Abstract
Purpose
We investigated the effects of mirodenafil, a phosphodiesterase-5 inhibitor developed in South Korea, on the female rat bladder in a partial bladder outlet obstruction (BOO) model.
Materials and Methods
Thirty-six female Sprague-Dawley rats were divided into four groups: the control group, BOO without medication group, BOO with mirodenafil 1 mg/kg group, and BOO with mirodenafil 4 mg/kg group. Mirodenafil was administered orally for 2 weeks after the induction of BOO. Two weeks after BOO, the rats in each group underwent cystometry under urethane anesthesia. After cystometry, the bladder was excised to perform immunohistochemical staining for connexin 43.
Results
The three BOO groups showed significant increases in mean bladder weight compared with the control group. Baseline pressure, threshold pressure, and maximum contraction pressure were not significantly different between the four groups. Although the contraction interval was decreased in all BOO groups compared with the control group, it was prolonged in the two groups treated with mirodenafil compared with the untreated BOO group. In the immunohistochemical examination, connexin 43 staining intensity in the lamina propria increased in the three BOO groups compared with the control group. The two groups treated with mirodenafil, however, showed decreased connexin 43 staining compared with the untreated BOO group.
Conclusions
Mirodenafil may increase the contraction intervals of female rat bladders in a partial BOO model. Decreasing bladder overactivity by mirodenafil may be related to intracellular communication mechanisms involving connexin 43.
Keywords: Connexin 43, Overactive urinary bladder, Phosphodiesterase inhibitors, Urinary bladder neck obstruction
INTRODUCTION
Phosphodiesterase-5 (PDE-5) inhibitors have been used to treat patients with lower urinary tract symptoms (LUTS) and concomitant erectile dysfunction. According to a meta-analysis of five randomized controlled trials, several PDE-5 inhibitors (e.g., sildenafil, tadalafil, vardenafil) result in improvement in the International Prostate Symptom Score compared with placebo in men with LUTS/benign prostatic hyperplasia (BPH) [1]. Recently, a randomized, placebo-controlled study revealed that the combination of tamsulosin and vardenafil was more effective for improving both LUTS and erectile function than was tamsulosin alone in LUTS/BPH patients [2]. However, the exact mechanism of action of PDE-5 inhibitors on LUTS remains to be clarified.
Eleven different PDE isoenzymes have been identified, and their relaxation effects have been observed in smooth muscle of the corpus cavernosum, vessels, and even bladder [3]. PDE isoenzymes have been identified in rat urinary bladder, and an organ bath study found that the relaxation effects on rat bladder strips may be associated with cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) [4].
Connexin is a gap junctional protein. Expression of connexin 43 is increased in the overactive neurogenic bladder [2]. In the bladder outlet obstruction (BOO) animal model, expression of connexin 43 is also increased in the persistently unstable bladder after the relief of BOO [5].
Mirodenafil is a new PDE-5 inhibitor developed in South Korea. According to an internal report of the company that produced mirodenafil, the IC50 of PDE-5 inhibition of mirodenafil is lower than that of other PDE-5 inhibitors such as sildenafil and tadalafil. After the completion of a phase III clinical trial in 2006, mirodenafil was marketed in Korea. In this study, we investigated the effects of mirodenafil on female rat bladders in a partial BOO model by use of cystometry and immunohistochemistry.
MATERIALS AND METHODS
1. Animals
This study was approved by the Institutional Animal Care and Use Committee of the Clinical Research Institute at Seoul National University Hospital (IACUG No. 07-0205). The facility used for the animal studies was accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Thirty-six female Sprague-Dawley rats (body weight, 200 to 300 g) were divided into four groups: the control group (sham operation), BOO without medication group (BOO for 2 weeks), BOO with 1 mg/kg group (1 mg/kg mirodenafil after 2 weeks of BOO), and BOO with 4 mg/kg group (4 mg/kg mirodenafil after 2 weeks of BOO). We determined the dose of mirodenafil according to our preliminary experiments and the advice of a researcher at the mirodenafil development company. We dissolved 30 mg or 120 mg mirodenafil in 50 mL purified drinking water and made two solutions: 0.6 mg/mL and 2.4 mg/mL. According to the body weight of the rats in each group, we administered about 0.3 to 0.5 mL of each solution by use of an oral Zonde needle. Mirodenafil was administered orally for 2 weeks until the day of cystometry. Mirodenafil was obtained from SK Chemical Corporation in Korea.
2. BOO induction
To induce a partial obstruction of the urethra, we modified the method of Mattiason and Uvelius [6]. Rats were anesthetized with isoflurane, and the bladder and proximal urethra were carefully exposed with a lower midline incision. After the urethra was identified, a blunt tip 22 G angiocatheter needle was inserted into the urethra from the meatus. The entire urethra was ligated with 4/0 black silk between the urethrovesical junction and the urethra while ligation tension was minimally applied. After the catheter was removed, the abdominal wall was closed in layers and 150 mg/kg ampicillin was injected to prevent infection. Sham operations were performed by use of the same method without ligating the urethra. After the operations, two rats of each group were bred in one cage. After recovery, the rats in the two mirodenafil groups were given oral doses of mirodenafil once daily for 2 weeks.
3. Anesthetized cystometry
Two weeks after BOO induction, the rats in all groups underwent cystometry while under urethane anesthesia (1.2 g/kg). Before cystometry, rats were anesthetized with isoflurane and a low midline incision was made. The bladder was exposed and a PE-50 polyethylene catheter was inserted into the bladder through the bladder dome. The PE-50 catheter was secured with purse string sutures and the open end was closed with a three-way stopcock. After closure of the abdomen, isoflurane administration was stopped and urethane (1.2 g/kg) was injected subcutaneously. After 1 hour of stabilization, cystometry was performed. The catheter was connected to an infusion pump (CFU-2100, Nihon-Kohden Co., Tokyo, Japan) for a continuous infusion of saline (flow rate of 4 mL/h) and to a pressure transducer (#1030, PowerLab Co., Colorado Springs, CO, USA) for measuring intravesical pressure. Basal pressure, threshold pressure, and maximal contraction pressure were analyzed. The contraction interval was measured between two voiding contractions. After cystometry, the bladder was excised and stored in formalin solution before immunohistochemical staining for connexin 43.
4. Immunohistochemical staining for connexin 43
For the immunohistochemical studies, we used the standard avidinbiotin indirect immunoperoxidase method with a commercially available kit (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Formalin-fixed paraffin-embedded blocks were cut and positioned on p-lysin-labeled slides. After deparaffinization with xylene, the sections were placed in 100% ethanol for 10 seconds three times and then incubated in a 10% citrate buffer solution at 120℃ for 10 minutes. Endogenous peroxidase activity was blocked by incubation in 3% H2O2 in methanol for 5 minutes at room temperature. The sections were then incubated with a rabbit polyclonal antihuman connexin 43 antibody (#3512, Cell Signaling Technology, Danvers, MA, USA) at a 1:400 dilution for 1 hour at room temperature. After the slides were rinsed in phosphate buffered saline, the sections were subsequently exposed to a biotinylated antimouse immunoglobulin antibody (DAKO, Carpinteria, CA, USA) for 10 minutes. Antibody binding was detected by using a streptavidin peroxidase detection system (Zymed, Carlsbad, CA, USA) according to the manufacturer's instructions. The slides were stained with chromogen 3,3'-diaminobenzidine (Research Genetics Inc., Huntsville, AL, USA), counterstained with Meyer's hematoxylin, and mounted. All slides were examined by a pathologist (E.K.K) and the results were classified as negative (0-10% staining), one positive (11-30% staining), or two positive (over 30%).
5. Statistical analysis
All results were expressed as the mean±standard deviation (SD). Data were analyzed by Mann-Whitney U test with SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). p-values less than 0.05 were considered statistically significant.
RESULTS
1. Body weight and bladder weight
The body weights and bladder weights of each group are presented in Table 1. The bladder weight per 100 g body weight was calculated for each group and compared (Table 1). Bladder weight was significantly increased in the BOO groups compared with the control group (p<0.05). There was no significant difference between the groups treated with mirodenafil and the untreated BOO group in body weight or bladder weight.
TABLE 1.
Body weight and bladder weight of each experiment group
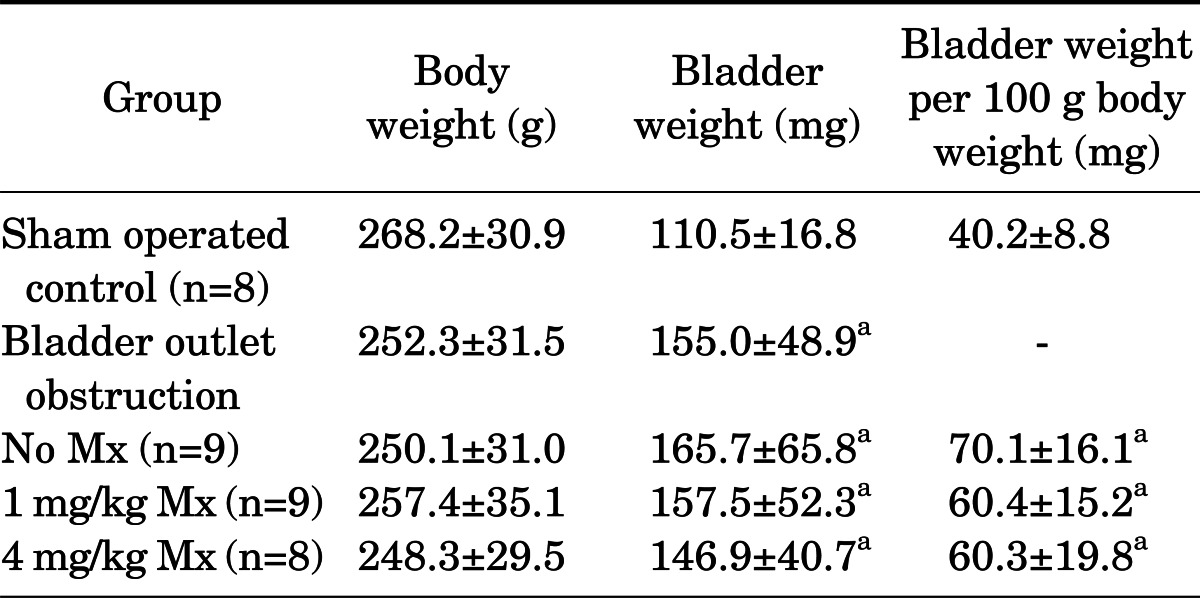
Values are presented as mean±standard deviation.
Mx, medication of mirodenafil.
a:p<0.05 compared to the control group.
2. Cystometry results
The cystometry results for all groups are shown in Table 2. Basal and threshold pressure were not significantly different in any experimental group. The change in threshold and basal pressure was also not significantly different in any experimental group. There was no significant difference in maximal contraction pressure between any groups. Contraction intervals (mean±SD) were 421.6±88.0, 92.4±37.6, 151.2±69.7, and 228.4±55.1 seconds in the control group, untreated BOO group, BOO with 1 mg/kg group, and BOO with 4 mg/kg group, respectively. The contraction intervals of the BOO groups were reduced compared with that of the control group. The contraction intervals in the groups treated with mirodenafil were significantly increased compared with that in the untreated group. The contraction interval in the group treated with 4 mg/kg mirodenafil increased more than that in the group treated with 1 mg/kg mirodenafil, but this difference was not significant.
TABLE 2.
Cystometric parameters of each group
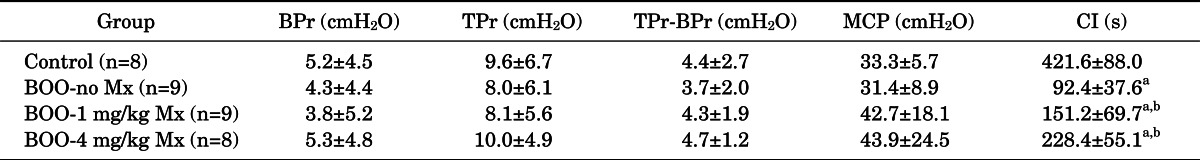
Values are presented as mean±standard deviation.
BPr, baseline pressure; TPr, threshold pressure; MCP, maximum contraction pressure; CI, contraction interval; BOO, bladder outlet obstruction; Mx, medication of mirodenafil.
a:p<0.05 (vs. the control). b:p<0.05 (vs. BOO-no Mx).
3. Immunohistochemistry results
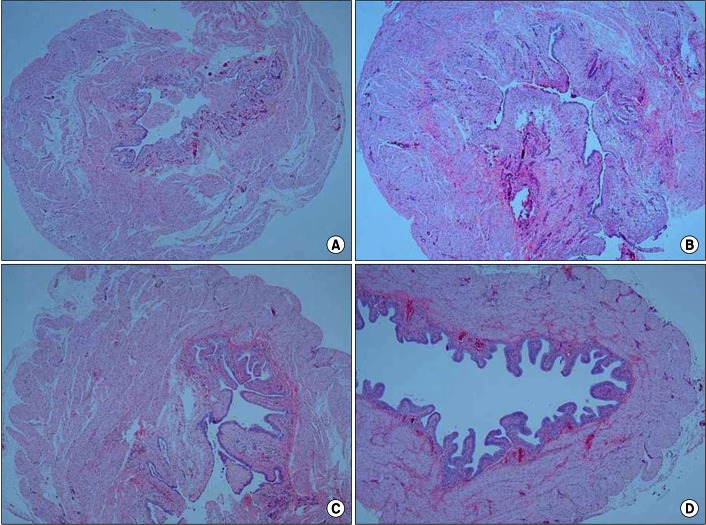
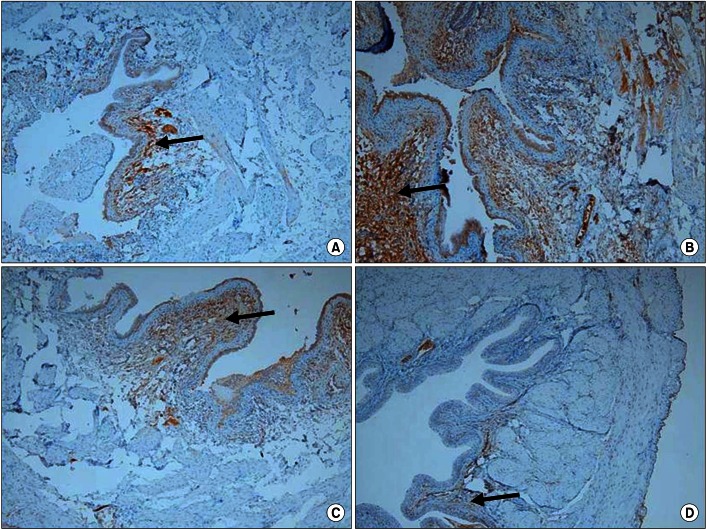
Hematoxylin and eosin staining showed that the bladder walls of the BOO groups were thickened compared with the bladder walls of the control group (Fig. 1). Connexin 43 was mainly distributed in the lamina propria compared with the smooth muscles. In the BOO groups, immunostaining for connexin 43 was definitely increased in the lamina propria. After 2 weeks of mirodenafil treatment, decreased connexin 43 immunostaining was found compared with that in the untreated BOO group (Fig. 2). The scores for all immunostaining results are presented in Table 3.
FIG. 1.
Representative photograph of hematoxylin and eosin staining of each group (×40). Bladder wall thickness of the 3 bladder outlet obstruction (BOO) groups increased compared with that of the control group. (A) Control group, (B) BOO without medication group, (C) BOO with 1 mg/kg mirodenafil group, and (D) BOO with 4 mg/kg mirodenafil group.
FIG. 2.
Representative photograph of immunohistochemical staining for connexin 43 in each group (×100). Connexin 43 was mainly localized in the lamina propria (arrow). Staining intensity increased in the three bladder outlet obstruction (BOO) groups compared with the control group, but was decreased in the two groups treated with mirodenafil compared with the untreated BOO group. (A) Control group, (B) BOO without medication group, (C) BOO with 1 mg/kg mirodenafil group, and (D) BOO with 4 mg/kg mirodenafil group.
TABLE 3.
Immunohistochemical staining results
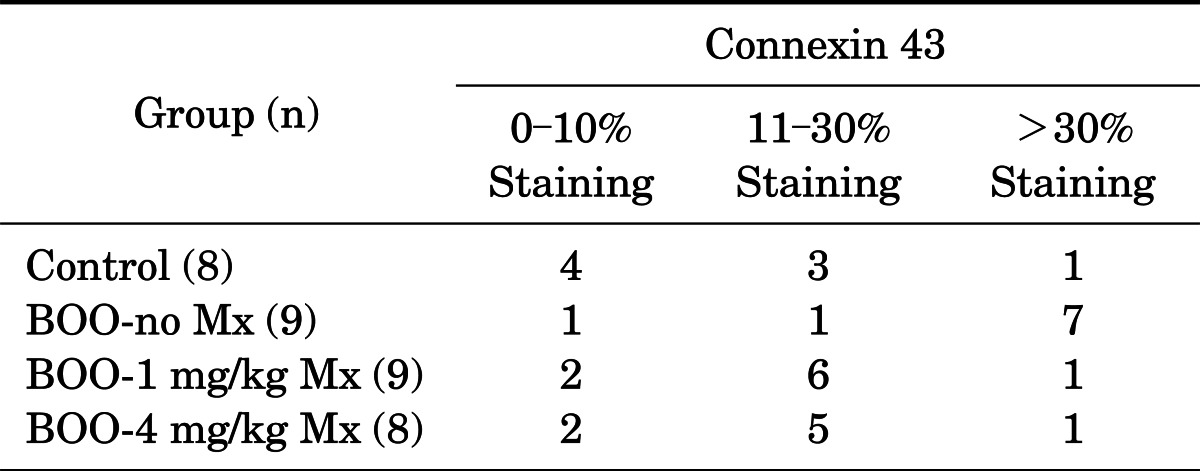
BOO, bladder outlet obstruction; Mx, medication of mirodenafil.
DISCUSSION
Gap junctions are important for cellular communication and are composed of connexon, a hexamer of connexin protein. Among the various connexins, connexin 43 is known to be associated with bladder overactivity [7-9]. In female spinal cord transected rat, the expression of connexin 43 is increased and is distributed mainly in the lamina propria. Miyazato et al. [8] reported that expression of connexin 43 mRNA is increased after BOO in female rats and that bladder contraction intervals are simultaneously decreased. Therefore, partial BOO resulting from detrusor overactivity may be dependent on increased intercellular communication via gap junctions in the bladder.
In our study, connexin 43 was mainly distributed in the lamina propria and was occasionally found in the interfascicula area. Similar to the spinal cord transected rat model, our study examined changes in the bladder after 2 weeks of BOO. We speculate that 2 weeks is a short period and that changes to the bladder appear mainly in the lamina propria. Sui et al. [10] reported that a network of interstitial cells extensively linked by connexin 43-containing gap junctions may operate as a functional syncytium that integrates signals and responses in the bladder wall. Whole bladder mount experiments showed that spontaneous activities induced by stretch and carbachol are organized and originate in the urothelium-suburothelium near the dome [11]. Thus, we suggest that the lamina propria or submucosa is the main area in which early change occurs as a result of BOO. In our study, the staining intensity of connexin 43 was increased in the BOO group and decreased after mirodenafil administration.
Mirodenafil treatment reduced connexin 43 staining. Mirodenafil is not an NO donor or inducer; thus, reduced expression of connexin 43 might be secondary to decreased detrusor overactivity appearing as reduced contraction intervals.
In female rats with BOO, intravenous administration of IC486051, a PDE-4 selective inhibitor, decreases the number and amplitude of nonvoiding bladder contractions [12]. Qiu et al. [3] reported that whereas relaxation of bladder smooth muscle is mainly mediated by the cAMP pathway, the cGMP pathway is also involved. Furthermore, PDE-5 inhibitors have some effects on other PDE isoenzymes such as PDE-3 or PDE-4. Therefore, PDE-5 inhibitors including mirodenafil may have some effects for relieving detrusor overactivity through other isoenzyme activities.
In our study, bladder contraction pressure was not changed in the BOO group compared with the control group. Mirodenafil did not increase contraction pressure. Tinel et al. [13] reported that sildenafil, vardenafil, and tadalafil reduce contractions of isolated bladder strips in a dose-dependent manner. Unlike in the strip study, cystometry contraction pressure cannot represent contractility in general. The degree of obstruction may have also varied among the rats, although we tried to exert constant tension around the urethra. Therefore, further studies will be necessary to investigate this point.
In several clinical studies, commercial PDE-5 inhibitors proved to be effective for improving LUTS [14]. Gacci et al. [15] performed a pilot study in which a single 20-mg dose of vardenafil led to a significant decrease in maximum detrusor pressure, an improvement in maximum cystometric capacity, and a remarkable increase in detrusor overactivity volume in spinal cord injured patients. These patients received standard oral oxybutynin treatment; thus, vardenafil may exert additional effects that improve neurogenic overactive bladder.
PDE-5 inhibitors do not have strong enough effects for improving bladder overactivity to be used as a monotherapy, but the additive effects of these compounds may be useful. In our results, although the mirodenafil-treated group showed prolonged contraction intervals, these were still shorter than those of the control group. This result indicates that PDE-5 inhibitors have weak effects on improving detrusor overactivity. Therefore, PDE-5 inhibitors including mirodenafil have some roles of combination treatment for refractory patients who have not responded to monotherapy of anticholinergics.
Our study had several limitations. First, our study lacked results on contractility. Contraction pressures observed by cystometry do not represent the contractility of bladder smooth muscle. Therefore, additional strip studies may be necessary. Second, our animal experiment was not of overactive bladder but was a partial BOO model. Therefore, a clinical trial with overactive bladder patients may provide clearer evidence of the effects of PDE-5 inhibitors on bladder overactivity.
CONCLUSIONS
Mirodenafil, a new PDE-5 inhibitor, reduced the intercontraction interval in female rats in a partial BOO model. Decreased bladder overactivity by mirodenafil may correlate with intracellular communication through connexin 43. Thus, mirodenafil could be a possible option for effectively treating overactive bladder.
ACKNOWLEDGMENTS
This study was supported by Eulji University Research Grant 2009.
Footnotes
The authors have nothing to disclose.
References
- 1.Liu L, Zheng S, Han P, Wei Q. Phosphodiesterase-5 inhibitors for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review and meta-analysis. Urology. 2011;77:123–129. doi: 10.1016/j.urology.2010.07.508. [DOI] [PubMed] [Google Scholar]
- 2.Gacci M, Vittori G, Tosi N, Siena G, Rossetti MA, Lapini A, et al. A randomized, placebo-controlled study to assess safety and efficacy of vardenafil 10 mg and tamsulosin 0.4 mg vs. tamsulosin 0.4 mg alone in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Sex Med. 2012;9:1624–1633. doi: 10.1111/j.1743-6109.2012.02718.x. [DOI] [PubMed] [Google Scholar]
- 3.Qiu Y, Kraft P, Craig EC, Liu X, Haynes-Johnson D. Identification and functional study of phosphodiesterases in rat urinary bladder. Urol Res. 2001;29:388–392. doi: 10.1007/s00240-001-0221-6. [DOI] [PubMed] [Google Scholar]
- 4.Haferkamp A, Mundhenk J, Bastian PJ, Reitz A, Dorsam J, Pannek J, et al. Increased expression of connexin 43 in the overactive neurogenic detrusor. Eur Urol. 2004;46:799–805. doi: 10.1016/j.eururo.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Kim JC, Kim DB, Seo SI, Park YH, Hwang TK. Effects of connexin expression on unstable bladder after relief of bladder outlet obstruction in rat. Korean J Urol. 2003;44:585–591. [Google Scholar]
- 6.Mattiasson A, Uvelius B. Changes in contractile properties in hypertrophic rat urinary bladder. J Urol. 1982;128:1340–1342. doi: 10.1016/s0022-5347(17)53503-x. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol. 2007;293:F1018–F1025. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazato M, Sugaya K, Nishijima S, Kadekawa K, Machida N, Oshiro Y, et al. Changes of bladder activity and connexin 43-derived gap junctions after partial bladder-outlet obstruction in rats. Int Urol Nephrol. 2009;41:815–821. doi: 10.1007/s11255-008-9516-7. [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Kim JC, Hwang TK. Effect of ACE inhibitor on connexin expression after bladder outlet obstruction in rats. Korean J Urol. 2005;46:1205–1212. [Google Scholar]
- 10.Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002;90:118–129. doi: 10.1046/j.1464-410x.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- 11.Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, et al. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol. 2007;292:F1065–F1072. doi: 10.1152/ajprenal.00229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiguchi J, Kwon DD, Kaiho Y, Chancellor MB, Kumon H, Snyder PB, et al. Suppression of detrusor overactivity in rats with bladder outlet obstruction by a type 4 phosphodiesterase inhibitor. BJU Int. 2007;99:680–686. doi: 10.1111/j.1464-410X.2006.06643.x. [DOI] [PubMed] [Google Scholar]
- 13.Tinel H, Stelte-Ludwig B, Hutter J, Sandner P. Pre-clinical evidence for the use of phosphodiesterase-5 inhibitors for treating benign prostatic hyperplasia and lower urinary tract symptoms. BJU Int. 2006;98:1259–1263. doi: 10.1111/j.1464-410X.2006.06501.x. [DOI] [PubMed] [Google Scholar]
- 14.McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007;177:1401–1407. doi: 10.1016/j.juro.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Gacci M, Del Popolo G, Macchiarella A, Celso M, Vittori G, Lapini A, et al. Vardenafil improves urodynamic parameters in men with spinal cord injury: results from a single dose, pilot study. J Urol. 2007;178:2040–2043. doi: 10.1016/j.juro.2007.07.048. [DOI] [PubMed] [Google Scholar]