Abstract
Objective
Exposure to high-calorie foods may promote overeating by stimulating brain reward pathways and appetite. Abdominal fat has particularly adverse metabolic consequences and may alter brain pathways that regulate feeding behavior. Functional magnetic resonance imaging (fMRI) was used to test the hypothesis that high-calorie food cues activate brain reward regions and increase appetite, and to examine relationships between abdominal fat and brain reward responsiveness in Hispanic women.
Design and Methods
fMRI was performed while thirteen volunteers viewed twelve blocks of pictures of food and non-food items. Participants rated hunger and food desire after each block of pictures. Brain activation to high-calorie foods was determined by calculating a contrast of high-calorie food minus non-food images. Pearson’s correlations were used to test the relationship between brain reward activation and waist circumference.
Results
High-calorie food images activated brain reward regions (Z>2.3, p<0.05 corrected for multiple comparisons) and increased hunger (p=0.001), desire for sweet (p=0.012) and savory (p=0.009) foods. The striatal response to high-calorie foods positively correlated with waist circumference, independent of BMI (r=0.621, p=0.031).
Conclusions
Exposure to high-calorie food images activates brain reward pathways and increases appetitive drive in Hispanic females. Abdominal fat, independent of BMI, parallels striatal responsiveness to high-calorie food images.
Keywords: food images, fMRI, appetite, desire, obesity, reward, brain, hunger
Introduction
Obesity rates have risen dramatically, and there is a disproportionate prevalence of obesity in people of Hispanic origin (1). Changes in the food environment are likely contributing to the world-wide obesity epidemic. The ubiquity of high-calorie foods and food cues is hypothesized to promote overeating behavior by stimulating brain reward and motivation pathways (2). Indeed, neuroimaging studies in humans have shown that visual food cues can stimulate activity in brain regions involved in reward and motivation (3-8), and obese compared to normal-weight individuals have greater brain reward activation to visual food cues, especially appetizing, high-calorie foods (9). These neuroimaging findings are consistent with behavioral studies showing that the appetitive effects of food cues are stronger in overweight compared to normal-weight individuals (10, 11) and suggest that obese individuals may be particularly vulnerable to the reward-related appetitive drive elicited by visual food cues.
In addition to generalized obesity, body fat distribution may influence brain responses to visual food stimuli, and Hispanics have greater levels of abdominal fat compared with non-Hispanic Whites and African-Americans (12, 13). Abdominal obesity is associated with particularly deleterious health consequences, including a greater risk for diabetes, cardiovascular disease and dementia (14, 15). Recently, abdominal fat levels were found to be correlated with a greater hippocampal response to food images in adolescents (16). Abdominal obesity-associated inflammation is hypothesized to affect the brain and may promote overeating behavior through effects on brain pathways involved in the regulation of food intake (17).
To investigate the effects of viewing high-calorie food images on both the brain reward response as well as ratings of hunger and desire for food in obese individuals, we used a combined approach with functional magnetic resonance imaging (fMRI) and behavioral ratings of appetitive drive presented after each block of pictures of food and non-food items throughout the study. We hypothesized that high-calorie food cues would activate brain regions involved in motivation and reward processing (i.e., including the striatum, amygdala, insula and orbitofrontal cortex) and would result in increased hunger and desire for food in young, obese Hispanic females, a population at high-risk for continued weight gain and obesity (1). We also hypothesized that abdominal obesity would positively correlate with brain reward activation and desire for high-calorie food cues.
Materials and Methods
Participants
Thirteen young (23±2 years), obese (BMI 34±4kg/m2) Hispanic females (12 Mexican-American, 1 Central-American) with no history of eating disorders or medical illness participated in the study. Anthropometric measurements (see below) were not obtained on one research participant. Participants were all right-handed, non-smokers and were not on weight-loss diets or taking medications. Participants were asked to maintain their regular diet and physical activity levels during participation in the study. Participants were studied during the follicular phase of their menstrual cycle. The study was approved by the University of Southern California Institutional Review Board. All participants provided written informed consent before participation.
Experimental Protocol
Participants underwent fMRI scanning at the Dana & David Dornsife Cognitive Neuroscience Imaging Center at the University of Southern California in the morning after a 10-12 hour overnight fast. Standard anthropometric measurements were performed prior to scanning in twelve participants. Height was measured to the nearest 0.1 cm using a stadiometer and weight to the nearest 0.1 kg using a portable calibrated scale. BMI was calculated as the weight in kilograms divided by the square of the height in meters. Waist and hip circumference were measured to the nearest 0.1 cm. Waist circumference was measured at the midpoint between the iliac crest and lower costal margin in the midaxillary line. Hip circumference was measured by positioning the measuring tape around the maximum circumference of the buttocks. Waist-hip-ratio (WHR) was calculated by dividing waist circumference by hip circumference.
fMRI Paradigm
fMRI scanning was performed using a Siemens 3T Magneton Trio MRI system equipped with a quadrature radiofrequency head coil as subjects viewed images of food and non-food items. Twelve visual activation task runs (each run consists of 4 high-calorie food blocks, 4 low-calorie food blocks, and 4 non-food control blocks) were presented in a randomized block design. Each block consisted of 10 photographs presented in a random order. Each image was presented for 2 seconds with 1-second blank period between images. No image was presented more than once. At the end of each block, visual analogue scales (VAS) appeared and subjects clicked on a number (1 to 10) using a computer mouse-like device to rate their feelings of hunger and desire to eat sweet and savory foods. Length of time for each block was approximately 1 minute and the total time for imaging was approximately 30 minutes.
Imaging Parameters
Functional, blood oxygen level dependent (BOLD) signals were acquired with a single-shot gradient echo planar imaging (EPI) sequence. Thirty-two 4 mm thick slices that cover the whole brain were acquired using the following parameters: TR=2,000 msec, TE=25 msec, bandwidth=2520 Hz/pixel, flip angle=85°, field of view = 220×220 mm, matrix=64×64. A high resolution 3D Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence (TR=2530 ms; (TE=2.62 ms; bandwidth=240 Hz/pixel; flip angle= 9°; slice thickness=1mm; field of view=256×256 mm; matrix=256 × 256) was used to acquire images for multi-subject registration.
Visual stimuli
Visual stimuli were presented using a Matlab (Mathworks, Inc, Sherborn, MA) program that allows the pictures to be displayed on a screen within the scanner. Picture stimuli consisted of high-calorie, low-calorie, and non-food neutral pictures. Pictures were selected from various websites and from the International Affective Picture System (18) and were previously matched for visual appeal (19). High-calorie food pictures included items such as fries, cookies and ice cream. Examples of low-calorie food pictures included salads, broccoli and fruits. High-calorie food items were significantly greater than low-calorie food items in caloric density. The control stimuli consisted of non-food neutral pictures (e.g., building, basket). Visual analogue scales (VAS) to assess hunger and desire sweet and savory foods appeared after each block of pictures. Participants clicked on a number (1 to 10) using a computer mouse-like device to rate their feelings on a scale from 1 to 10 where 1 is “not at all” and 10 is” very much”. Prior studies have demonstrated good reproducibility and validity of these VAS scores for assessing subjective sensations of hunger and desire to eat specific types of food (20).
fMRI Analysis
Image processing was conducted using Functional MRI of the Brain (FMRIB) Software Library (www.fmrib.ox.ac.uk/fsl). Correction of fMRI data included spatial smoothing and temporal smoothing with a high pass filter and motion correction. Images were realigned to the anatomical images acquired within each scanning session and normalized to a template brain. A general linear model (GLM) was used to analyze the contribution of each experimental factor (high-calorie, low-calorie, and non-food images) to BOLD responses. Regional brain activation to food cues was determined by calculating a contrast of food minus non-food images, high minus non-food images, low minus non-food images, and high minus low calorie food images. Contrast maps were set at a standard threshold of Z>2.3, p<0.05, corrected for multiple comparisons for whole brain.
Region of Interest Analysis
Given the main interests of this paper on a) the effects of high-calorie food cues on brain reward center activation and food desire, and b) the association between abdominal fat levels and brain reward activation to high-calorie food cues, we performed correlation analyses confined to voxels within the brain reward regions (ie.striatum, amydala, insula, OFC, mPFC) that were found to be responsive to high calorie food images (vs. non-food images). Correlations were performed for the composite brain reward centers (ie.striatum, amydala, insula, OFC, mPFC) and for each individual region of interest. Functional regions of interest (ROIs) were definied by drawing 4 mm sphere around the peak coordinates within the region. Pearson’s correlations were used to test the relationships between brain reward activation to high-calorie food cues and a) ratings of hunger and food desire, b) waist circumference (as an indicator of abdominal obesity), and c) BMI. Correlation data was analyzed using SPSS (SPSS Inc. IL, USA). A p-value of <0.05 was considered significant.
Results
Anthropometric data
Participants ranged in BMI from 28 to 40 kg/m2 (M = 34, SD = 3.8 kg/m2); waist circumference ranged from 93.5 to 129.5 cm (M = 109.2, SD = 10.2 cm), hip circumference from 97.8 to 123.2 cm (M = 112.5, SD = 8.8 cm) and waist:hip ratio from 0.86 to 1.12 (M = 0.97, SD = 0.094). BMI was significantly correlated with waist circumference (r=0.841, p=0.001).
Neuroimaging data
Whole-brain analysis results
Food compared to Non-Food contrast (Figure 1)
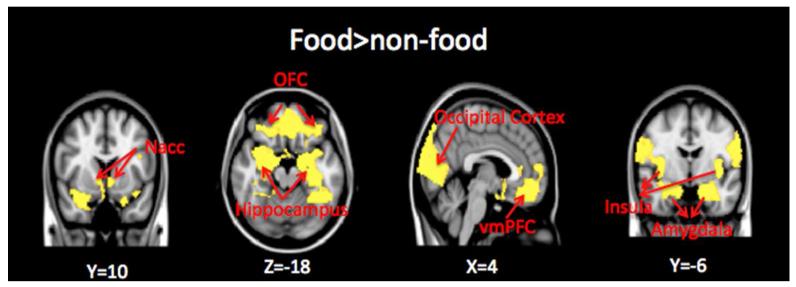
Figure 1.
Whole brain contrast map. Brain regions with greater response to food (high-calorie and low-calorie foods) compared to non-food images are shown in yellow (threshold of p<0.05, two-tailed, whole brain corrected). Nacc=nucleus accumbens; OFC=orbitofrontal cortex; vmPFC=ventral-medial prefrontal cortex. Montreal Neurological Institute (MNI) coordinates were used to define brain regions.
Brain regions in the bilateral orbitofrontal cortex (OFC), ventral-medial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC), bilateral insular, bilateral nucleus accumbens, bilateral amygdala, bilateral hippocampus and occipital cortex exhibited greater response to food vs. non-food images (Z>2.3, p<0.05 corrected for whole-brain multiple comparisons).
High-calorie food compared to Non-Food contrast (Figure 2, Table 1)
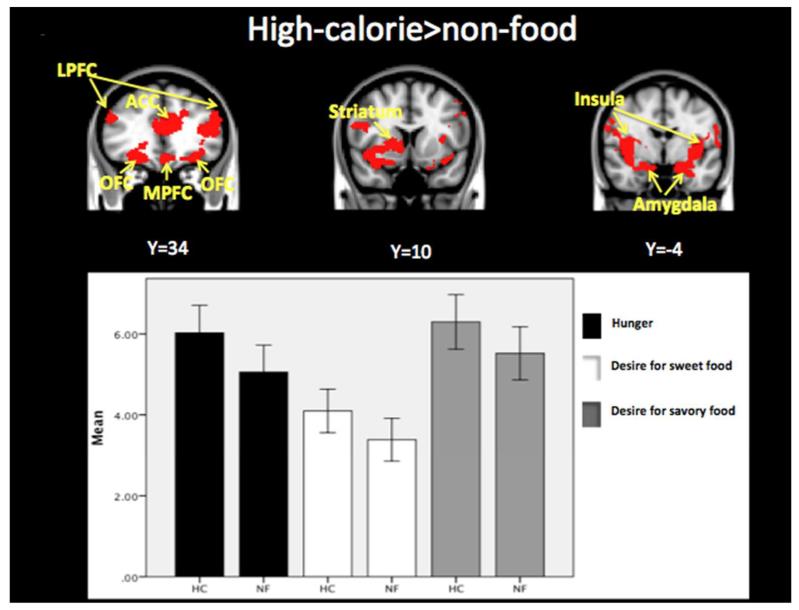
Figure 2.
Whole brain contrast map. Brain regions with greater response to high-calorie compared to non-food images are shown in red (threshold of p<0.05, two-tailed, whole brain corrected). High-calorie food images (HC) compared to non-food images (NF) resulted in significantly greater hunger (0.97 ± 0.23, p=0.001) and desire for sweet (0.71 ± 0.24, p=0.012) and savory (0.78 ± 0.25, p=0.009) foods. LPFC=lateral prefrontal cortex; ACC=anterior cingulate cortex; LPFC=lateral prefrontal cortex; OFC=orbitofrontal cortex. Montreal Neurological Institute (MNI) coordinates were used to define brain regions.
Table 1.
Brain regions with greater response to high-calorie foods compared to non-food images.
| Whole brain | x, y, z | Max Z | |
|---|---|---|---|
| High-calorie vs. Neutral pictures | L Amygdala/Hippocampus | −20, 2, −20 | 4.13 |
| R Amygdala/Hippocampus | 22, −2, −20 | 3.41 | |
| L Insular Cortex/Frontal Operculum Cortex/Central Opercular Cortex |
−40, −4, 2 | 3.88 | |
| R Insular Cortex/Frontal Operculum Cortex/Central Opercular Cortex |
40, −4, 0 | 4.57 | |
| L Orbital Frontal Cortex | −30, 34,−16 | 3.55 | |
| R Orbital Frontal Cortex | 26, 34,−10 | 3.45 | |
| L Occipital Cortex | −8, −96, 6 | 4.40 | |
| R Occipital Cortex | 14, −96,6 | 4.60 | |
| L Lateral Prefrontal Cortex | −44, −34, 14 | 4.41 | |
| R Lateral Prefrontal Cortex | 50, 10, 22 | 3.16 | |
| L Supramarginal Gyrus | −48, 46, 32 | 3.84 | |
| R Supramarginal Gyrus | 52, −32, 42 | 3.34 | |
| Medial Prefrontal Cortex | 0, 48, 04 | 3.10 | |
| Anterior Cingulate Cortex | 0, 36, 14 | 3.58 | |
| R Temporal Fusiform Cortex/Parahippocampal Gyrus/Hippocampus |
−36, −20, −24 | 3.89 | |
| R Putamen/Pallidum | 16, 8,0 | 3.09 | |
| R Nucleus Accumbens | 10, 10, −4 | 3.05 | |
| R Caudate | 10, 14, 8 | 2.87 |
Brain regions with a greater BOLD signal response to high-calorie food cues compared to non-food items included the bilateral insula, bilateral OFC, lateral PFC, ACC, bilateral amygdala and right striatum, regions implicated in motivation and reward processing (Z>2.3, p<0.05 corrected for whole-brain multiple comparisons).
Behavioral Data
3×3 ANOVA with “block type” (high-calorie, low-calorie, and neutral) and “question” (hunger, desire for sweet food, and desire for savory foods) as within-subject factors indicated a main effect of block type (F(2,24) =13.441, p=. 0001) and main effect of question (F(2,24) =9.98, p=.001).
Paired-t tests showed that hunger ratings were significantly greater after seeing pictures of high-calorie foods compared to non-food images (0.97 ± 0.23, p=0.001) (Figure 2). Desire for sweet (0.71 ± 0.24, p=0.012) and savory (0.78 ± 0.25, p=0.009) foods was also significantly greater in response to high-calorie foods compared to non-food visual cues. The pattern was the same although the response was attenuated after seeing pictures of low-calorie foods vs. non-food images: difference in hunger ratings (0.72 ± 0.27, p=0.02), desire for sweet (0.25 ± 0.26, p=0.05), and savory foods (0.47 ± 0.15, p=0.008).
High-calorie vs. Low-calorie contrast (Figure 3) and Low-calorie food vs. Non-food contrast
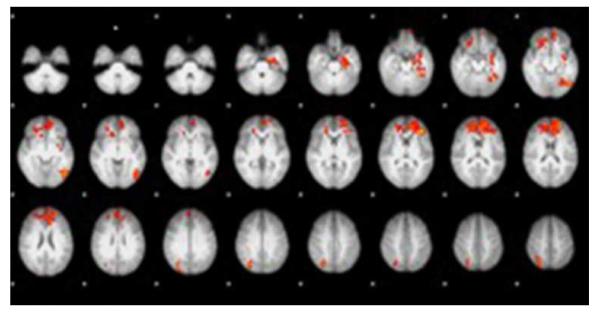
Figure 3.
High-calorie food images vs. Low-calorie food images whole brain contrast map. High-calorie food images resulted in greater activation in the anterior cingulate cortex, medial prefrontal cortex, orbitofrontal cortex, left hippocampus, left insula, and left lateral occipital cortex (Z>2.3, p<0.05 corrected for whole-brain multiple comparisons). Areas in red indicate greater activation to high-calorie compared to low-calorie food images.
Analysis contrasting high-calorie blocks with low-calorie blocks identified clusters including the ACC, medial PFC, OFC, left hippocampus, left insular and left lateral occipital cortex, and low-calorie compared to non-food blocks yielded activation in the lateral PFC, left supramarginal gyrus and occipital cortex (Z>2.3, p<0.05 corrected for whole-brain multiple comparisons).
Region of Interest (ROI) analysis results
Neurobehavioral Correlations
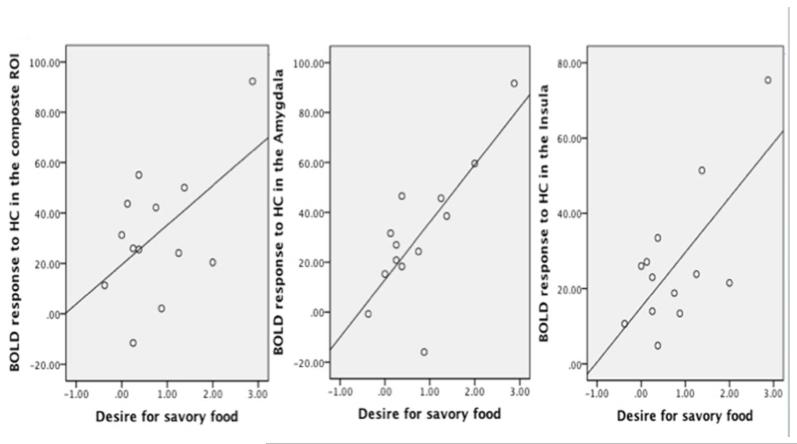
Brain response to high-calorie food images in reward regions (i.e., bilateral OFC, right striatum, bilateral insular, bilateral amygdala and mPFC) was positively correlated with the desire for savory foods (r=0.54, p=0.057) (Figure 4). Post-hoc exploratory analysis suggested this association was primarily driven by brain signal change in the bilateral amygdala (r=0.77) and bilateral insula (r=0.7). Correlation coefficients for ratings of hunger and food desire and all ROIs are presented in Table 2.
Figure 4.
Brain responses to high-calorie (HC) food images in A) composite reward regions of interest (ROI) (i.e., bilateral OFC, right striatum, bilateral insular, bilateral amygdala and mPFC) (r=0.54, p=0.057); B) amygdala (r=0.77, p=0.002); C) insula (r=0.70, p=0.008) were positively correlated with the desire for savory foods.
Table 2.
Correlation coefficients for ratings of hunger and food desire and all regions of interest (ROIs).
| Waist | BMI | Hunger | Desire for sweet foods |
Desire for savory foods |
||
|---|---|---|---|---|---|---|
| Composite ROI | Pearson Correlation | .621* | .412 | .150 | .438 | .539 |
| Sig. (2-tailed) | .031 | .162 | .625 | .134 | .057 | |
| N | 12 | 13 | 13 | 13 | 13 | |
| Striatum | Pearson Correlation | .613' | .293 | .159 | .475 | .450 |
| Sig. (2-tailed) | .034 | .331 | .605 | .101 | .123 | |
| N | 12 | 13 | 13 | 13 | 13 | |
| OFC | Pearson Correlation | .454 | .454 | .002 | .234 | .297 |
| Sig. (2-tailed) | .138 | .119 | .995 | .443 | .325 | |
| N | 12 | 13 | 13 | 13 | 13 | |
| Amygdala | Pearson Correlation | .642* | .529 | .414 | .566* | .766** |
| Sig. (2-tailed) | .024 | .063 | .159 | .044 | .002 | |
| N | 12 | 13 | 13 | 13 | 13 | |
| Insular | Pearson Correlation | .357 | .195 | .104 | .521 | .700** |
| Sig. (2-tailed) | .254 | .523 | .736 | .068 | .008 | |
| N | 12 | 13 | 13 | 13 | 13 | |
| MPFC | Pearson Correlation | .432 | .210 | -.002 | .129 | .225 |
| Sig. (2-tailed) | .161 | .490 | .996 | .674 | .459 | |
| N | 12 | 13 | 13 | 13 | 13 | |
Correlation is significant at the 0.0S level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Correlations between abdominal fat and neural response to high-calorie food cues
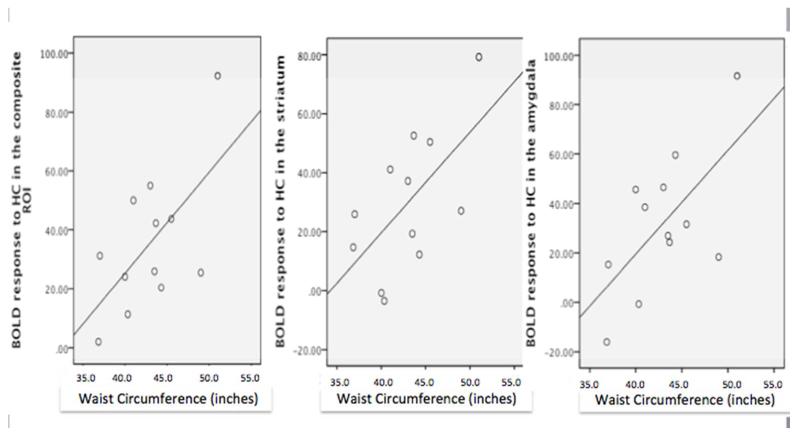
Brain response to high-calorie food cues in reward centers i.e., bilateral OFC, right striatum, bilateral insular, bilateral amygdala and mPFC) was significantly associated with waist circumference (r=0.621, p=0.031) (Figure 5). Post-hoc exploration of the basis for the significant association between waist circumference and activation in reward-related regions responsive to food cues suggest that the relationship was primarily driven by response in the striatum (r = 0.61) and amygdala (r = 0.64). Correlation coefficients for waist circumference and all ROIs are also presented in Table 2.
Figure 5.
Positive correlation between waist circumference (inches) and brain response to high-calorie (HC) food cues in A) composite reward regions of interest (ROI) (i.e., bilateral OFC, right striatum, bilateral insular, bilateral amygdala and mPFC) (r=0.62, p=0.03); B) striatum (r=0.61, p=0.03); C) amygdala (r=0.64, p=0.02).
BMI was not significantly correlated with the neural response to high-calorie food cues (Table 2). Correlation between the reward center (i.e., bilateral OFC, right striatum, bilateral insular, bilateral amygdala and mPFC) response to high-calorie food cues and waist circumference was slightly attenuated after controlling for BMI (p=0.063). Bilateral striatum was significantly correlated with waist circumference after controlling for BMI (p=0.007), but bilateral amygdala response was not (p=0.176).
Discussion
The present study was performed to examine the effects of visual food cues on brain activation, hunger and desire for food in young, obese Hispanic females. The results demonstrate that high-calorie food images (compared to non-food items) increase BOLD signal in brain regions implicated in the regulation of food reward and motivation. The pattern of brain responses to high-calorie food cues that we observed is consistent with previous studies (3, 5-9) and provides further support that these brain regions, including the striatum, PFC, OFC, insula and amygdala, are important mediators of feeding behavior. Moreover, we found that simply viewing pictures of high-calorie foods results in increased ratings of hunger and desire for sweet and savory foods. Taken together, these findings suggest a potential neurobiological mechanism to help explain how high-calorie food images may contribute to a reward-related appetitive drive in obese, young Hispanic females, a group disproportionately affected by obesity (1, 21).
Prior studies have shown that females have stronger reactivity to food-related stimuli than males (22-24) and obese compared to lean individuals have greater brain reward responses to appetizing visual food cues (6, 8, 9). At least two prior studies reported a significant positive association between BMI and neural responses to food cues in reward and motivation-related regions (6, 25). While the association was not significant in our relatively small sample, it is worth noting that the correlation was in the positive direction, and of an implied effect-size that is consistent with those prior findings (r (13) = 0.412, p = .162). Moreover, in the present study, we found that waist circumference, independent of BMI, positively correlated with the striatal response to high-calorie food images suggesting a link between abdominal fat accumulation and heightened brain reward signaling. It is notable that a recent fMRI study found a positive relationship between waist circumference and the hippocampal response to visual food cues in adolescents (16). In contrast, a separate study found that in older adults abdominal fat levels were correlated with reduced activation in the nucleus accumbens and caudate in response to a sucrose stimulus (26). Whether the finding observed in older adults is related to age-dependent factors or the specific food-related stimulus (ie. sucrose taste) will require additional study. Nevertheless, the present study supports previous findings suggesting an association between abdominal fat accumulation and the central response to food cues and is the first to demonstrate that abdominal fat levels, independent of BMI, are predictive of striatal responsivity to high-calorie food cues.
It is worth noting that recent literature has shown a link between visceral abdominal obesity and alterations in brain structure and function (27-30). Studies have shown that abdominal obesity is associated with decreased total brain volume (27), decreased hippocampal volume and increased white matter hyperintensities (28) as well as cognitive impairment (29) (30) suggesting that the vascular and metabolic changes related to visceral fat may affect brain structure and function.
Although potential mechanisms for the association between visceral abdominal fat and greater brain reward responses are speculative, they could include visceral fat associated pro-inflammatory changes, alterations in the expression of adipose-tissue derived hormones, and insulin resistance (15, 31). Visceral fat is characterized by an enhanced rate of lipolysis and an increased release of free fatty acids and proinflammatory adipokines, which potentiate peripheral insulin resistance. Elevated levels of circulating free fatty acids also result in an excess lipid flux to the brain, which can initiate a central proinflammatory response and disrupt hypothalamic energy signaling pathways (32). Given the nature of this correlation finding, it is unclear whether a greater brain reward response to food cues leads to excess food intake and resultant excess abdominal adiposity or whether greater levels of abdominal fat result in changes in the brain reward response to food cues. Longitudinal studies will be necessary investigate the causal relationship between brain responses to food images and abdominal fat accumulation. Our results are consistent with either of these possibilities.
This study was performed in the morning hours while participants were in a fasted state, a time when reward reactivity is most likely to be engaged (4, 22, 24, 33-35). Consistent with prior reports, we found greater responsiveness in limbic and prefrontal regions to high-calorie compared to low-calorie food images (5, 24, 35). The heightened neural response to high-calorie compared to low-calorie food cues that we observed is in line with the behavioral ratings in our participants, which showed a more robust appetitive response to high-calorie foods. Interestingly, we found that the amygdala and insula responses to high-calorie food cues were predictive of the desire for savory foods. The insula is the primary gustatory complex and involved in interoceptive processing, and it is thought to play a key role in addictive behavior (36). The amygdala helps regulate the emotional responses to food and mediates stimulus-reward learning (37). Our findings provide further evidence of the importance of the amygdala and insula on regulation of the brain and appetitive responses to high-calorie food cues.
This is the first study to investigate brain responses to food cues in the Hispanic population, a group with a high prevalence of obesity and type 2 diabetes (21, 38). Abdominal fat distribution is greater in Hispanics compared to Non-Hispanic Whites and African Americans (12, 13), and greater levels of visceral abdominal fat may play a role in the increased risk for type 2 diabetes in the Hispanic population (12). Recent data shows that Mexican-American women are more affected by obesity and have had a greater rise in obesity prevalence over the last 12 years than Mexican-American men and Non-Hispanic White men and women (21). Environmental factors likely contribute to these disparities. For example, Mexican-American women were found to have lower physical activity levels than Non-Hispanic White women (39), and studies have shown an association of acculturation with poor dietary quality and obesity in Hispanic-Americans (40). Future studies will be necessary to determine whether there are ethnic differences in brain responses to food stimuli and whether ethnic differences vary by gender.
Limitations
The sample size is small, however the imaging findings reported are significant after correcting for search space. The study was limited to young, obese, Hispanic females limiting the generalizability of the findings. Future studies should include a normal-weighted control group in order to further establish the association between heightened brain responses to high-calorie food cues and overeating. Waist circumference measurements were used to estimate abdominal fat levels, and visceral and subcutaneous fat depots were not directly measured. Blood sampling was not performed thus we could not assess whether higher free fatty acid levels and/or the infammatory nature of visceral fat are associated with greater brain reward activation to food cues. Energy balance prior to imaging was not controlled although participants were asked to maintain their regular diet and physical activity levels during participation in the study.
Conclusions
These data suggest that viewing high-calorie food images activates brain reward regions and increases hunger and desire for food, and that higher levels of abdominal fat are predictive of greater brain reward reactivity in response to high-calorie food cues. These findings suggest that the current food environment, which is inundated with images of high-calorie foods, influences brain pathways that control eating behavior and may promote food intake in obese, young Hispanic women, a group at high-risk for continued weight gain and obesity (1, 21).
Acknowledgments
We thank Jiancheng Zhuang, PhD for assitance with MRI operations, Dongju Seo, PhD from Yale University for providing the pictures used in this study, and the volunteers who participated in the study.
The study was funded by the National Center on Minority Health and Health Disparities (P60 MD002254-01).
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Gordon-Larsen P, The NS, Adair LS. Longitudinal trends in obesity in the United States from adolescence to the third decade of life. Obesity (Silver Spring) 2010;18(9):1801–4. doi: 10.1038/oby.2009.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33(6):653–61. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. Eur J Neurosci. 2004;20(5):1411–8. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- 5.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high-versus low-calorie foods. Neuroimage. 2003;19(4):1381–94. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 6.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–21. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15(10):1602–8. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- 8.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Scharmuller W, Ubel S, Ebner F, Schienle A. Appetite regulation during food cue exposure: A comparison of normal-weight and obese women. Neurosci Lett. 2012 doi: 10.1016/j.neulet.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 10.Ferriday D, Brunstrom JM. ‘I just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. Int J Obes (Lond) 2011;35(1):142–9. doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- 11.Tetley A, Brunstrom J, Griffiths P. Individual differences in food-cue reactivity. The role of BMI and everyday portion-size selections. Appetite. 2009;52(3):614–20. doi: 10.1016/j.appet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Hasson RE, Adam TC, Davis JN, Weigensberg MJ, Ventura EE, Lane CJ, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab. 2010;95(8):4048–51. doi: 10.1210/jc.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maligie M, Crume T, Scherzinger A, Stamm E, Dabelea D. Adiposity, Fat Patterning, and the Metabolic Syndrome among Diverse Youth: The EPOCH Study. J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 15.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl 1):16S–9S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 16.Wallner-Liebmann S, Koschutnig K, Reishofer G, Sorantin E, Blaschitz B, Kruschitz R, et al. Insulin and hippocampus activation in response to images of high-calorie food in normal weight and obese adolescents. Obesity (Silver Spring) 2010;18(8):1552–7. doi: 10.1038/oby.2010.26. [DOI] [PubMed] [Google Scholar]
- 17.Heber D, Carpenter CL. Addictive genes and the relationship to obesity and inflammation. Mol Neurobiol. 2011;44(2):160–5. doi: 10.1007/s12035-011-8180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang PJBM, Cuthbert BN, editors. International affective picture system (IAPS): Technical manual and affective ratings Gainesville. University of Florida, Center for Research in Psychophysiology; 2001. [Google Scholar]
- 19.Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, et al. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 2011;121(10):4161–9. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 21.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 22.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169(1):111–9. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99(4):538–43. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res. 2010;1350:159–66. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Grosshans M, Vollmert C, Vollstadt-Klein S, Tost H, Leber S, Bach P, et al. Association of leptin with food cue-induced activation in human reward pathways. Arch Gen Psychiatry. 2012;69(5):529–37. doi: 10.1001/archgenpsychiatry.2011.1586. [DOI] [PubMed] [Google Scholar]
- 26.Green E, Jacobson A, Haase L, Murphy C. Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Res. 2011;1386:109–17. doi: 10.1016/j.brainres.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debette S, Beiser A, Hoffmann U, Decarli C, O’Donnell CJ, Massaro JM, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68(2):136–44. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62(10):1545–8. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 29.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4(2):111–6. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 30.Kanaya AM, Lindquist K, Harris TB, Launer L, Rosano C, Satterfield S, et al. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch Neurol. 2009;66(3):329–35. doi: 10.1001/archneurol.2008.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan KK, Woods SC, Seeley RJ. Central nervous system mechanisms linking the consumption of palatable high-fat diets to the defense of greater adiposity. Cell Metab. 2012;15(2):137–49. doi: 10.1016/j.cmet.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 34.Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30(8):1625–35. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 35.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198(1):149–58. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 36.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 38.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 39.Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988-1994. JAMA. 1998;280(4):356–62. doi: 10.1001/jama.280.4.356. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Escamilla R. Acculturation, nutrition, and health disparities in Latinos. Am J Clin Nutr. 2011;93(5):1163S–7S. doi: 10.3945/ajcn.110.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]