Abstract
Large elastic artery stiffness is an independent predictor of age-related cardiovascular events that is attributable to structural remodeling throughout the artery. The intima, media and adventitial layers of the artery uniquely remodel with advancing age and all contribute to arterial stiffening. The specific expression of the extracellular matrix proteins collagen and elastin, and post-translational modifications of these proteins by advanced glycation end-products are key mechanisms in arterial stiffening with age and will be reviewed in the context of region-specific expression. In addition, interventions for attenuating age-related arterial stiffness and novel imaging advances for translating basic findings to older clinical populations will be discussed.
Keywords: Intima, Media, Adventitia, Collagen, Elastin, Advanced glycation endproducts, inflammation, oxidative stress
Aging is the major risk factor for cardiovascular diseases (CVD) that is largely due to dysfunctional arteries (1,2). Stiffening of the large elastic arteries, one expression of arterial dysfunction, is emerging as an independent predictor of CVD events (3,4,5,6). Much of the stiffening observed with aging is attributable to structural remodeling within the artery (7). The structural alterations within the intimal, medial and/or adventitial layers of the artery include increased collagen deposition, reductions in elastin and greater cross-linking by advance glycation end-products (AGEs) (1, 8–12). Both oxidative stress (11–17) and inflammatory (18–20) signaling cascades are associated with advancing age that may, in turn, contribute to arterial stiffening with aging.
Novel interventions attenuating large elastic artery stiffness in older individuals hold promise for reducing mortality due to CVD. As such, aerobic exercise and nutraceutical interventions have been shown to reduce arterial stiffening and are associated with reductions in cardiovascular events (21–23). Only recently have studies begun to elucidate the cellular and molecular mechanisms for the beneficial effects of aerobic exercise and nutraceuticals improving arterial stiffness with older age. Here current evidence is reviewed for the extracellular matrix (ECM) remodeling within the intimal, medial, and adventitial layers of the artery as well as the role of increased oxidative and inflammatory signaling in age-related large elastic artery stiffening. Additionally, aerobic exercise and nutraceuticals used as interventions, and the associated down-stream cellular and molecular mechanisms will also be discussed in the context of translating basic findings to the aged human population.
Extracellular Matrix Modifications with Aging
Large elastic artery stiffness with aging is associated with arterial collagen deposition, reductions in elastin and AGEs accumulation (1,7); however, only recently have these ECM expressions been examined in a layer-by-layer analysis in arteries to determine the region-specific source. The arterial wall is composed of an intima where endothelial cells reside, a medial layer composed of multiple layers of smooth muscle cells and the adventitia containing a sparse population of fibroblasts. The cells within each layer have unique functions and preferentially express, secrete and/or synthesize the extracellular matrix (ECM) molecules collagen and elastin based on cell type. As such, adventitial fibroblasts have greater collagen synthesis compared with smooth muscle cells (24); however, adventitial fibroblasts and endothelial cells typically have less elastin production compared with smooth muscle cells (25,26). Endothelial cells also express collagen (27), but the expression in relation to other vascular cells is unknown. In contrast to collagen and elastin, AGEs is a non-specific post-translational modification of proteins throughout the arterial wall expressed in endothelial cells, smooth muscle cells and adventitial fibroblasts (11,12,28).
Collagen
Increased collagen deposition with aging has been observed in all three layers of the artery: intima, media and adventitia (11,12,29–31). Type I collagen, the major arterial collagen sub-type conferring mechanical stiffness (7) has a greater expression in the aortic intima of older compared with younger adults and is associated with the increased expression of the smooth muscle marker, smooth muscle alpha actin (SMαA) (30). Because smooth muscle cells from arteries of older animals have greater migratory properties it has been proposed the intimal cells are smooth muscle cells migrating from the medial layer (32). Interestingly cultured senescent endothelial cells, a cell model of replicative aging, have greater collagen I and SMαA expressions, suggesting a novel hypothesis of endothelial cell contribution to intimal collagen deposition with aging (27). Despite the cellular origin, intimal cells contribute to collagen deposition with aging.
Studies assessing collagen deposition in the medial layer with advancing age have divergent results showing either an increase or no change in these ECM proteins (11,12,29,30, 33–35). Animal studies have demonstrated increases in total collagen content in the medial layer (33, 35); however, most collagen sub-types have shown a decrease or no change in animal models (11,12,29,33,35). This is markedly different when compared with the medial layer from aged humans demonstrating an increase in all collagen sub-types examined (30). Although both animals and humans have increased total medial collagen deposition with aging, the sub-type expression profile explaining the overall increase in this layer appear to be species specific.
Fibroblasts in the adventitia, or outer-most layer of the artery, synthesize more collagen when compared with smooth muscle cells in vitro (24); suggesting adventitial fibroblasts, if activated, may significantly contribute to collagen deposition with advancing age. Recently it has been demonstrated that aging promotes adventitial collagen I expression in aortic histological segments (11,12,29) and in primary cultured aortic fibroblasts (11). Additionally, advancing age induces adventitial transforming growth factor beta 1 (TGF-β1) expression that, in turn, stimulates the pro-synthetic myofibroblast phenotype and collagen I expression in cultured fibroblasts from young animals (29). The age-related increase in adventitial collagen deposition is consistent with observations implicating this arterial layer in vascular stiffening with advancing age (36). However, direct in vivo evidence for this adventitial signaling pathway to promote collagen deposition and arterial stiffness is lacking.
Elastin
Elastin provides elasticity to vessels and is expressed in all cells within the artery. However, compared with the other arterial cell types medial smooth muscle cells express greater amounts of elastin (11,12,26,29). Aging reduces elastin in arteries (26,31,33,35,37–39) that is largely attributable to reductions within the smooth muscle medial layer (29,33). Moreover, lysyl oxidase (LOX), a key enzyme responsible for elastin maturation (40, 41) has a reduced medial expression, and the elastin degrading enzyme matrix metalloproteinase-2 (MMP-2) is greater in whole arteries (34), and within the medial layer (29,39) of aged arteries. In contrast to the medial layer, aging does not affect elastin expression, or the regulating enzymes LOX and MMP-2 in the adventitial layer (29). Collectively the diminished medial elastin expression with aging is related to dysregulated enzyme expressions that either prevent maturation or promote degradation.
AGEs
Advancing age promotes the accumulation of AGEs, a non-enzymatic glycosylation of proteins that, in turn, promotes large elastic artery stiffness. AGEs non-specifically cross-link proteins throughout the artery and have been shown to be expressed in all layers of the artery (12,28,42). Recent investigations have begun to elucidate a direct causal role for AGEs in the pathogenesis of large elastic artery stiffness with advancing age (10,12,43). Compounds that break AGEs cross-links, such as Alagebrium, have been shown to attenuate arterial stiffening in older adults (43) and rodents (10). Moreover, treatment of biologically active AGEs ex vivo produce greater mechanical stiffness in cultured aortic rings from young mice further supporting a role of AGEs in arterial stiffening (12). Although AGEs contribute to arterial stiffening, less in known about the mechanisms leading to the increased in glycosylation with aging and arterial stiffness.
Oxidative Stress
Oxidative stress, or greater reactive oxygen species in relation to antioxidant capacity, has been shown to be present in arteries of older rodents (11–14,17,44–46), and plasma markers of oxidative stress are independent predictors of arterial stiffness in healthy humans (47). Increased whole artery aortic expression of the p67 subunit of the pro-oxidant enzyme NADPH oxidase and reductions in the anti-oxidant enzyme manganese superoxide dismutase (MnSOD) are also a consequence of aging contributing to the increased oxidative burden (11,17). In addition, greater superoxide bioavailability is associated with markers of oxidative stress, enhanced collagen deposition, reductions in elastin and greater AGEs accumulation (11,12,29). Interestingly when the antioxidant TEMPOL, a mimetic of superoxide dismutase (SOD), was given in drinking water to old mice aortic superoxide bioavailability, arterial stiffness and adventitial collagen deposition were reduced to levels not different from young controls (11). Moreover, adventitial fibroblasts isolated and cultured from old rodents demonstrate greater collagen I expression that was attenuated with SOD treatment. In contrast, the addition of pyrogallol, a superoxide generator, to fibroblasts isolated from young rodents induced collagen I expression that was abolished with SOD pre-treatment (11). Although collagen within the medial layer was not increased, TEMPOL reduced this expression in both young and old mice. In addition to direct superoxide-scavenging effects, TEMPOL may also reduce oxidative stress by attenuating pro-oxidant expressions (11). Collectively, these data demonstrate a distinct role for oxidative signaling, specifically superoxide, in age-related arterial stiffening and adventitial collagen deposition.
Despite reductions in age-associated arterial stiffening and collagen deposition, elastin expression is not improved with TEMPOL treatment in old mice. Moreover, young mice ingesting TEMPOL demonstrate lower elastin expressions in the media without having an effect on arterial stiffness or adventitial elastin expression (11), suggesting superoxide is an important signaling radical in the maintenance of elastin by smooth muscle cells. This observation is in agreement with others showing TEMPOL attenuates elastin in the lungs of rats with pulmonary hypertension (48). TEMPOL also has no effect on AGEs accumulation in the medial and adventitial layers of older mice (11). This, in part, may be due to a short, 3-week treatment protocol, whereas a longer TEMPOL intervention would potentially result in reductions of AGEs. Additionally, it is unknown if oxidative signaling contributes to AGEs abundance in the intima by endothelial cells with advancing age, and further investigation is warranted.
Inflammation
Advancing age results in greater whole artery activation of the pro-inflammatory transcription factor NFκB that, in turn, promotes the expression of pro-inflammatory cytokines such as IL-1β, IL-6, IFN-γ and TNF-α (15,17,18,19,20). Additionally, macrophage infiltration is notably higher in the adventitia and surrounding adipose tissue that is also implicated in arterial inflammation (15). Although numerous studies have observed greater inflammation in arteries, no investigations have directly determined whether reductions in inflammation attenuate large elastic artery stiffening with age. Others have demonstrated that indirect anti-inflammatory treatments targeting NFκB improve some expressions of arterial dysfunction (49). However, direct causal studies linking inflammation with age-related arterial stiffening and ECM remodeling are lacking and further investigation is needed.
Interventions
Aerobic Exercise
Aerobic exercise reduces the risk for CVD, and attenuates age-related arterial stiffening in older adults and aged rodents (10,31,50,51,52). The mechanisms for exercise-related improvements in arterial stiffness with aging have largely been unexplored. However, recent evidence has begun to elucidate the structural, cellular and molecular mechanisms underlying the large elastic artery de-stiffening effects of exercise with aging.
Collagen has long been implicated in the pathogenesis of age-associated arterial stiffening, and as a result is a prime target for exercise in attenuating arterial stiffness. Indeed, older mice given access to running wheels results in reduced medial and adventitial collagen I and III sub-type expressions, and is associated with an attenuation of adventitial TGF-β1 expression (29). Moreover, the age-related increase of the pro-oxidant p67 subunit of NADPH oxidase is attenuated, and anti-oxidant SOD activities are increased in whole arterial segments of older voluntary exercised mice (45). Interestingly, TGF-β1 induces collagen in adventitial fibroblasts via a superoxide dependent mechanism that has been shown to mediate functions through NADPH oxidase (29,53). Albeit not tested, the beneficial effects of exercise for attenuating large artery stiffness and adventitial collagen expression may, in part, be mediated via reductions in a TGF-β1-NADPH oxidase-superoxide signaling cascade; however, further investigation is needed to test this hypothesis. Additionally, the effects of exercise on intimal collagen deposition with advancing age are unknown warranting further research. Collectively, these data suggest the collagen-lowering effect of exercise in the medial and adventitial layers is an important mechanism underlying the de-stiffening of large elastic arteries with aging.
Decreases in elastin with aging contribute to greater arterial stiffening. However, older exercise trained rodents do not have increased elastin in whole aortic segments or the medial layer despite reductions in arterial stiffness (29,31). The elastin modulating proteins LOX and MMP-2 are also unaffected with exercise training (29). Moreover, adventitial elastin expression is unaltered with exercise training and no current data exists for the effects of exercise on intimal elastin with aging. Taken together, elastin contributes to age-related arterial stiffening, but not to the de-stiffening effects of aerobic exercise.
Few studies have determined the effects of exercise on arterial AGEs accumulation. Recently, exercise and the AGEs cross-link breaker Alagebrium have been shown to independently attenuate arterial stiffness, and in combination reverse vascular stiffness with aging (10). These data suggest exercise decreases arterial stiffness either by preventing new AGEs formation as stated by the authors or via a mechanism independent of AGEs. Further investigations are required to determine if exercise-related reductions in arterial stiffening are due to breakdown of established AGEs, prevention of new AGEs formation or mediated via an independent mechanism.
Novel Interventions
Aerobic exercise training is recognized as an intervention having profound effects on slowing and/or reversing large elastic artery stiffness. However, exercise can be contra-indicted in some older populations and not all older individuals are willing to perform exercise on a regular basis. To treat these individuals other novel interventions need to be established. Interventions using active compounds derived from plant sources that may confer health benefits is one potential option. Two novel plant-derived compounds, nitrites and curcumin, have recently been shown to attenuate large elastic artery stiffness in mice and will be discussed herein with an insight to the potential mechanisms of action.
Sodium Nitrite
Nitrates are found naturally in fruits, vegetables and grains, and are considered to be beneficial for health as well as safe (54). Interestingly when consumed, dietary nitrates are converted to nitrite in the mouth and gastrointestinal tract (54). Thus, it has been suggested that the positive effects of vegetable-derived nitrates are, in part, mediated by nitrites. As such, administration of sodium nitrite has been shown to exert protective qualities in animals.
Although studies have examined the effects of sodium nitrite in disease models (55–57), little data exists for the effects of sodium nitrite in older animals. Recent evidence, however, has demonstrated that a short-term, 3-week sodium nitrite supplementation in older mice reduces arterial stiffness, which was associated with attenuated whole artery AGEs accumulation and oxidative signaling (12,17). The reduced oxidative burden with sodium nitrite was related to reduced p67 subunit expression of NADPH oxidase and greater SOD activity in the whole aorta (17). Moreover, in an ex vivo model, sodium nitrite prevented the superoxide-mediated increase in AGEs, and AGEs-induced mechanical stiffening of aortic segments (12). In contrast, sodium nitrite did not attenuate the age-related alterations in adventitial collagen I, whole artery TGF-β1, or medial elastin expressions (12). Sodium nitrite did, however, decrease medial elastin expression in young mice without increasing arterial stiffness (12). Despite the reductions of elastin in young mice, sodium nitrite still remains a novel translational intervention for the treatment of age-related arterial stiffening that deserves further investigation.
Curcumin
Curcumin is the active ingredient in the Indian spice turmeric that is used widely in Indian cooking. Found naturally in the root of Curcuma longa, curcumin has been utilized in traditional Indian medicine to treat skin, liver and gastrointestinal tract ailments (58). More recently curcumin supplementation in animal models of disease have been shown to improve arterial function, including arterial stiffness in young rodents (59–61). Importantly, curcumin is generally recognized as safe by the FDA, and is non-toxic at doses as high as 8,000 milligrams/day (62).
Few studies have assessed the effectiveness of curcumin in treating arterial dysfunction, and none have determined the effects in older rodents until recently. In this study, curcumin supplemented chow given to older mice for 4 weeks attenuated arterial stiffness. The reductions in stiffness were associated with attenuations of adventitial collagen type I and whole artery AGEs expressions in old treated mice without affecting medial elastin expression (63). Additionally, old curcumin treated mice had reduced aortic superoxide bioavailability and p67 subunit of NADPH oxidase expression, and greater whole artery MnSOD expression compared with old control mice (63). These findings provide initial evidence that curcumin supplementation may be a novel intervention for the treatment of arterial stiffness. However, clinical studies assessing the effects of curcumin supplementation in older populations are currently lacking.
Translation to Humans
Translating novel pre-clinical mechanisms and interventions to human populations is of great biomedical importance. Of notable importance is the observation that the adventitia is related to arterial stiffening with age (36), and older animals have greater adventitial remodeling with increased collagen deposition and AGEs accumulation (11,12,29). The translation of arterial remodeling is difficult to assess non-invasively. However, recent advances in imaging techniques have shown that greater adventitial thickness, assessed with non-invasive imaging, is correlated with cardiovascular risk factors in humans (64). Translation of novel pre-clinical interventions improving arterial stiffness and adventitial remodeling have not currently been performed in older adults, but may provide mechanistic insight to the de-stiffening effects of interventions with aging.
Conclusions
In conclusion, large elastic artery stiffness with aging is a complex process involving intimal, medial and adventitial remodeling that is summarized in the proposed working model (Figure 1). Recent evidence has elucidated the novel mechanism for adventitial remodeling in arterial stiffening with age. Combined with novel imaging modalities and plant-based interventions, pre-clinical findings assessing adventitial remodeling can readily be translated to older human populations.
Figure 1.
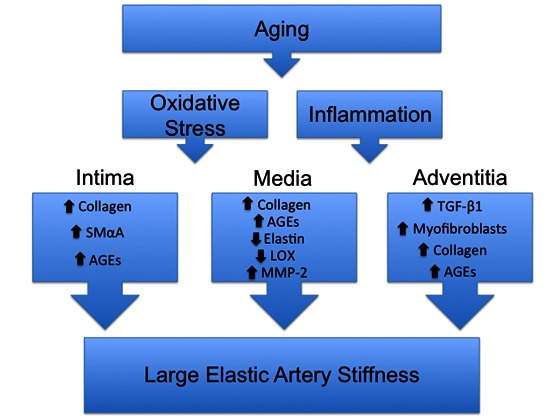
Summary of region-specific modifications with age and proposed mechanisms contributing to artery stiffness. Aging results in greater oxidative stress and inflammation that promotes region specific remodeling in the intimal, medial and adventitial layers that contributes to arterial stiffening. The intima has greater collagen, smooth muscle alpha actin (SMαA) and advanced glycation endproduct (AGEs) expressions. The increased SMαA may be a result of smooth muscle cell migration from the media or a novel endothelial phenotypic change. Within the medial layer collagen and AGEs accumulation are greater, whereas elastin is reduced. The elastin modulating enzymes Lysyl Oxidase (LOX) and matrix metalloproteinase-2 (MMP-2) are also modulated in the media to promote elastin degradation. Adventitial transforming growth factor beta 1 (TGF-β1), myofibroblasts, collagen and AGEs expressions are greater. TGF-β1 induces a myofibroblast phenotype and collagen in fibroblasts.
References
- [1].Lakatta EG, Levy D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part I: Aging Arteries: A “Set Up” for Vascular Disease. Circulation. 2003;107:139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- [2].Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Moxaffarian D, Mussolino ME, Nichol G, Paynter NP, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics -- 2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mattace-Raso FUS, van der Cammen TJM, Hofman A, van Popele NM, Bos ML, Schalekamp MADH, Asmar R, Reneman RS, Hoeks APG, Breteler MMB, Witteman JCM. Arterial Stiffness and Risk of Coronary Heart Disease and Stroke: The Rotterdam Study. Circulation. 2006;113:657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- [4].Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial Stiffness and Cardiovascular Events: The Framingham Heart Study. Circulation. 2010;121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated Aortic Pulse Wave Velocity, a Marker of Arterial Stiffness, Predicts Cardiovascular Events in Well-Functioning Older Adults. Circulation. 2005;111:3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- [6].Willum Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic Value of Aortic Pulse Wave Velocity as Index of Arterial Stiffness in the General Population. Circulation. 2006;113:664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- [7].Diez J. Arterial stiffness and extracellular matrix. Adv Cardiol. 2007;44:76–95. doi: 10.1159/000096722. [DOI] [PubMed] [Google Scholar]
- [8].Zieman SJ, Melenovsky V, Kass DA. Mechanisms, Pathophysiology, and Therapy of Arterial Stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–43. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- [9].Zieman SJ, Vojtech M, Clattenburg L, Corretti MC, Capriotti AR, Gerstenblith G, Kass DA. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens. 2007;25:577–83. doi: 10.1097/HJH.0b013e328013e7dd. [DOI] [PubMed] [Google Scholar]
- [10].Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, Nyhan SM, Lussman C, Gupta G, White AR, Daher JP, Shoukas AA, Levine BD, Berkowitz Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol. 2012;47:565–72. doi: 10.1016/j.exger.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 2012;11:269–276. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: Role of normalization of advanced glycation end-products. Exp Gerontol. 2012;47:588–94. doi: 10.1016/j.exger.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc Gene Protects Against Age-Related Endothelial Dysfunction. Circulation. 2004;110:2889–95. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- [14].Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide Excess in Hypertension and Aging : A Common Cause of Endothelial Dysfunction. Hypertension. 2001;37:529–34. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- [15].Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2011;301:H1025–H32. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–97. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–37. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zou Y, Yoon S, Jung KJ, Kim CH, Son TG, Kim MS, Kim YJ, Lee J, Yu BP, Chung HY. Upregulation of aortic adhesion molecules during aging. J Gerontol A Biol Sci Med Sci. 2006;61:232–44. doi: 10.1093/gerona/61.3.232. [DOI] [PubMed] [Google Scholar]
- [19].Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-κB. J Appl Physiol. 2008;105:1333–41. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of Vascular Aging: New Perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–41. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marik PE, Varon J. Omega-3 Dietary Supplements and the Risk of Cardiovascular Events: A Systematic Review. Clin Cardiol. 2009;32:365–72. doi: 10.1002/clc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Miura H, Nakagawa E, Takahashi Y. Influence of group training frequency on arterial stiffness in elderly women. Eur J Appl Physiol. 2008;104:1039–44. doi: 10.1007/s00421-008-0860-1. [DOI] [PubMed] [Google Scholar]
- [23].Okada S, Hiuge A, Makino H, Nagumo A, Takaki H, Konishi H, Goto Y, Yoshimasa Y, Miyamoto Y. Effect of Exercise Intervention on Endothelial Function and Incidence of Cardiovascular Disease in Patients with Type 2 Diabetes. J Atheroscler Thromb. 2010;17:828–33. doi: 10.5551/jat.3798. [DOI] [PubMed] [Google Scholar]
- [24].Patel S, Shi Y, Niculescu R, Chung EH, Martin JL, Zalewski A. Characteristics of Coronary Smooth Muscle Cells and Adventitial Fibroblasts. Circulation. 2000;101:524–32. doi: 10.1161/01.cir.101.5.524. [DOI] [PubMed] [Google Scholar]
- [25].Ruckman JL, Luvalle PA, Hill KE, Giro MG, Davidson JM. Phenotypic stability and variation in cells of the porcine aorta: collagen and elastin production. Matrix Biol. 1994;14:135–45. doi: 10.1016/0945-053x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- [26].Sauvage M, Hinglais N, Mandet C, Badier C, Deslandes F, Michel JB, Jacob MP. Localization of elastin mRNA and TGF-ß1 in rat aorta and caudal artery as a function of age. Cell Tissue Res. 1998;291:305–14. doi: 10.1007/s004410051000. [DOI] [PubMed] [Google Scholar]
- [27].Fleenor BS, Marshall KD, Rippe C, Seals DR. Replicative aging induces endothelial to mesenchymal transition in human aortic endothelial cells: Potential role of inflammation. J Vasc Res. 2012;49:59–64. doi: 10.1159/000329681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–22. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- [29].Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971–82. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang M, Zhang J, Jiang L-Q, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory Profile Within the Grossly Normal Aged Human Aortic Wall. Hypertension. 2007;50:219–27. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- [31].Nosaka H, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol. 2003;28:204–12. doi: 10.1139/h03-016. [DOI] [PubMed] [Google Scholar]
- [32].Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat Aortic MCP-1 and Its Receptor CCR2 Increase With Age and Alter Vascular Smooth Muscle Cell Function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- [33].Wang M, Lakatta EG. Altered Regulation of Matrix Metalloproteinase-2 in Aortic Remodeling During Aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- [34].Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II Activates Matrix Metalloproteinase Type II and Mimics Age-Associated Carotid Arterial Remodeling in Young Rats. Am J Pathol. 2005;167:1429–42. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, Peppas A, Shen YT, Vatner DE, Vatner SF. Mechanism of Gender-Specific Differences in Aortic Stiffness With Aging in Nonhuman Primates. Circulation. 2007;116:669–76. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- [36].Schulze-Bauer CAJ, Regitnig P, Holzapfel GA. Mechanics of the human femoral adventitia including the high-pressure response. Am J Physiol Heart Circ Physiol. 2002;282:H2427–40. doi: 10.1152/ajpheart.00397.2001. [DOI] [PubMed] [Google Scholar]
- [37].Qiu H, Tian B, Resuello RG, Natividad FF, Peppas A, Shen YT, Vatner DE, Vatner SF, Depre C. Sex-specific regulation of gene expression in the aging monkey aorta. Physiol Genomics. 2007;29:169–80. doi: 10.1152/physiolgenomics.00229.2006. [DOI] [PubMed] [Google Scholar]
- [38].Cox RH. Age-related changes in arterial wall mechanics and composition of NIA Fischer rats. Mech Ageing Dev. 1983;23:21–36. doi: 10.1016/0047-6374(83)90096-9. [DOI] [PubMed] [Google Scholar]
- [39].Behmoaras J, Slove S, Seve S, Vranckx R, Sommer P, Jacob MP. Differential expression of lysyl oxidases LOXL1 and LOX during growth and aging suggests specific roles in elastin and collagen fiber remodeling in rat aorta. Rejuvenation Res. 2008;11:883–9. doi: 10.1089/rej.2008.0760. [DOI] [PubMed] [Google Scholar]
- [40].Bedell-Hogan D, Trackman P, Abrams W, Rosenbloom J, Kagan H. Oxidation, cross-linking, and insolubilization of recombinant tropoelastin by purified lysyl oxidase. J Biol Chem. 1993;268:10345–50. [PubMed] [Google Scholar]
- [41].Kothapalli CR, Ramamurthi A. Lysyl oxidase enhances elastin synthesis and matrix formation by vascular smooth muscle cells. J Tissue Eng Regen Med. 2009;3:655–61. doi: 10.1002/term.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Halushka MK, Selvin E, Lu J, Macgregor AM, Cornish TC. Use of human vascular tissue microarrays for measurement of advanced glycation endproducts. J Histochem Cytochem. 2009;57:559–66. doi: 10.1369/jhc.2009.953273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved Arterial Compliance by a Novel Advanced Glycation End-Product Crosslink Breaker. Circulation. 2001;104:1464–70. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- [44].Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NFkB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- [45].Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–85. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 Mice Are a Suitable Model of Oxidative Stress-Mediated Impaired Endothelium-Dependent Dilation With Aging. J Gerontol A Biol Sci Med Sci. 2009;64A:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, Dabhadkar K, Brigham K, Hooper WC, Alexander RW, Jones DP, Quyyumi AA. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218:90–5. doi: 10.1016/j.atherosclerosis.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Klemm DJ, Majka SM, Crossno JT, Jr, Psilas JC, Reusch JEB, Garat CV. Reduction of Reactive Oxygen Species Prevents Hypoxia-induced CREB Depletion in Pulmonary Artery Smooth Muscle Cells. J Cardiovasc Pharmacol. 2011;58:181–91. doi: 10.1097/FJC.0b013e31821f2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate Treatment Improves Age-Associated Vascular Endothelial Dysfunction: Potential Role of Nuclear Factor κB and Forkhead Box O Phosphorylation. J Gerontol A Biol Sci Med Sci. 2011;66A:409–18. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–62. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- [51].Tanaka H, DeSouza CA, Seals DR. Absence of Age-Related Increase in Central Arterial Stiffness in Physically Active Women. Arterioscler Thromb Vasc Biol. 1998;18:127–32. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- [52].Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, Habitual Exercise, and Dynamic Arterial Compliance. Circulation. 2000;102:1270–5. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- [53].Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Lassegue B, Griendling KK. Nox4 Is Required for Maintenance of the Differentiated Vascular Smooth Muscle Cell Phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–8. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Machha A, Schechter A. Dietary nitrite and nitrate: a review of potential mechanisms of cardiovascular benefits. Eur J Nutr. 2011;50:293–303. doi: 10.1007/s00394-011-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Montenegro MF, Amaral JH, Pinheiro LC, Sakamoto EK, Ferreira GC, Reis RI, Marcal DMO, Pereira RP, Tanus-Santos JE. Sodium nitrite downregulates vascular NADPH oxidase and exerts antihypertensive effects in hypertension. Free Radic Biol Med. 2011;51:144–52. doi: 10.1016/j.freeradbiomed.2011.04.005. [DOI] [PubMed] [Google Scholar]
- [56].Ataya B, Tzeng E, Zuckerbraun BS. Nitrite-generated nitric oxide to protect against intimal hyperplasia formation. Trends Cardiovasc Med. 2011;21:157–62. doi: 10.1016/j.tcm.2012.05.002. [DOI] [PubMed] [Google Scholar]
- [57].Alef MJ, Vallabhaneni R, Carchman E, Morris SM, Shiva S, Wang Y, Kelley EE, Tarpey MM, Gladwin MT, Tzeng E, Zuckerbraun BS. Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. J Clin Invest. 2011;121:1646–56. doi: 10.1172/JCI44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Miriyala S, Panchatcharam M, Rengarajulu P. Cardioprotective effects of curcumin. Adv Exp Med Biol. 2007;595:359–77. doi: 10.1007/978-0-387-46401-5_16. [DOI] [PubMed] [Google Scholar]
- [59].Majithiya JB, Balaraman R. Time-Dependent Changes in Antioxidant Enzymes and Vascular Reactivity of Aorta in Streptozotocin-Induced Diabetic Rats Treated With Curcumin. J Cardiovasc Pharmacol. 2005;46:697–705. doi: 10.1097/01.fjc.0000183720.85014.24. [DOI] [PubMed] [Google Scholar]
- [60].Nakmareong S, Kukongviriyapan U, Pakdeechote P, Donpunha W, Kukongvirilyapan V, Kongylingyoes B, Sompamit K, Phisalaphong C. Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficency. Hypertension Res. 2011;383:519–29. doi: 10.1038/hr.2011.180. [DOI] [PubMed] [Google Scholar]
- [61].Rungseesantivanon S, Thenchaisri N, Ruangvejvorachai P, Patumraj S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement Altern Med. 2010;10:57. doi: 10.1186/1472-6882-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cheng AL, Hsu C, Lin J, Hsu MM, Ho Yf, Shen TS, Ko JY, Lin JT, Lin Br, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang TJ, Tsai CC, Hsieh CY. Phase I clincial trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- [63].Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR. Curcumin amerliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol. 2012 doi: 10.1016/j.exger.2012.10.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Skilton MR, Boussel L, Bonnet F, Bernard S, Douek PC, Moulin P, Serusciat A. Carotid intima-media and adventitial thickening: Comparison of new and established ultrasound and magnetic resonance imaging techniques. Atherosclerosis. 2011;215:405–10. doi: 10.1016/j.atherosclerosis.2010.12.036. [DOI] [PubMed] [Google Scholar]


