Abstract
Background
Due to the aging population in low- and middle-income countries, cerebrovascular disease is expected to remain a leading cause of death. Little has been published about stroke in Peru.
Aims
We conducted a retrospective cohort study of hospitalized stroke patients at a referral center hospital in Lima, Peru to explore factors associated with functional outcome among stroke patients.
Methods
We identified 579 patients hospitalized for ischemic stroke or intracerebral hemorrhage stroke at the National Institute of Neurologic Sciences in Lima, Peru in 2008 and 2009. A favorable outcome was defined as a modified Rankin score of ≤2 at discharge.
Results
The mean age was 63.3 years; 75.6% had ischemic stroke; the average length of stay was 17.3 days. At hospital discharge, 231 (39.9%) had a favorable outcome. The overall mortality rate was 5.2%. In multivariate models, the likelihood of having a favorable outcome decreased linearly with increasing age (p=0.02) and increasing NIHSS (p=0.02). Favorable outcome was also associated with male gender (relative risk [RR]=1.2; 95% confidence interval [CI]: 1.0, 1.5) and divorced status (RR=1.3, 95% CI: 1.1, 1.7). Patients on Salud Integral de Salud (public assistance-type insurance, SIS) (RR=0.7, 95% CI: 0.5,1.0) were also less likely to have a favorable outcome.
Conclusions
Favorable outcome after stroke was independently associated with younger age, lower NIHSS score, male gender, being divorced, and not being on SIS insurance. These findings suggest further study of worse functional outcomes in patients with SIS insurance and confirm the importance of risk adjustment for age, stroke severity (NIHSS) and other socioeconomic factors in outcomes studies. Future studies should preferentially assess outcome at 30-days and 6-months to provide more reliable comparisons and allow additional study of Peruvian end-of-life decision-making and care.
INTRODUCTION
The World Health Organization estimates non-communicable diseases will comprise three-quarters of all deaths worldwide by 2030.1 Due to the aging population in low- and middle-income countries, cerebrovascular disease is expected to remain a leading cause of death. A recent study of population-based stroke incidence studies found an alarming increase in stroke incidence over the past four decades in the developing world: while stroke incidence decreased by 42% in high-income countries, there was a 100% increase in incidence in low and middle-income countries.2
Little has been published about stroke in Peru. One population-based study among 1,933 residents 65 years and older living in Peru reported a prevalence of 6.8% of self-reported stroke in urban sites and 2.7% in rural sites.3 The same population-based study reported that stroke was the most common cause of death, accounting for 28.6% of death among residents in urban sites and 13.7% in rural sites.3 A community screening for stroke among residents living in Cuzco City, a Peruvian Andean town 3,380 meters above sea level, reported a crude stroke prevalence of 0.647% among 3,246 people 15 years and older, corresponding to 5.74 per 1000 adjusted to the World Health Organization population,4 which is within the range of estimates (1.74 to 6.51 per 1000) reported by other community-based studies in other South American countries.5
Although mortality rates due to stroke have declined steadily over the last three decades throughout the Americas, decreases have been less dramatic in Latin America, where mortality rates remain two to four times higher than in the U.S. or Canada.6 These differences may partially reflect decreased public awareness of stroke, limited health service capacity to offer acute interventions or affordable treatments, 6 or underlying fundamental differences in the populations.
In a first step towards improving stroke outcomes in Peru, our objective in this study was to identify factors associated with good functional outcome. Such factors might identify targets for interventions, and may serve as risk adjustment variables in future studies of comparative effectiveness.
METHODS
We conducted a retrospective cohort study in which we identified patients hospitalized for evaluation and treatment of ischemic stroke or intracerebral hemorrhage (ICH) at the National Institute of Neurologic Sciences (ICN) in Lima, Peru – a national referral center for patients with neurologic diseases. In Lima, patients may present with stroke at 14 other hospitals: 3 in the Social Security (public health for indigent patients) system, 8 in the network of Ministry of Health hospitals, and 3 in the military system. Approximately 60% of patients admitted to the ICN are referred from other hospitals. We included all patients who were admitted between January 1, 2008 and December 31, 2009. Those with evidence of stroke, such as sensory deficit, motor and speech impairment, or other signs of stroke underwent brain imaging and were diagnosed with ischemic stroke or ICH.
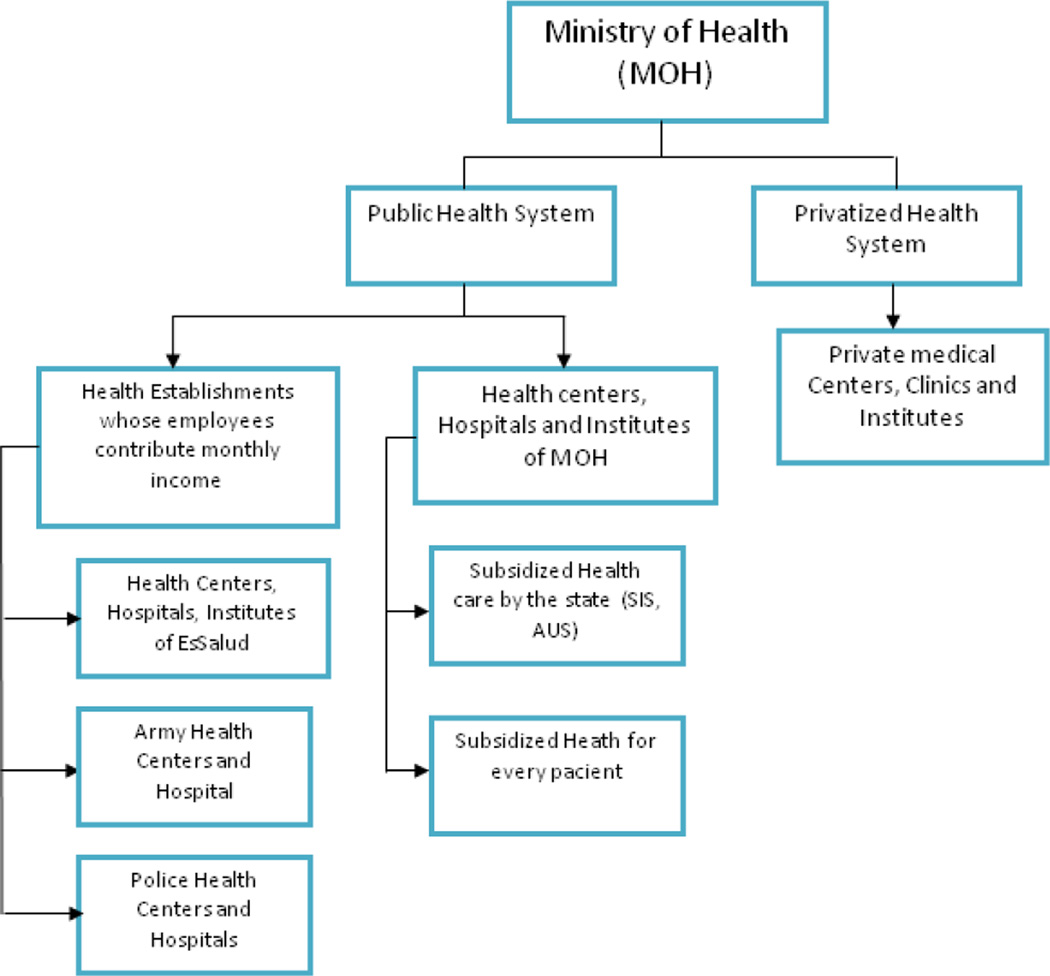
The ICN is managed by the Ministry of Health (MOH) (Figure 1) and serves patients from hospitals and clinics throughout Peru regardless of insurance status. The Department of Cerebrovascular Diseases within the ICN offers specialized care for any stroke patient and typically serves patients who are transferred from other hospitals. Transfer to the ICN is affected by distance and travel to Lima; patients traveling from the coast or Andean highlands travel between 2 to 16 hours while those from the jungle require several days of travel. Air transfer, however, is not a practical means for most patients. Once admitted, patients with stroke are hospitalized according to level of care required: in the intensive care unit for severe patients, in the stroke unit for acute cases within 48 hours of stroke onset or patients with mild to moderate severity, or in the inpatient wards for stroke patients arriving after 48 hours with mild to moderate severity. We abstracted information from medical records including risk factors, gender, age, NIH Stroke Scale (NIHSS) scores, length of stay, hospital ward, status and functional outcome at discharge. We also collected information on whether patients receive coverage from Salud Integral de Salud (SIS), a governmental program that provides health insurance to indigent people, similar to Medicaid in the U.S. All research procedures were approved by the Humans Subjects Committee of the National Institute of Neurological Sciences in Lima, Peru.
Figure 1.
Organization of the Health System in Peru
We defined functional outcome based on the modified Rankin scale (mRS) assessed at discharge and dichotomized between 2 and 3. A favorable outcome was defined as a mRS score of less than or equal to 2, and an unfavorable outcome, 3 or greater. We examined frequencies and means (SD) to characterize our patient population. For pairwise comparisons, we used Pearson’s chi-square test and Fisher’s exact test for categorical variables, and Student’s t-test for continuous variables. Because the outcome was common, we used generalized linear models with a log link, robust standard errors, and a Poisson distribution for the variance to estimate relative risk (RR) and 95% confidence intervals (CI). We used multivariate models and selected variables in a backward stepwise procedure to identify independent predictors of functional outcome at discharge. Predictors of interest were initially placed into the full model and removed sequentially if corresponding P-value of the Wald test exceeded 0.10. All tests were two-sided, and statistical significance was defined as P-value < 0.05. All analyses were conducted in Stata 11.1 (College Station, TX).
RESULTS
We identified 579 patients in 2008–2009, 321 (55.4%) of whom were men. At baseline, the average age of patients was 63.3 years (SD = 16.2). The majority of patients were diagnosed with ischemic stroke (75.6%), and over half (57.8%) were admitted to the cerebrovascular unit. The average NIHSS was 10.1 (SD = 7.1), and differed significantly between ischemic strokes and ICH (9.5 vs. 12.0, P = 0.0003). The average length of stay was 17.3 days, with four patients remaining in the hospital more than 100 days. At discharge, 231 (39.9%) had a favorable outcome. Of the 349 with an unfavorable outcome, 30 patients died in the hospital. The overall in-hospital mortality rate among all patients enrolled in this study was 5.2%; 3.2% of patients with ischemic stroke died whereas 11.4% of patients with hemorrhagic stroke died in the hospital (p<0.001).
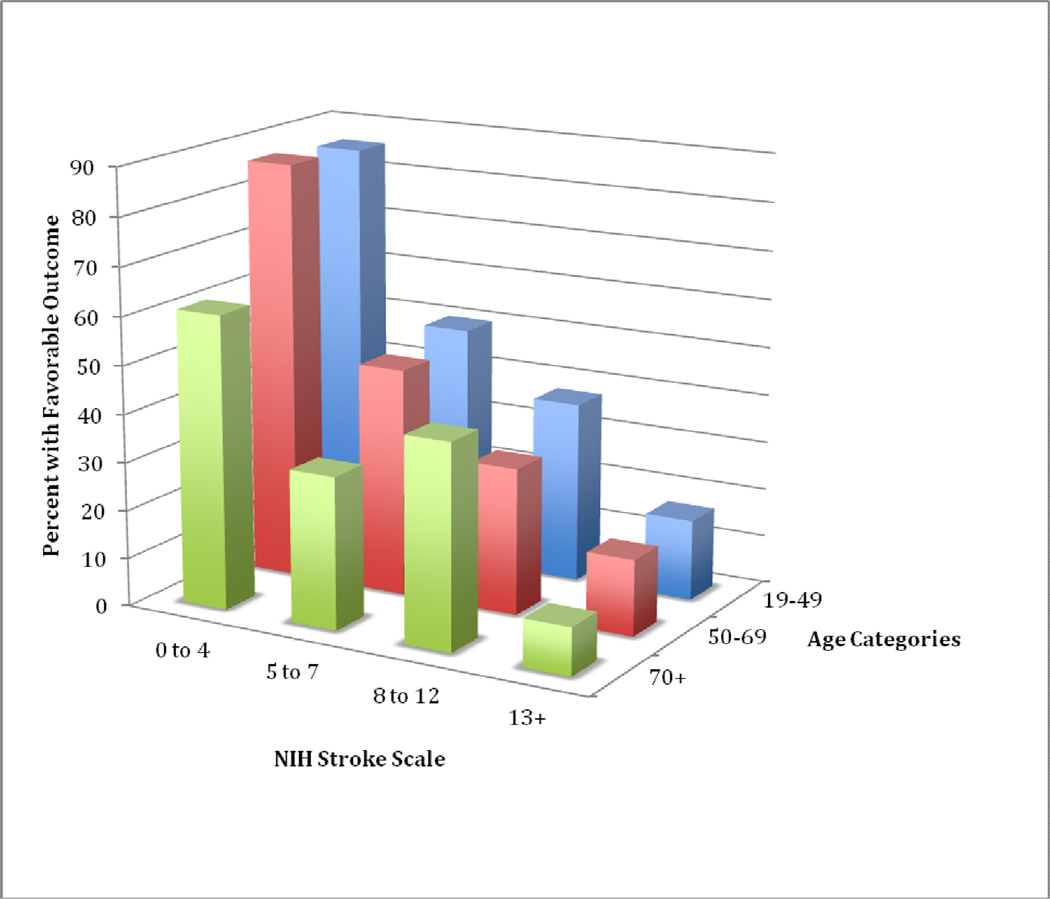
Compared to patients with unfavorable outcomes, patients with favorable outcomes at discharge were significantly younger, had shorter lengths of hospitalization, had lower NIHSS scores, were more likely to be dyslipidemic, and had a higher prevalence of alcohol use (Table 1). Percent of patients with favorable outcome decreased according to increasing age and increasing NIHSS severity (Figure 2).
Table 1.
Characteristics of Stroke Outcome at Discharge Among 579 Patients As Defined By Modified Rankin Scale, Lima, Peru, 2008–2009
| Favorable Outcome mRS ≤ 2 (n=231) |
Unfavorable Outcome mRS > 2 (n=348) |
||||
|---|---|---|---|---|---|
| Characteristic | N* | (%) | N* | (%) | P- value** |
| Male | 137 | (59.3) | 184 | (52.8) | 0.09 |
| Stroke Subtype | 0.6 | ||||
| Ischemic | 176 | (76.5) | 260 | (74.9) | |
| Intracerebral Hemorrhage | 54 | (23.4) | 87 | (25.1) | |
| Age in years | <0.001 | ||||
| 19–49 | 56 | (24.2) | 49 | (14.1) | |
| 50–69 | 107 | (46.3) | 140 | (40.2) | |
| 70+ | 68 | (29.4) | 159 | (45.7) | |
| Marital Status | 0.6 | ||||
| Married/Cohabitating | 139 | (60.2) | 222 | (63.8) | |
| Single | 46 | (19.9) | 60 | (17.2) | |
| Separated/Divorced | 46 | (19.9) | 66 | (19.0) | |
| Hospital Ward | 0.8 | ||||
| Other (Surgery, ICU, other) | 99 | (42.9) | 145 | (41.7) | |
| Cerebrovascular | 132 | (57.1) | 203 | (58.3) | |
| Medicaid-type insurance | 14 | (6.1) | 34 | (9.8) | 0.11 |
| Time from onset to hospitalization | 0.4 | ||||
| < 21 hours | 50 | (21.6) | 92 | (26.4) | |
| 21–47 hours | 44 | (19.1) | 74 | (21.3) | |
| 48–119 hours | 73 | (31.6) | 91 | (26.2) | |
| 120+ hours | 64 | (27.7) | 91 | (26.2) | |
| Length of stay in days | 0.01 | ||||
| 1–9 | 64 | (27.4) | 80 | (23.0) | |
| 10–12 | 54 | (23.4) | 70 | (20.1) | |
| 13–19 | 67 | (29.0) | 87 | (25.0) | |
| 20+ | 46 | (19.9) | 111 | (31.9) | |
| NIHSS on admission | <0.001 | ||||
| 0–4 | 83 | (35.9) | 20 | (5.8) | |
| 5–7 | 74 | (32.0) | 100 | (28.7) | |
| 8–12 | 54 | (23.4) | 92 | (26.4) | |
| 13+ | 20 | (8.7) | 136 | (39.1) | |
| Previous Stroke | 19 | (8.2) | 42 | (12.1) | 0.2 |
| Hypertension | 168 | (72.7) | 264 | (75.9) | 0.4 |
| Diabetes | 44 | (19.2) | 66 | (19.0) | 0.9 |
| Dyslipidemia | 70 | (30.3) | 79 | (22.7) | 0.04 |
| Obesity | 16 | (6.9) | 20 | (5.8) | 0.6 |
| Hyperuricemia | 1 | (0.4) | 4 | (1.2) | 0.7 |
| Metabolic Syndrome | 3 | (1.3) | 2 | (0.6) | 0.7 |
| Alcohol Use | 36 | (15.6) | 35 | (10.1) | 0.047 |
| Sedentary Lifestyle | 5 | (2.2) | 5 | (1.4) | 0.5 |
| Tobacco Use | 21 | (9.1) | 29 | (8.3) | 0.8 |
Numbers may not add to column total due to missing values.
Pearson chi-square test or Fishers' exact test (small numbers) for nominal categorical variables; Student's t-test for continuous variables; score test for trend for ordinal and interval variables
Figure 2.
Percent With Favorable Outcome By Age and NIHSS
In our final multivariate model, independent predictors of favorable outcome included male gender, younger age, marital status, insurance, and lower NIHSS at time of admission (Table 2). As expected, increasing age and NIHSS score were associated with a reduced likelihood of favorable outcome. Even after controlling for these predictors of outcome, men were 22% more likely than women, divorced were 30% more likely than married, and uninsured were 30% more likely than SIS-insured to have a favorable outcome. Stroke subtype, length of stay, and risk factors such as dyslipidemia and alcohol use were not significant independent predictors of functional outcome in the fully adjusted model.
Table 2.
Independent Predictors of Favorable Outcome At Discharge Among 579 Stroke Patients in Lima, Peru, 2008–2009
| Unadjusted |
Adjusted* |
|||||
|---|---|---|---|---|---|---|
| Characteristic | RR | 95% CI | Test of Trend |
RR | 95% CI | Test of Trend |
| Male | 1.2 | (1.0, 1.4) | 1.2 | (1.0, 1.5) | ||
| Age | ||||||
| 19–49 | 1.0 | Reference | <0.001 | 1.0 | Reference | 0.02 |
| 50–69 | 0.8 | (0.6, 1.0) | 0.9 | (0.7, 1.1) | ||
| 70+ | 0.6 | (0.4, 0.7) | 0.7 | (0.5, 0.9) | ||
| Marital Status | ||||||
| Cohabitating/Married | 1.0 | Reference | 1.0 | Reference | ||
| Single | 1.2 | (0.9, 1.5) | 1.0 | (0.8, 1.2) | ||
| Divorced | 1.1 | (0.8, 1.4) | 1.3 | (1.1, 1.7) | ||
| Insurance | 0.7 | (0.5, 1.1) | 0.7 | (0.5, 1.0) | ||
| NIHSS | ||||||
| 0–4 | 1.0 | Reference | <0.001 | 1.0 | Reference | <0.001 |
| 5–7 | 0.5 | (0.4, 0.6) | 0.5 | (0.4, 0.6) | ||
| 8–12 | 0.5 | (0.4, 0.6) | 0.5 | (0.4, 0.6) | ||
| 13+ | 0.2 | (0.1, 0.2) | 0.2 | (0.1, 0.3) | ||
Adjusted for all other variables
DISCUSSION
In this first English-language report on hospital discharge outcomes in a referral stroke population at the National Institute of Neurologic Sciences in Lima, Peru, increasing age and increased stroke severity at arrival to the hospital were associated with decreasing likelihood of favorable outcome, as expected. We also observed a higher likelihood of favorable outcome in men, in those who were divorced, and those without SIS – a Medicaid-type insurance. In multivariate models that adjusted for severity and other factors, there were no significant associations between favorable outcome and stroke subtype or with hospital ward where patients were treated.
The overall in-hospital mortality rate in our study was 5.2%; These mortality rates are slightly lower than what has been reported in a prior Peruvian study. A prospective cohort study of 1,517 stroke patients hospitalized between April 1987 and December 1998 in the Neurologic ward of the Guillermo Almenara Hospital in Peru reported a mortality rate of 5.4% for patients with ischemic stroke and 24.1% for patients with hemorrhagic stroke. 7 Also higher than our mortality rates, the Hospital Nacional Cayetano Heredia documented mortality of patients hospitalized for ICH or ischemic stroke of 19.8%.8
The lower in-hospital mortality rate that we observed in our hospital based cohort may reflect a selection bias where less severe strokes are preferentially referred to ICN. In a population-based study in Chile in which all patients with stroke were identified within the community, 71% were admitted to the hospital admission. 9 About half of those patients not admitted to the hospital were identified through death certificates. Thus, the low mortality rate in our study may be attributed, in part, to a similarly high percentage of people with severe strokes dying in the community or other hospitals prior to referral to the ICN.
Another possible explanation for the low mortality rate is the lack of “do not resuscitation” (DNR) orders or “withdrawal of life support” (WOLS) in Peru. In the US, DNAR, WOLS, and palliative measures are commonly instituted when physicians prognosticate a poor outcome, thereby enabling a “self-fulfilling prophecy” of a poor outcome. 10, 11 WOLS and DNAR orders account for up to 77% of in-hospital ICH deaths in the U.S. 12–14 No formal studies have been published on the practice of DNR or WOLS in Peru, but patients with severe neurologic sequelae of stroke are typically stabilized and treated before being discharged, with many potentially dying at home thereafter
Similar to stroke outcome studies conducted in other parts of the world, increasing stroke severity and older age were strongly associated with unfavorable outcome. 15–17 In data from the US-based TOAST randomized trial in ischemic stroke patients, approximately 88% of stroke patients with an initial NIHSS of 0–3 had a favorable outcome (“excellent” or “good” in their terminology) at 7 days, which is similar to our rate of favorable outcome at discharge (81%) in patients with NIHSS scores between 0–4 (calculated from data in Table 1) 15. For patients presenting with an initial NIHSS score > 13, our estimate of 13% favorable outcome is also similar to estimates from the TOAST data of approximately 9% favorable outcome in patients with NIHSS > 16 and approximately 20% for those with NIHSS > 11. In addition, age was an important predictor of outcome, similar to the results from re-analysis of the NINDS tPA trial data and from stroke prognostic models developed in Germany.16, 17 In our study, men were 20% more likely to have a favorable outcome than women. These findings are consistent with reports of more favorable outcome in men by other stroke outcome studies. 18–20 The role of risk factors unique to women such as estrogen, hormone replacement therapy, pregnancy, or systematic delays in recognition have all been considered as possible explanations.19 Although this gender-specific stroke outcome has been recently recognized, the biological and social explanations for the gender difference in disability after stroke have not been fully elucidated.
We observed a decreased likelihood of favorable outcome for patients with SIS insurance. Having coverage by SIS in this study is a surrogate for lower socioeconomic status since the Peruvian government provides health insurance to indigent people – a program similar to Medicaid in the U.S. The observed association between SIS and decreased likelihood of favorable outcome is likely driven by the influence of low socioeconomic status on stroke outcomes, which has been observed worldwide and was recently reviewed.21 We also observed an interesting association in which divorcees were more likely to have a favorable outcome compared to their married counterparts. This association could be driven by unmeasured confounding that warrants more research, or could represent a spurious association in our sample.
Several limitations are notable. The patient sample was referral hospital-based rather than population-based and is therefore not representative of the full spectrum of stroke and stroke outcomes in Lima, Peru. Survival bias may have affected our mortality rate although it is unclear in which direction this bias may have been. Those with very severe stroke may have died before reaching the hospital, thereby decreasing our in-hospital mortality rate. In Lima, patients may present with stroke at 14 other hospitals: 3 in the Social Security (public health for indigent patients) system, 8 in the network of Ministry of Health hospitals, and 3 in the military system. Approximately 60% of patients admitted to the ICN are referred from other hospitals, leading to the above described selection bias.” Patients admitted to one of the 14 hospitals with a more severe stroke may have been referred preferentially to our hospital, thereby potentially increasing the mortality rate. Given that we observed a rather low mortality rate in our study, we expect that many patients died before reaching our hospital.
Furthermore, the selection of less severe patients into this study may overestimate the prevalence of favorable outcome in a more representative stroke sample from Lima. Despite this bias, stroke severity remained significantly associated with unfavorable outcome, and our results likely represent a conservative estimate of this association. The lack of DNAR and WOLS orders in Peru may also result in many patients being discharged to home with the anticipation of death occurring in their homes. If so, an improved measure of mortality would be the mortality rate at 30 days after stroke; these data were not collected for this study.
CONCLUSIONS
In conclusion, favorable outcome of stroke was independently associated with younger age, lower NIHSS score at time of admission, male gender, being divorced, and not being on SIS insurance. We also observed an in-hospital mortality rate somewhat lower than what has been reported elsewhere in Peru, with referral bias and cultural factors related to non-use of DNAR orders or WOLS as possible explanations. These findings suggest further work should be done to investigate the cause, and propose solutions for the worse functional outcomes in patients with SIS insurance. Also, in future studies of comparative effectiveness of interventions for stroke in Peru, our findings confirm the importance of risk adjustment for age, stroke severity (NIHSS) and other socioeconomic factors. Future studies should preferentially assess outcome at 30-days and 6-months to provide more reliable comparisons across different studies and improved understanding of the predictors of long-term stroke survival in Peru and allow additional study of the cultural variations of medical practice surrounding end-of-life decision-making and care.
ACKNOWLEDGMENTS
We are grateful to neurology residents Rosa Ecos and Frank Solis for their support with data collection.
Grant Support:
Supported by: NIH Fogarty International Center grant RO1NS55627 to Joseph R. Zunt, University of Washington and by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental & Craniofacial Research, National Institute On Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases Health, and NIH Office of Women’s Health and Research through the International Clinical Research Fellows Program at Vanderbilt University (R24 TW007988).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. Dr. Silvia Montano is an employee of the U.S. Government. This work was prepared as part of her official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
CONFLICTS OF INTEREST
None of the authors have any conflicts of interest to disclose.
REFERENCES
- 1.World Health Organization. World Health Statistics. Geneva, Switzerland: World Health Organization; 2005. Part Ii. Highlighted Topics; pp. 34–45. [Google Scholar]
- 2.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide Stroke Incidence and Early Case Fatality Reported in 56 Population-Based Studies: A Systematic Review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 3.Ferri CP, Schoenborn C, Kalra L, Acosta D, Guerra M, Huang Y, Jacob KS, Llibre Rodriguez JJ, Salas A, Sosa AL, Williams JD, Liu Z, Moriyama T, Valhuerdi A, Prince MJ. Prevalence of Stroke and Related Burden among Older People Living in Latin America, India and China. J Neurol Neurosurg Psychiatry. 2011;82:1074–1082. doi: 10.1136/jnnp.2010.234153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaillard AS, Hommel M, Mazetti P. Prevalence of Stroke at High Altitude (3380 M) in Cuzco, a Town of Peru. A Population-Based Study. Stroke. 1995;26:562–568. doi: 10.1161/01.str.26.4.562. [DOI] [PubMed] [Google Scholar]
- 5.Saposnik G, Del Brutto OH. Stroke in South America: A Systematic Review of Incidence, Prevalence, and Stroke Subtypes. Stroke; a journal of cerebral circulation. 2003;34:2103–2107. doi: 10.1161/01.STR.0000088063.74250.DB. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez T, Malvezzi M, Chatenoud L, Bosetti C, Levi F, Negri E, La Vecchia C. Trends in Mortality from Coronary Heart and Cerebrovascular Diseases in the Americas: 1970–2000. Heart. 2006;92:453–460. doi: 10.1136/hrt.2004.059295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deza L, Aldave R, Barrera J. Historia Natural De La Enfermedad Vascular Cerebral En El Peru. Estudio Intrahospitalario De 1517 Pacientes. Revista de Neuro-Psiquiatria del Peru. 2001;64:105–132. [Google Scholar]
- 8.Castaneda-Guarderas A, Beltran-Ale G, Casma-Bustamante R, Ruiz-Grosso P, Malaga G. Registry of Patients with Stroke Stated in a Public Hospital of Peru, 2000–2009. Revista peruana de medicina experimental y salud publica. 2011;28:623–627. doi: 10.1590/s1726-46342011000400008. [DOI] [PubMed] [Google Scholar]
- 9.Lavados PM, Sacks C, Prina L, Escobar A, Tossi C, Araya F, Feuerhake W, Galvez M, Salinas R, Alvarez G. Incidence, 30-Day Case-Fatality Rate, and Prognosis of Stroke in Iquique, Chile: A 2-Year Community-Based Prospective Study (Piscis Project) Lancet. 2005;365:2206–2215. doi: 10.1016/S0140-6736(05)66779-7. [DOI] [PubMed] [Google Scholar]
- 10.Longstreth WT., Jr. Predicting Outcomes after Intracerebral Hemorrhage. Stroke. 1991;22:955–956. [PubMed] [Google Scholar]
- 11.Longstreth WT., Jr. Prediction of Outcomes after Intracerebral Hemorrhage. Stroke. 1993;24:1761. [PubMed] [Google Scholar]
- 12.Zurasky JA, Aiyagari V, Zazulia AR, Shackelford A, Diringer MN. Early Mortality Following Spontaneous Intracerebral Hemorrhage. Neurology. 2005;64:725–727. doi: 10.1212/01.WNL.0000152045.56837.58. [DOI] [PubMed] [Google Scholar]
- 13.Becker KJ, Baxter AB, Cohen WA, Bybee HM, Tirschwell DL, Newell DW, Winn HR, Longstreth WT., Jr. Withdrawal of Support in Intracerebral Hemorrhage May Lead to Self-Fulfilling Prophecies. Neurology. 2001;56:766–772. doi: 10.1212/wnl.56.6.766. [DOI] [PubMed] [Google Scholar]
- 14.Zahuranec DB, Brown DL, Lisabeth LD, Gonzales NR, Longwell PJ, Smith MA, Garcia NM, Morgenstern LB. Early Care Limitations Independently Predict Mortality after Intracerebral Hemorrhage. Neurology. 2007;68:1651–1657. doi: 10.1212/01.wnl.0000261906.93238.72. [DOI] [PubMed] [Google Scholar]
- 15.Adams HP, Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD. Baseline Nih Stroke Scale Score Strongly Predicts Outcome after Stroke: A Report of the Trial of Org 10172 in Acute Stroke Treatment (Toast) Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 16.Ingall TJ, O'Fallon WM, Asplund K, Goldfrank LR, Hertzberg VS, Louis TA, Christianson TJ. Findings from the Reanalysis of the Ninds Tissue Plasminogen Activator for Acute Ischemic Stroke Treatment Trial. Stroke. 2004;35:2418–2424. doi: 10.1161/01.STR.0000140891.70547.56. [DOI] [PubMed] [Google Scholar]
- 17.Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. German Stroke Study C. Age and National Institutes of Health Stroke Scale Score within 6 Hours after Onset Are Accurate Predictors of Outcome after Cerebral Ischemia: Development and External Validation of Prognostic Models. Stroke. 2004;35:158–162. doi: 10.1161/01.STR.0000106761.94985.8B. [DOI] [PubMed] [Google Scholar]
- 18.Chong JY, Lee HS, Boden-Albala B, Paik MC, Sacco RL. Gender Differences in Self- Report of Recovery after Stroke: The Northern Manhattan Study. Neurology. 2006;67:1282–1284. doi: 10.1212/01.wnl.0000238161.71591.e9. [DOI] [PubMed] [Google Scholar]
- 19.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender Differences in Stroke Incidence and Poststroke Disability in the Framingham Heart Study. Stroke. 2009;40:1032–1037. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roth DL, Haley WE, Clay OJ, Perkins M, Grant JS, Rhodes JD, Wadley VG, Kissela B, Howard G. Race and Gender Differences in 1-Year Outcomes for Community- Dwelling Stroke Survivors with Family Caregivers. Stroke. 2011;42:626–631. doi: 10.1161/STROKEAHA.110.595322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Addo J, Ayerbe L, Mohan KM, Crichton S, Sheldenkar A, Chen R, Wolfe CD, McKevitt C. Socioeconomic Status and Stroke: An Updated Review. Stroke; a journal of cerebral circulation. 2012;43:1186–1191. doi: 10.1161/STROKEAHA.111.639732. [DOI] [PubMed] [Google Scholar]