2−/− mice: A model for low phospholipid-associated cholelithiasis. Hepatology. 2004;39:117–128. doi: 10.1002/hep.20022. [DOI] [PubMed] [Google Scholar]
- 56.Wang R, et al. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coelho D, et al. Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nat. Genet. 2012;44:1152–1155. doi: 10.1038/ng.2386. [DOI] [PubMed] [Google Scholar]
- 58.Kemp S, et al. X-linked adrenoleukodystrophy: Clinical, metabolic, genetic and pathophysiological aspects. Biochim. Biophys. Acta. 2012;1822:1465–1474. doi: 10.1016/j.bbadis.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Cartier N, et al. Chapter ten - Lentiviral Hematopoietic Cell Gene Therapy for X-Linked Adrenoleukodystrophy. In: Theodore F, editor. Methods in Enzymology. Academic Press; 2012. pp. 187–198. [DOI] [PubMed] [Google Scholar]
- 60.Tarling EJ, Edwards PA. Dancing with the sterols: Critical roles for ABCG1, ABCA1, miRNAs, and nuclear and cell surface receptors in controlling cellular sterol homeostasis. Biochim. Biophys. Acta. 2012;1821:386–395. doi: 10.1016/j.bbalip.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Berge KE, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adajacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 62.Yu L, et al. Expression of ABCG5 and ABCG8 Is required for Regulation of Biliary Cholesterol Secretion. J. Biol. Chem. 2005;280:8742–8747. doi: 10.1074/jbc.M411080200. [DOI] [PubMed] [Google Scholar]
- 63.Wilund KR, et al. High-level expression of ABCG5 and ABCG8 attenuates diet-induced hypercholesterolemia and atherosclerosis in Ldlr−/− mice. J. Lipid Res. 2004;45:1429–1436. doi: 10.1194/jlr.M400167-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Glavinas H, et al. Utilization of membrane vesicle preparations to study drug-ABC transporter interactions. Exp. Opin. Drug Metab. Toxicol. 2008;4:721–732. doi: 10.1517/17425255.4.6.721. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, et al. Purification and reconstitution of sterol transfer by native mouse ABCG5 and ABCG8. Biochemistry. 2008;47:5194–5204. doi: 10.1021/bi800292v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagao K, et al. Lipid outward translocation by ABC proteins. FEBS Lett. 2010;584:2717–2723. doi: 10.1016/j.febslet.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 67.Tang C, Oram JF. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim. Biophys. Acta. 2009;1791:563–572. doi: 10.1016/j.bbalip.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmeyer S, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]