Summary
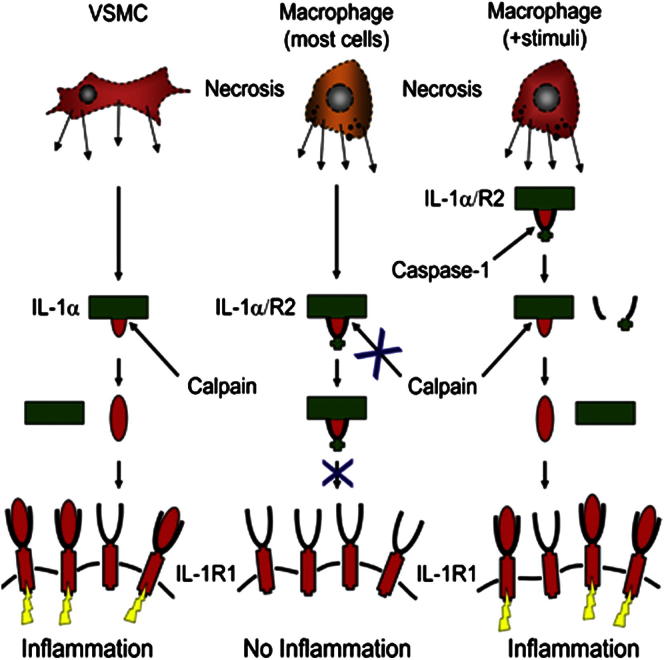
Necrosis can induce profound inflammation or be clinically silent. However, the mechanisms underlying such tissue specificity are unknown. Interleukin-1α (IL-1α) is a key danger signal released upon necrosis that exerts effects on both innate and adaptive immunity and is considered to be constitutively active. In contrast, we have shown that necrosis-induced IL-1α activity is tightly controlled in a cell type-specific manner. Most cell types examined expressed a cytosolic IL-1 receptor 2 (IL-1R2) whose binding to pro-IL-1α inhibited its cytokine activity. In cell types exhibiting a silent necrotic phenotype, IL-1R2 remained associated with pro-IL-1α. Cell types possessing inflammatory necrotic phenotypes either lacked IL-1R2 or had activated caspase-1 before necrosis, which degraded and dissociated IL-1R2 from pro-IL-1α. Full IL-1α activity required cleavage by calpain after necrosis, which increased its affinity for IL-1 receptor 1. Thus, we report a cell type-dependent process that fundamentally governs IL-1α activity postnecrosis and the mechanism allowing conditional release of this blockade.
Graphical Abstract
Highlights
► Necrosis-induced IL-1α inflammatory activity is highly cell type dependent ► Full activity requires calpain cleavage of IL-1α, which increases receptor affinity ► Intracellular IL-1R2 binds IL-1α, preventing cleavage and activity postnecrosis ► Caspase-1 cleaves IL-1R2, reversing binding and restoring IL-1α-dependent responses
Introduction
Understanding why the immune system responds to necrosis and how this is controlled is critical in unraveling multiple human diseases. The “danger” model proposes that immunity responds to nonphysiological cell death, damage, or stress (Matzinger, 1994). Accordingly, necrotic death releases damage-associated molecular patterns (DAMPs), which are sensed as danger and act as universal signals to activate immunity (Chen and Nuñez, 2010; Kono and Rock, 2008; Rock et al., 2010). DAMPs are retained in healthy cells and during apoptosis (Basu et al., 2000; Cohen et al., 2010; Scaffidi et al., 2002), whereas necrosis releases them into the extracellular milieu. Interleukin-1α (IL-1α) is an important DAMP that activates immunity postnecrosis (Chen et al., 2007; Clarke et al., 2010; Cohen et al., 2010; Eigenbrod et al., 2008; Kono et al., 2010; Rao et al., 2007), driving pathologies as diverse as tumorigenesis (Sakurai et al., 2008), atherosclerosis (Clarke et al., 2010; Kamari et al., 2007), graft rejection (Rao et al., 2007, 2008), toxic liver insults (Chen et al., 2007), and ischemia-reperfusion injury (Cohen et al., 2010; Luheshi et al., 2011).
The prototypic IL-1 family is ancient, with homologs identified back to echinoderms (Beck and Habicht, 1986). IL-1α, one of the principal ligands, is expressed by most lineages as a signal peptide-less protein, is not readily secreted (Dinarello, 2009), and is actively retained during apoptosis (Cohen et al., 2010). Once released into the extracellular milieu, IL-1α ligation of the type 1 IL-1 receptor (IL-1R1) leads to multiple proinflammatory effects (Dinarello, 2009), including cytokine secretion, neutrophil recruitment, and upregulation of major histocompatibility complex (MHC) and costimulatory molecules on antigen-presenting cells. IL-1α also has powerful effects on adaptive immunity by enhancing expansion and survival of T cells, differentiation of T helper 17 (Th17) cells, and effector T cell proliferation in the presence of regulatory T cells (Sims and Smith, 2010). These potent effects mean that activity is tightly controlled at multiple levels. Mice deficient in IL-1α or IL-1β exhibit no phenotype. However, mice lacking the IL-1 receptor antagonist (IL-1RA) have small litters and retarded growth and develop spontaneous arthritis-like polyarthropathy, arteritis, and cancer (Dinarello, 2009). Indeed, increased IL-1 activity is a hallmark of many chronic inflammatory conditions, including rheumatoid arthritis, gout, diabetes, atherosclerosis, and psoriasis (Dinarello, 1996, 2009; Duewell et al., 2010; Rajamäki et al., 2010).
IL-1 family members are synthesized as inactive precursors unable to bind their receptor, providing an initial level of control. IL-1β and IL-18 are activated by caspase-1, which requires inflammasome formation. In contrast, IL-33 processing by caspase-3 or caspase-1 results in inactivation (Cayrol and Girard, 2009; Lüthi et al., 2009). Pro-IL-1α (p33) is processed to mature IL-1α (p17) by calpain (Kobayashi et al., 1990), but the biological consequences of cleavage are unknown given that p33 is reported to be fully active. This finding is credited to two papers, but one only discusses p33 activity (March et al., 1985), whereas activity within the second study may be compromised by p33 degradation (Mosley et al., 1987). Interestingly, calpain is activated upon loss of plasma membrane integrity (Wang, 2000), suggesting that calpain cleavage of IL-1α could be a control point for activity postnecrosis. Although a recent study reports increased IL-1α activity after granzyme B cleavage (Afonina et al., 2011), differential efficacy of p33 and p17 IL-1α is still controversial (Gross et al., 2012), and no mechanism to explain this has been reported.
We report that necrosis-induced IL-1α-dependent responses are highly cell type dependent and correlate with calpain cleavage of IL-1α during necrosis. Contrary to current understanding, p33 requires calpain processing for full biological activity, which increases its affinity for IL-1R1. Cell type dependency occurs due to expression of an intracellular form of IL-1R2 that binds IL-1α, preventing calpain cleavage and cytokine activity. After inflammasome activation, caspase-1 specifically cleaves IL-1R2, which abrogates IL-1α binding, allows calpain cleavage, and completely restores IL-1α-dependent responses. Regulated secretion of IL-1α also requires IL-1R2 cleavage. Thus, we report an important cell type-dependent mechanism that fundamentally governs IL-1α activity postnecrosis and the mechanism allowing conditional release of this blockade.
Results
Necrosis-Induced Sterile Inflammation Is Cell Type Specific and Correlates with Calpain Cleavage of pro-IL-1α
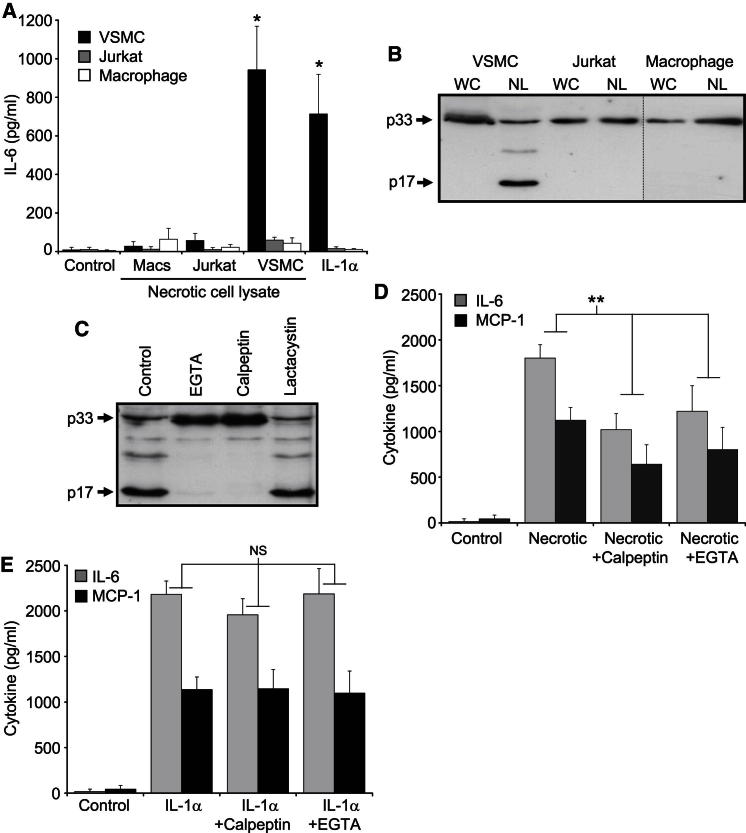
We have recently shown that IL-1α released during vascular smooth muscle cell (VSMC) necrosis is a powerful DAMP that induces local vessel inflammation (Clarke et al., 2010). To determine which other cell types could drive IL-1α-dependent sterile inflammation, we analyzed IL-6 release from VSMCs, Jurkat cells, and primary macrophages treated with lysates from these cell types undergoing necrosis. Only necrotic VSMCs induced significant IL-6 release from viable VSMCs, previously shown to be IL-1α dependent (Clarke et al., 2010), and only VSMCs responded to IL-1α (Figure 1A). Little IL-1α activity was found in membrane fractions of necrotic macrophages or Jurkat cells (Figure S1A available online) or in lysates from cells undergoing hypoxia-induced necrosis (Figure S1B). Although comparable amounts of IL-1α were found in all three cell types, only VSMCs processed p33 to p17 IL-1α (Figure 1B). Calpain cleaves p33 to p17 (Kobayashi et al., 1990); however, the relevance of cleavage to IL-1α activity is unknown. Several reports indicate that processing is not required for secretion (Brough et al., 2003; Prudovsky et al., 2003), and cytokine activity is supposedly independent of cleavage (March et al., 1985; Mosley et al., 1987). VSMCs rapidly cleaved p33 to p17 during necrosis, which was prevented by the calpain inhibitors calpeptin and EGTA but not the proteasomal inhibitor lactacystin (Figure 1C). Necrotic VSMC lysates prepared with calpain inhibition produced significantly reduced responses (Figure 1D), whereas calpeptin or EGTA treatment did not inhibit cytokine production in response to IL-1α (Figure 1E). However, prolonged incubation at 37°C in the presence of calpeptin resulted in some processing of p33 to p17 (Figure S1C), which could contribute to the apparent IL-1α activity seen with calpeptin-treated VSMCs (Figure 1D). Nevertheless, taken together this suggests that, contrary to the accepted literature, p33 is not fully active until cleaved.
Figure 1.
Necrosis-Induced Sterile Inflammation Is Cell Type Specific
(A) IL-6 concentrations in conditioned media of macrophages (Macs), Jurkat cells, or VSMCs incubated with lysates from their respective necrotic cells or with IL-1α.
(B and C) Immunoblots of IL-1α content and processing in whole cell (WC) or necrotic lysates (NL) (B) or in necrotic cell lysates pretreated with protease inhibitors as indicated (C).
(D and E) IL-6 and MCP-1 concentrations in conditioned media of VSMCs incubated with necrotic VSMC lysates made in the presence of protease inhibitors (D), or with IL-1α alone, or with calpeptin or EGTA (E).
Data represent mean ± SD; ∗p ≤ 0.007 versus control, n = 3; ∗∗p ≤ 0.03 (MCP-1), p ≤ 0.002 (IL-6), n ≥ 4. NS, not significant. See also Figure S1.
Calpain-Cleaved p33 and Recombinant p17 Are More Active than p33 IL-1α
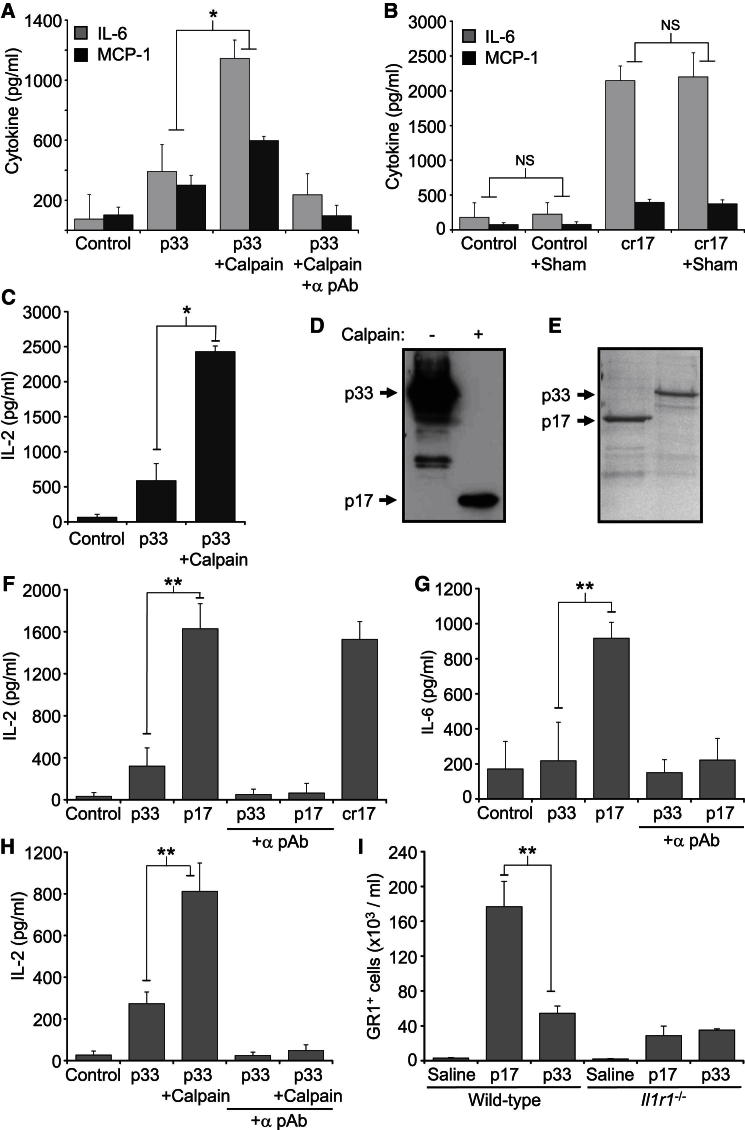
To directly compare p17 and p33 activity, we purified HIS-tagged recombinant proteins. Although we could express and purify p17 in E. coli, p33 was very insoluble and prone to aggregation (not shown)—a finding reported by others (Tokunaga et al., 2010). After denaturation in urea, p33 could be purified and renatured to a soluble protein after sequential dialysis, but this made comparison between p17 and p33 impossible because of the inability to control for refolding efficiency between different proteins. To circumvent this, we cleaved p33 in vitro with calpain, which increased cytokine release compared to uncleaved p33 (Figure 2A). Importantly, a neutralizing antibody to IL-1α reduced responses to control (Figure 2A), whereas a “calpain sham” reaction without p33 neither promoted nor inhibited cytokine release (Figure 2B), indicating that the increased activity is due to processing of p33 to p17. These data were also reproduced cross-species with the classic IL-1-sensitive murine cell line EL4 (Figure 2C), suggesting a conserved requirement for calpain processing of IL-1α. Although calpain cleavage of IL-1α increased activity, these differences represent an underestimate because of the nonstoichiometric conversion of p33 to p17 during in vitro cleavage (Figure 2D).
Figure 2.
Calpain-Cleaved p33 and Recombinant p17 Is More Active than p33 IL-1α
(A) Cytokine concentrations in conditioned media of VSMCs treated with p33 or calpain-cleaved p33, ± IL-1α neutralizing antibody (α pAb).
(B) VSMCs were also treated with calpain sham reactions (no p33) or commercial recombinant p17 IL-1α (cr17) ± calpain sham.
(C) IL-2 concentration in conditioned media of murine EL4 cells treated with p33 ± calpain.
(D) Immunoblot of in vitro cleavage of p33 by calpain.
(E) Coomassie stain of purified soluble p17- and p33-GST fusion proteins.
(F and G) IL-2 (F) or IL-6 (G) concentrations in conditioned media of EL4 or VSMCs, respectively, treated with 1 nM p17- or p33-GST ± α pAb.
(H) IL-2 concentrations in conditioned media of EL4 cells incubated with p33-GST ± calpain, and ± α pAb.
(I) GR1+ cells recruited intraperitoneally in wild-type or Il1r1−/− mice injected with saline or 29 fmol/g p17 or p33.
Data represent mean ± SD or mean ± SEM (I); ∗p ≤ 0.0003, n = 4 (A), n = 3 (B, C) protein preparations and cleavage reactions; ∗∗p ≤ 0.007, n = 4. NS, not significant.
By fusing IL-1α to GST, we purified small amounts of p17 and p33 as soluble proteins (∼145 μg/l) (Figure 2E). Comparison at equal molarities demonstrated significant increases in IL-2 release from EL4 cells (Figure 2F) and IL-6 release from VSMCs for p17 compared to p33 (Figure 2G). IL-1α neutralization reduced responses to control indicating IL-1α-dependent stimulation, excluding effects from copurified bacterial PAMPs (Figures 2F and 2G). To eliminate whether GST-p33 was “functionally dead” as a result of a purification artifact or intrinsic instability, we cleaved it with calpain or incubated it at 37°C for 16 hr, respectively. Cleavage of GST-p33 significantly restored activity (Figure 2H), whereas incubation did not result in protein degradation (not shown), again supporting an inherent lower activity of p33. Lastly, injection of p17 into the peritoneum resulted in significantly increased neutrophil recruitment compared to injection of p33 in wild-type mice, a result that was not seen in Il1r1−/− mice (Figure 2I).
p33 IL-1α Shows Minimal Activity and Behaves as a Partial Agonist due to a Lower Receptor Affinity
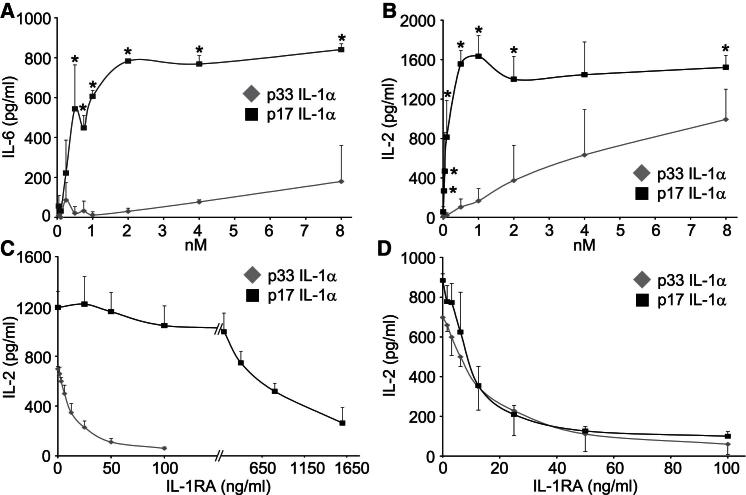
To understand why p33 is less active than p17, we generated cytokine concentration-response curves. VSMCs responded to p17 at 50 pM and saturated at 2 nM (Figure 3A). In contrast, p33 generated responses only at supraphysiological amounts and failed to saturate (Figure 3A). At physiological concentrations of 1 nM, p17 was ∼50 times more active than p33. EL4 cells are very sensitive to IL-1α, with responses detectable with fM amounts. p17 activity was detectable at 5 pM and rapidly saturated at 0.5 nM (Figure 3B). However, p33 gave a linear concentration response, such that 65% of the maximal response was obtained at 8 nM but did not reach saturation (Figure 3B). At 1 nM p17 was ∼10 times more active than p33. To exclude steric hindrance of fused GST only on p33, we specifically cleaved off GST. Comparison of p33 with or without GST revealed identical efficacies at a range of concentrations (Figure S2). Together, this suggests that p33 can bind IL-1R1 at higher concentrations and thus acts as a partial agonist.
Figure 3.
p33 IL-1α Shows Minimal Activity at Physiological Concentrations because of Lower Receptor Affinity
(A and B) Concentration-response curves to p17 and p33, minus control, in VSMCs (50 pM to 8 nM) (A) or EL4 cells (5 pM to 8 nM) (B).
(C and D) IL-2 concentrations in conditioned media from EL4 cells treated with p17 or p33 at 4 nM (C), and 0.1 nM p17 or 4 nM p33 (D), with increasing concentrations of IL-1RA.
Data represent mean ± SD; ∗p ≤ 0.03, n = 3 (A), n ≥ 4 (B); for difference in IC50 p < 0.0001 (C), p = 0.52 (D), n = 3. See also Figure S2.
To investigate whether p33 binds IL-1R1 but fails to induce downstream signaling, we conducted competition experiments with a fixed concentration of p17 (1 nM) and increasing concentrations of p33 (up to 8 nM). However, even 8 nM p33 failed to inhibit cytokine responses (data not shown), suggesting a stronger affinity of p17 for the receptor. IL-1RA binds IL-1R1 but does not induce signaling (Dinarello, 2009). Therefore the difference in concentration of IL-1RA required to inhibit 50% of the cytokine response induced by p17 or p33 (the IC50) directly relates to the difference in affinity of the two ligands for IL-1R1. Responses to 4 nM p33 were completely inhibited by 100 ng/ml IL-1RA (IC50 of 13.7 ± 3.8 ng/ml) (Figure 3C). In contrast, 1,600 ng/ml IL-1RA failed to inhibit responses to 4 nM p17, but 50% inhibition occurred at 633.3 ± 16.7 ng/ml (Figure 3C). This indicates that p33 has an affinity for IL-1R1 46.3 times lower than does p17 (p < 0.0001). To exclude p17 responses from another receptor insensitive to IL-1RA blockade, we repeated with 0.1 nM p17. Under these conditions 100 ng/ml IL-1RA completely inhibited cytokine release and gave an IC50 of 10.3 ± 2.9 ng/ml (Figure 3D). Thus, a 40-fold difference in concentration gave near superimposable curves (p = 0.52), correlating well with the value above. Together these data show that, in contrast to previous reports, IL-1α is not fully active until processed by calpain and that cell types unable to cleave IL-1α induce much smaller IL-1α-dependent responses after necrosis.
An Intracellular Form of IL-1R2 Protects p33 IL-1α from Calpain Processing
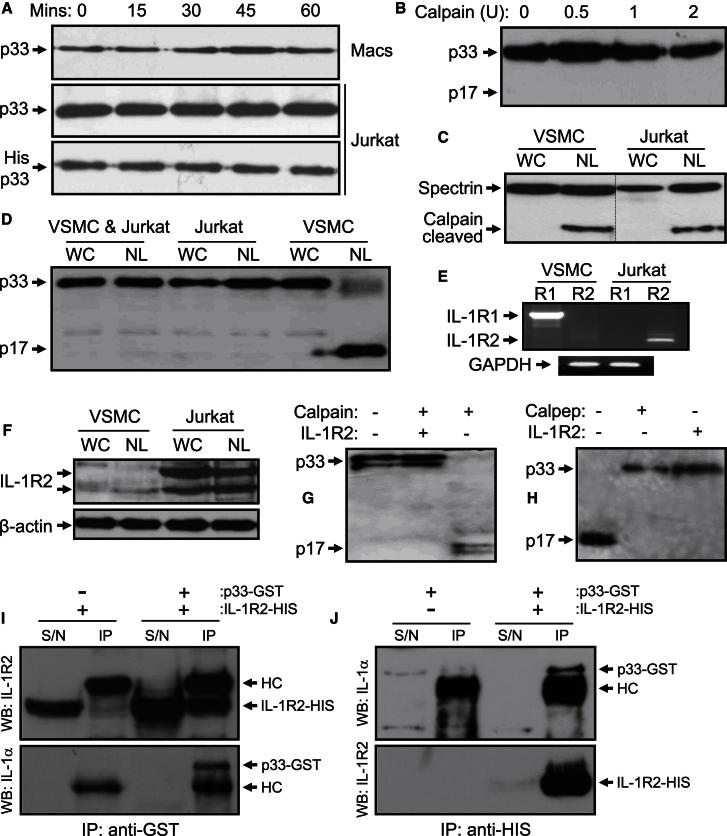
We hypothesized that cell type-specific cleavage of IL-1α could occur through modification of enzyme or substrate, excess of a calpain inhibitor, or a binding partner that protects IL-1α from processing. Jurkat and macrophage necrotic lysates incubated at 37°C for extended periods still showed no processing of endogenous p33 (Figure 4A), whereas recombinant His-p33 spiked into Jurkat lysates also remained uncleaved (Figure 4A), excluding a modification to the cell-derived cytokine. Similar results were also found with primary T lymphocytes (Figure S3A). An excess of calpain inhibitor was excluded, because high calpain activity was found in all necrotic cell types (Figure S3B). Addition of purified calpain to Jurkat necrotic lysates also failed to cleave p33 (Figure 4B), whereas analysis of spectrin, an alternative calpain substrate, revealed calpain-dependent processing in both Jurkat and VSMCs necrotic lysates (Figure 4C).
Figure 4.
An Intracellular Form of IL-1R2 Protects IL-1α from Calpain Processing
(A and B) Immunoblot for endogenous IL-1α processing in necrotic lysates of primary macrophages (Macs) and Jurkat cells (A), for exogenous His-p33 in necrotic Jurkat lysates (A), or for Jurkat necrotic lysates treated with increasing amounts of purified calpain (B).
(C and D) Immunoblot of whole cell (WC) and necrotic (NL) Jurkat and VSMC lysates for α-spectrin (C) or IL-1α after mixing of lysates from both cell types (D).
(E and F) RT-PCR (E) or immunoblot (F) for IL-1 receptors in VSMCs and Jurkat cells.
(G and H) Immunoblot for IL-1α cleavage in a cell-free system with purified calpain (G) and in necrotic VSMC lysates (H), ± 250 ng IL-1R2.
(I and J) Coimmunoprecipitation of IL-1R2 with p33-GST (I) and p33 with IL-1R2-HIS (J) in transfected HEK cell lysates.
See also Figure S3.
Protection of recombinant p33 by Jurkat necrotic lysates suggested that an excess of a binding partner must be present, and therefore that it could be transferred. Indeed, necrotic lysates made from mixed Jurkat and VSMCs displayed no p33 cleavage (Figure 4D). Known binding partners of IL-1α include the type 1 and 2 IL-1 receptors; therefore we treated Jurkat necrotic lysates with a large excess of IL-1RA, which resulted in calpain-specific cleavage of p33 (Figure S3C). The only known receptors for IL-1RA are IL-1R1 and IL-1R2, suggesting that IL-1α may be bound to an intracellular IL-1R. RT-PCR revealed that VSMCs express only IL-1R1, whereas Jurkat cells (Figure 4E) and macrophages (data not shown) express IL-1R2. Immunoblots confirmed IL-1R2 expression in Jurkat cells (Figure 4F), macrophages, and T cells (Figure S3D), whereas addition of recombinant IL-1R2 to a cell-free cleavage reaction (Figure 4G) or necrotic VSMC lysates (Figure 4H) prevented calpain-dependent p33 processing in a dose-dependent manner (Figure S3E). IL-1R2 also antagonized p33 in a dose-dependent manner (Figure S3F). Importantly, IL-1R2 coimmunoprecipitated with p33-GST and p33 with IL-1R2-HIS (Figures 4I and 4J), whereas a proximity ligation assay demonstrated association of endogenous proteins in situ (Figure S3G), supporting a direct interaction. Finally, IL-1R2 also protected IL-1α from cleavage by the inflammatory proteases granzyme B, chymase, and elastase (Figure S3H).
IL-1R2 Silencing Enables Calpain Cleavage of p33 IL-1α and Restores Inflammatory Response to Necrotic Cells
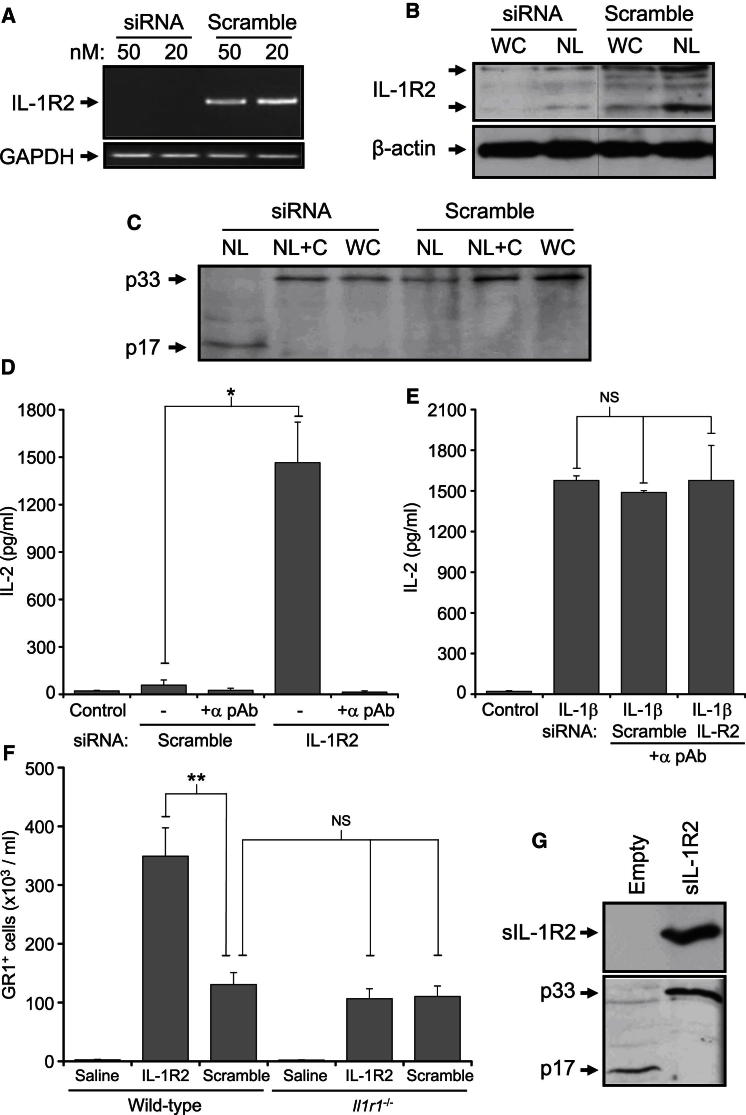
Low transfection efficiency and suspension growth made Jurkat cells unsuitable for siRNA silencing. Therefore, necrotic HeLa cells were tested for p33 cleavage (Figure S4A), calpain activity (data not shown), and IL-1R2 expression (Figures 5A and 5B), which indicated that they responded similarly. siRNA to IL-1R2 reduced both IL-1R2 mRNA (Figure 5A) and protein (Figure 5B), which was not seen with scrambled control. After IL-1R2 silencing, necrotic HeLa lysates displayed calpain-dependent processing of p33 to p17 (Figure 5C) and restoration of IL-1α-dependent responses (Figure 5D), whereas scrambled control cells did not. Because IL-1R2 functions as a decoy receptor that limits bioavailability of IL-1 (Colotta et al., 1993), we examined whether IL-1R2 silencing had reversed “general” IL-1 antagonism within necrotic lysates. By neutralizing all IL-1α activity within necrotic lysates, we compared responsiveness to IL-1β with or without scrambled or IL-1R2-silenced necrotic lysates present (Figure 5E). This revealed no significant difference in IL-2 production, implying that IL-1R2 silencing hadn’t simply reduced general IL-1 antagonism. Sterile peritonitis induced with scrambled control necrotic lysates recruited equal numbers of neutrophils in wild-type and Il1r1−/− mice, therefore representing IL-1-independent responses (Figure 5F). However, IL-1R2-silenced necrotic lysates recruited 3-fold more cells, which was IL-1 dependent because of loss of response in Il1r1−/− mice (Figure 5F). Because previously described forms of IL-1R2 retain a signal peptide (Liu et al., 1996), they would be expected to be in the exocytic pathway. Therefore we determined whether cell surface-shed IL-1R2 (Orlando et al., 1997) protects IL-1α by inhibiting metalloproteases with BB-94; p33, however, remained uncleaved (Figure S4B). Furthermore, immunofluorescence revealed a large amount of IL-1R2 to be intracellular (Figure S4C) in multiple cell types (Figure S4D) and endogenous p33 and IL-1R2 to be highly colocalized (Figure S4E) throughout the cytoplasm (Figure S4F). In addition, subcellular fractionation (Figure S4G) and protease K protection assays (Figure S4H) revealed a native pool of IL-1R2 in the cytosol without internal membrane disruption, as evidenced by a lack of calreticulin in cytosolic fractions. Finally, expression of exogenous soluble IL-1R2 in VSMCs prevented p33 cleavage (Figure 5G). Together these results demonstrate that a cytosolic complex of p33 and IL-1R2 exists in many cell types. During necrosis this prevents calpain cleavage of p33 to the fully active p17 form, which reduces IL-1α-dependent responses.
Figure 5.
Silencing of IL-1R2 Enables IL-1α Processing and Restores Necrotic Cell-Induced Inflammation
(A–C) siRNA-mediated silencing reduces IL-1R2 mRNA (A) and protein in whole cell (WC) and necrotic lysates (NL) (B), which leads to calpain-dependent IL-1α cleavage upon necrosis (C) that is inhibited with calpeptin (+C).
(D and E) IL-2 concentrations in conditioned media of EL4 cells incubated with necrotic lysates from HeLa cells treated with siRNA to IL-1R2 or scrambled, either alone (D) or with IL-1β treatment (E), ± α pAb. Data represent mean ± SD; ∗p = 0.0007, n = 3 independent silencings and treatments. NS, not significant.
(F) GR1+ cells recruited intraperitoneally in wild-type or Il1r1−/− mice injected with saline or 8.3 × 105 control or IL-1R2-silenced necrotic HeLa cells. Data represent mean ± SEM; ∗∗p ≤ 0.006, n ≥ 4. NS, not significant.
(G) Immunoblot for IL-1R2 and IL-1α in necrotic lysates of empty vector or soluble IL-1R2-transfected VSMCs.
See also Figure S4.
Caspase-1 Specifically Cleaves IL-1R2, which Restores IL-1α-Dependent Inflammation Postnecrosis
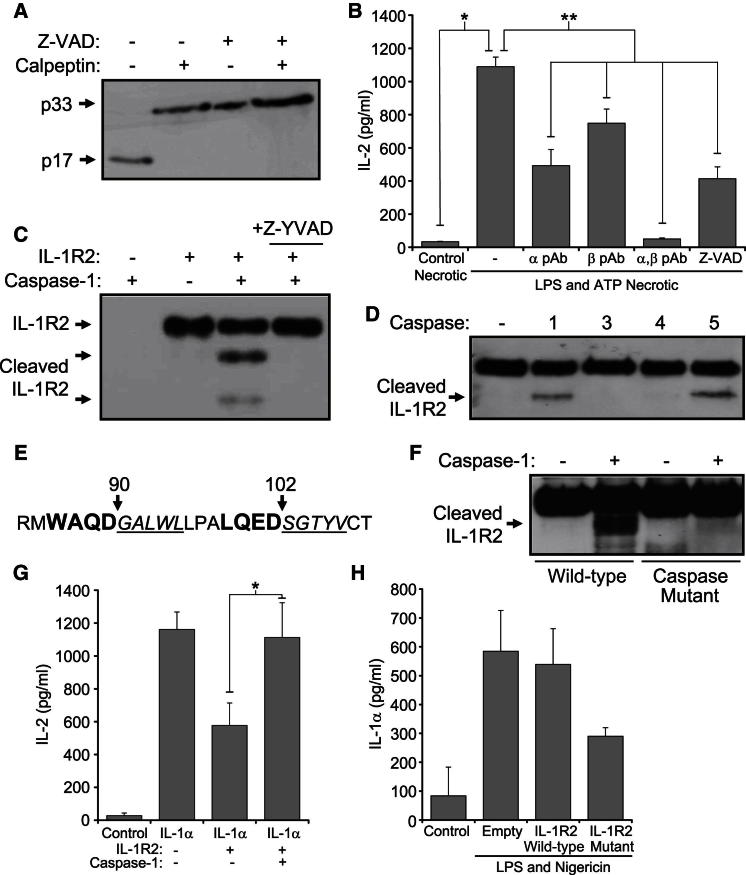
Macrophages are suggested to be the sensors of necrotic-derived DAMPs, which then release IL-1α to drive sterile inflammation (Chen et al., 2007; Kono et al., 2010). However, our current data indicate that macrophage IL-1α is bound to IL-1R2, and therefore is nonfunctional. Because recent work demonstrates that IL-1α secretion requires inflammasome activation (Fettelschoss et al., 2011), we investigated whether this could overcome IL-1R2 blockade. Necrotic lysates made from LPS and ATP-stimulated macrophages displayed processing of p33, which could be prevented by the caspase inhibitor Z-VAD-fmk or calpeptin (Figure 6A). Furthermore, after LPS and ATP treatment, macrophage necrotic lysates induced IL-2 release from EL4 cells, which was blocked with Z-VAD-fmk or IL-1α- and IL-1β-neutralizing antibodies (Figure 6B). IL-1R2 contains many predicted caspase sites (Figure S5A) and cell-free cleavage resulted in caspase-1-specific processing (Figure 6C). IL-1R2 could be cleaved by inflammatory caspase-1 and caspase-5, but not by caspase-4 or the apoptotic caspase-3 (Figure 6D). To confirm the cleavage site, we mutated the P1 position Asp to Ala, resulting in a nonconsensus sequence for caspases. Individual mutation of all sites that could give the correct sized products did not prevent IL-1R2 processing (data not shown). However, Edman degradation of the C-terminal IL-1R2 fragment sequenced two products at equal abundance corresponding to cleavage at two separate Asp residues 12 amino acids apart (Figure 6E), and subsequent mutation of both these sites prevented cleavage (Figure 6F). The crystal structure of IL-1R2 complexed to IL-1 (Wang et al., 2010) revealed that cleavage at these sites would remove the D1 domain vital for binding. Indeed, antagonistic activity toward IL-1α was lost upon processing (Figure 6G), whereas sham cleavage reactions neither promoted nor inhibited IL-2 release (Figure S5B), confirming that cleaved IL-1R2 cannot bind IL-1α. Finally, caspase-1 cleavage of IL-1R2 appears to be a prerequisite for physiological IL-1α secretion, as indicated by the fact that expression of the noncleavable IL-1R2 mutant reduced IL-1α release (Figure 6H). Therefore, after exposure to stimuli that activate inflammasomes, intracellular IL-1R2 is cleaved by caspase-1, causing it to dissociate from IL-1α. Subsequently, during necrosis calpain can now cleave IL-1α to the fully active form, which allows the generation of robust IL-1α-dependent responses.
Figure 6.
Caspase-1 Cleavage of IL-1R2 Restores Necrotic Cell-Induced IL-1α-Dependent Inflammation
(A) Immunoblot for IL-1α in necrotic lysates from LPS- and ATP-treated macrophages ± calpeptin (during lysis) or ± caspase inhibitor Z-VAD (during LPS and ATP treatment).
(B) IL-2 concentrations in conditioned media of EL4 cells incubated with necrotic lysates from control or LPS- and ATP-treated macrophages ± α and/or β pAb, or ± Z-VAD (during LPS and ATP treatment). Data represent mean ± SEM; ∗p < 0.00004, ∗∗p ≤ 0.02, n ≥ 3.
(C and D) Immunoblot for IL-1R2 cleavage after incubation with active caspase-1 ± Z-YVAD (C) or a panel of active caspases (D).
(E) Edman degradation of cleaved IL-1R2 detected two sequences (italic underlined) corresponding to processing at two tetrapeptide sites (large bold).
(F) Immunoblot for cleaved IL-1R2 after mutation of both caspase-1 sites.
(G) IL-2 concentrations in conditioned media of EL4 cells incubated with IL-1α, and IL-1R2 ± caspase-1 cleavage. Data represent mean ± SD; ∗p = 0.009, n = 3.
(H) IL-1α concentration in conditioned media of activated THP-1 cells transfected with caspase site mutant IL-1R2, or as indicated. Data representative of mean ± SD from n = 2.
See also Figure S5.
Discussion
Although an immune response to necrosis may resolve the original pathology and initiate repair, recruited leukocytes can damage the surrounding tissue. This sterile inflammatory response can also lead to unwanted activation of adaptive immunity. Many human diseases are driven by activation of these pathways, including ischemia-reperfusion injury, Alzheimer’s disease, atherosclerosis, and toxic insults to liver and lung (Chen and Nuñez, 2010; Kono and Rock, 2008; Stewart et al., 2010). Therefore, tight regulation of both release and activity of DAMPs is critical for host defense.
The current understanding is that p33 is fully active and therefore requires only cell lysis to signal after necrosis. In contrast, we find that activity is kept under tight control at multiple levels in a cell type-dependent and conditional manner. Blocking calpain processing of endogenous p33 during necrosis significantly decreases activity, and comparison of recombinant proteins demonstrates up to ∼50-fold increased activity of p17 compared to p33. This difference occurs at the receptor, with a ∼50-fold greater affinity of p17 for IL-1R1. IL-1α cleavage and activity after necrosis is cell type dependent because of expression of a cytosolic IL-1R2, which binds and protects IL-1α from cleavage and prevents IL-1α activity. IL-1R2 also protected IL-1α from granzyme B, chymase, and elastase cleavage, suggesting that the primary control over IL-1α activity postnecrosis is by IL-1R2 binding. Lastly, active caspase-1 specifically cleaves IL-1R2, causing dissociation from IL-1α, calpain processing, and complete restoration of IL-1α activity after necrosis or during regulated secretion.
DAMPs need to be retained on apoptosis, released on necrosis, and able to activate the immune system. IL-1α is near universally expressed (Dinarello, 2009), lacks a signal peptide, is rarely extracellular under normal physiology, and also associates with chromatin during apoptosis, resulting in retention (Cohen et al., 2010). Other identified DAMPS include heat shock proteins, uric acid, HMGB1, ATP, and dsDNA (Kono and Rock, 2008). These DAMPs utilize specific cognate receptors, such as RAGE, or pattern recognition receptors, such as TLRs, which have a limited expression pattern that restricts cell types able to respond. In contrast, IL-1R1 is widely expressed throughout many lineages (Dinarello, 2009), enabling IL-1α to act as a “universal” DAMP. Although this may provide more efficient detection of necrosis, it could predispose the immune system to overreact, perhaps explaining the extensive control over IL-1α activity via release, IL-1R2 binding, and calpain cleavage. This cell type and conditional dependency for IL-1α activity after necrosis perhaps suggests an immune advantage in allowing only some cell types to utilize IL-1α as a DAMP. Previous studies concluded that IL-1α is only a secondary signaling molecule released by macrophages during sterile inflammation (Chen et al., 2007; Kono et al., 2010). However, this finding might have occurred due to the use of necrotic cell types that express IL-1R2 and therefore cannot cleave and utilize IL-1α (Y.Z., M.H., and M.C.H.C.; data not shown). Indeed, whereas control necrotic lysates induce an equivalent peritonitis in both wild-type and Il1r1−/− mice (representing the net effect of all other DAMPS, independent of IL-1), necrotic cells without IL-1R2 induced a 3-fold greater response, which was all IL-1 dependent. This key observation implies that without IL-1R2 expression, the single most powerful DAMP within necrotic cells is IL-1α.
Our studies also identify multiple mechanisms that could give a tissue- and cell type-dependent response to necrosis. Given that p33 has some activity, dependent on its concentration and sensitivity of the responding cells, extensive necrosis in an IL-1R1-rich tissue (e.g., liver) could activate immunity regardless of IL-1α cleavage. Conversely, minimal necrosis in an IL-1R1-low tissue (e.g., kidney) that cannot cleave p33 may not respond to IL-1α at all. If necrosis occurs with a stimulus that activates caspase-1 (e.g., infection), IL-1R2 blockade is abrogated and cleaved fully active p17 can signal to any IL-1R1-expressing cell. Necrosis of cells that can cleave IL-1α within IL-1R1-dense tissues would give extensive immune activation (e.g., vascular tissue). Where the amount of IL-1R2, IL-1R1, and necrosis preclude IL-1α activity, the response to necrosis could be deferred to other DAMPs and specialist immune cells, perhaps giving more control over reactions. Additionally, activation of IL-1α during necrosis could act as a “tipping point” that drives an inflammatory response toward adaptive immunity. Indeed, this is the case in graft-versus-host disease, where injured endothelial cell-derived IL-1α induces intimal T cell recruitment and IL-17 production, driving human artery allograft rejection (Rao et al., 2007, 2008).
Necrosis is induced by many stimuli including physical trauma, chemical stress, and bacterial toxins and is characterized by loss of plasma membrane integrity (Kono and Rock, 2008). Calpains are activated by Ca2+ entry after loss of membrane integrity (Wang, 2000), and in VSMCs p33 cleavage occurs immediately upon necrosis. Granzyme B is proposed to activate IL-1α extracellularly (Afonina et al., 2011); but given its inefficiency to process IL-1α relative to calpain (Afonina et al., 2011), cleavage within the necrotic body by calpain probably predominates when IL-1R2 is absent. IL-1α and IL-1β arose from ancient gene duplications (Dinarello, 2009) but have diverged molecularly. IL-1α is a DAMP that is activated by a protease intrinsically linked to necrosis, whereas IL-1β is activated in response to PAMPs by a protease family intrinsically linked to apoptosis and inflammation. However, these current data indicate that in many cells IL-1α function requires both calpain and caspase-1. Secretion of IL-1α from macrophages (Fettelschoss et al., 2011) or dendritic cells (DCs) (Gross et al., 2012) requires inflammasome activation, but given that IL-1α is not a caspase substrate, this has been puzzling (Keller et al., 2008). We suggest that IL-1R2 is the likely target of caspase-1 during physiological IL-1α release. Indeed, expression of an uncleavable IL-1R2 mutant reduces IL-1α release, whereas Casp1−/− mice have long been known to be deficient in IL-1α secretion (Kuida et al., 1995; Li et al., 1995).
An important finding of this work is that IL-1R2 binds p33 in the cytosol. Although the previously described splice variant of IL-1R2 (Liu et al., 1996) loses the transmembrane domain, it still codes for a signal peptide and should be secreted. Despite this, our data show that a large pool of IL-R2 exists in the cytosol. One explanation for this is that the IL-1R2 signal peptide is short (13 amino acids) and is predicted to be relatively weak (Signal P; Y-max = 0.316) compared to other secreted proteins (e.g., IL-8; Y-max = 0.790), and many proteins with signal peptides can be found in the cytosol (Davis et al., 2006). Indeed, multiple factors determine how much of a signal peptide-containing protein ends up nontargeted, including the signal peptide and flanking amino acid sequences and cell type and growth condition effects (Levine et al., 2005). Clearly further work is needed to elucidate the specific form of IL-1R2 that binds p33, the mechanisms that generate it, and how this is controlled.
Cell type-specific effects of IL-1R2 expression may occur in a number of diseases. Induction of antitumor immunity in vivo is dependent upon the mode of cell death, whereby necrosis is more immunogenic than apoptosis (Melcher et al., 1998) and local IL-1 activity (Ghiringhelli et al., 2009; Michaud et al., 2011). Necrosis can induce maturation of DCs and expression of costimulatory molecules and can stimulate T cells (Basu et al., 2000; Sauter et al., 2000)—all processes critical for antitumor immunity and all known activities of IL-1. Indeed, differences are reported between the necrotic cell type and ability to induce DC maturation (Sauter et al., 2000), perhaps reflecting IL-1R2 expression and thus ability of necrotic cell-derived IL-1α to modulate antigen-presenting cell function. Intriguingly, IL-1R2 is upregulated in some tumors including pancreatic ductal adenocarcinoma (Rückert et al., 2010) and ovarian cancer, where it provides a powerful distinction between primary and recurrent tumors (Laios et al., 2008). Finally, atherosclerosis is highly dependent on IL-1 (Chamberlain et al., 2009; Chi et al., 2004; Duewell et al., 2010), although recent work casts doubt on IL-1β’s role (Menu et al., 2011), suggesting that IL-1α could be the major ligand affecting atherosclerotic plaques. Indeed, necrotic VSMC-released IL-1α is a potent inducer of local vessel inflammation in vivo (Clarke et al., 2010), and given that VSMCs express high amounts of IL-1α and IL-1R1 but little IL-1R2, their death is highly inflammatory.
In summary, in addition to acting as a decoy receptor, IL-1R2 also plays a hitherto unreported role that fundamentally controls IL-1α activity postnecrosis. Cells expressing intracellular IL-1R2 release little active IL-1α after necrosis without prior activation of caspase-1, which processes IL-1R2. In contrast, necrotic cells devoid of IL-1R2 are inherently powerful inducers of sterile inflammation able to fully activate IL-1α upon calpain cleavage. Thus, changes in intracellular IL-1R2 expression may underlie and modulate many chronic inflammatory diseases or other pathologies involving cellular necrosis.
Experimental Procedures
All materials are from Sigma-Aldrich unless otherwise stated.
Cell Culture
VSMCs, EL4, HEK, and HeLa cells were cultured in DMEM and Jurkat and THP-1 cells in RPMI 1640, all supplemented with penicillin, streptomycin, L-glutamine, and 10% FCS. Human monocyte-derived macrophages were differentiated as described previously (Brown et al., 2000). Cells were treated as indicated with Calpeptin (30 μM), Lactacystin (10 μM; both Biomol), IL-1α pAb (1 μg/ml), IL-1α and IL-1β (all PeproTech), IL-1RA (Amgen), IL-1R2 (250 ng; R&D), Z-YVAD, Z-VAD-fmk (both 10 μm; Bachem), LPS (1 μg/ml), EGTA (5 mM), and BB-94 (1 μg/ml; Tocris). Cells in serum-free (SF) DMEM were disrupted by freeze thaw in liquid N2, clarified, and stored at −80°C. Cells were also made necrotic by incubation with 7-BIO (25 μM; Enzo) or digitonin (0.1%) (data not shown) or by overnight hypoxic exposure. To activate inflammasomes, cells were treated with LPS (1 μg/ml; 4 hr), followed by ATP (5 mM) or Nigericin (20 μM) for 30 min. Calpain activity was determined with Calpain-Glo (Promega). VSMCs, HEK, and THP-1 cells were transfected with pcDNA3 (Invitrogen) with nucleofection (Amaxa) or FugeneHD (Promega).
Protein Expression and Purification
Human p33 (1-271) or p17 (119-271) was cloned into pET15b (Novagen) or pGEX-4T-3 (GE). Human IL-1R2 (1-296) was cloned into pGEX-4T-3. For His purification, IPTG-induced cultures were lysed in BugBuster (Novagen) with benzonase, lysozyme, and protease inhibitors (10 min, RT). Urea (6 M) was added and incubated (10 min) before clarification and purification on a Ni2+ column. Columns were washed (40 mM imidazole) and eluted (250 mM imidazole). Concentrated protein (Vivaspin) was dialyzed against decreasing urea (4 M, 2 M, 0 M) in 10 mM Tris (pH 8.0), 50 mM NaCl. For GST purification, IPTG-induced cultures were lysed in 50 mM Na2HPO4 (pH 7.5), 150 mM NaCl, 1 mM DTT, 1 mM EDTA, with benzonase, lysozyme, and protease inhibitors (30 min, RT), clarified, applied to glutathione agarose (QIAGEN), washed, eluted (50 mM reduced glutathione), concentrated, and dialyzed against 10 mM Tris (pH 8.0), 50 mM NaCl. GST tag was removed with biotinylated thrombin (Novagen). Protein concentration was determined (660 nm; Thermo Scientific), checked by Coomassie staining, and if necessary adjusted and rechecked. Proteins were stored in 10% glycerol (−80°C).
Protease Cleavage
His- or GST-p33 was incubated in 10 mM Tris (pH 7.5), 150 mM NaCl, 1 mM DTT, 2 mM CaCl2, with calpain (Calbiochem) ± calpeptin or IL-1R2 (RT). IL-1R2 was incubated with active caspase-1, caspase-3, caspase-4, or caspase-5 (Promokine) in 50 mM HEPES (pH 7.2), 50 mM NaCl, 0.1% CHAPS, 10 mM EDTA, 5% glycerol, and 10 mM DTT (37°C). Jurkat lysates were also incubated with granzyme B (100 nM; Cambridge Bioscience), chymase, or elastase (100 nM; both Enzo).
Cytokine Release Assay
VSMCs were plated, adhered overnight, and incubated in SF DMEM (∼24 hr). Fresh SF DMEM and treatments were added and incubated (6 hr). EL4 cells were washed and plated in SF DMEM with treatments and incubated (24 hr). 17.5 × 103 necrotic VSMCs or macrophages or 5.25 × 104 necrotic Jurkat or HeLa cells were used per 500 μl. Supernatants were clarified and cytokines assayed by ELISA (PeproTech) or Cytomix (eBioscience).
Immunoblotting, Edman Degradation, and Co-IP
Whole cell or necrotic lysates from 1 × 105 VSMCs or macrophages, or 3 × 105 Jurkat, HeLa, or T cells were loaded per lane. Antibodies used were IL-1α (PeproTech), His (GE), α-spectrin (Millipore), β-actin (Sigma), calreticulin (Cell Signaling), HDAC1 (Santa Cruz), and IL-1R2 (R&D). Caspase-1-cleaved IL-1R2 was separated, electroblotted to PVDF, and stained and bands were excised for Edman degradation (ABI Procise 494HT). For co-IP, transfected HEK cells were incubated on ice (50 mM Tris [pH 8], 150 mM NaCl, 1% Triton X-100, protease inhibitors), freeze thawed, clarified, and incubated with 2 μg of anti-His or anti-GST (GE) (16 hr, 4°C). Immunocomplexes were precipitated with magnetic protein-G beads (Dynal) and beads washed before elution with Laemmli buffer.
RT-PCR
RNA was extracted with TRI reagent, DNase treated (Ambion), and reverse transcribed (Promega) before PCR with the following primers: IL-1R1, AGGAGACGGAGGACTTGTGT and GCGTCATAGGTCTTTCCATC; total IL-1R2, CATTACAAGCGGGAGTTCAG and TAGTGCAGACGTAGGTGCCA; soluble IL-1R2, TGGCACCTACGTCTGCACTA and TGTCTCCAAAAGGAAGAGCGA; GAPDH, TGTTGCCATCAATGACCCCTT and CTCCACGACTGACTCAGCG.
siRNA Silencing
IL-1R2 silencing was performed with SMARTpool siRNA and controls (Dharmacon). In brief, HeLa cells were transfected with 20 nM of siRNA via HiPerFect (QIAGEN), retransfected after 48 hr, and harvested 48 hr later.
Immunofluorescence
For IL-1R2, cells were fixed in 2% formaldehyde and permeabilized with 0.5% NP-40. For IL-1R2 and p33, cells were fixed in 2% formaldehyde, washed, and fixed with methanol. All were blocked in 1% BSA, incubated with IL-1R2 mAb (R&D) and anti-N-terminal IL-1α (Aviva Systems Biology), washed, incubated with Alexa Fluor antibodies (Molecular Probes), washed, and imaged on a BX51 (Olympus) or a TCS SP2 AOBS (Leica) microscope. PLA assays with the primary antibodies as above were conducted according to the manufacturer (Olink). For flow cytometry, cells were fixed in 2% formaldehyde, washed, blocked in 1% BSA and 0.05% sodium azide, stained with anti-IL-1R2, washed, stained with secondary antibodies, washed, and analyzed on a C6 flow cytometer (Accuri). Intracellular staining steps had 0.5% saponin present.
Animal Protocols
Experiments were conducted under UK Home Office licensing. Mice were injected intraperitoneally with saline, 0.29 fmol/g body weight of p17 or p33, or 8.3 × 104 necrotic HeLa cells. After 6 hr, the peritoneal cavity was lavaged with 5 ml of PBS. GR-1+ cells were counted by FACS after anti-GR-1-FITC staining (Biolegend).
Statistics
Parametric tests were employed for analysis of continuous ELISA and peritonitis data, conducted with a one-way, two-tailed ANOVA (Excel).
Acknowledgments
We gratefully acknowledge monocyte donations from E. Chilvers’ laboratory and J. Skepper for help with confocal. This study was supported by British Heart Foundation Grants FS/09/005/26845 (M.C.H.C.), PG/06/024/20354, and RG 04/001 (M.R.B.), and NIHR Cambridge BRC.
Published: February 7, 2013
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2013.01.008.
Supplemental Information
References
- Afonina I.S., Tynan G.A., Logue S.E., Cullen S.P., Bots M., Lüthi A.U., Reeves E.P., McElvaney N.G., Medema J.P., Lavelle E.C., Martin S.J. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1α. Mol. Cell. 2011;44:265–278. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Binder R.J., Suto R., Anderson K.M., Srivastava P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Beck G., Habicht G.S. Isolation and characterization of a primitive interleukin-1-like protein from an invertebrate, Asterias forbesi. Proc. Natl. Acad. Sci. USA. 1986;83:7429–7433. doi: 10.1073/pnas.83.19.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough D., Le Feuvre R.A., Wheeler R.D., Solovyova N., Hilfiker S., Rothwell N.J., Verkhratsky A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J. Immunol. 2003;170:3029–3036. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- Brown S.B., Clarke M.C., Magowan L., Sanderson H., Savill J. Constitutive death of platelets leading to scavenger receptor-mediated phagocytosis. A caspase-independent cell clearance program. J. Biol. Chem. 2000;275:5987–5996. doi: 10.1074/jbc.275.8.5987. [DOI] [PubMed] [Google Scholar]
- Cayrol C., Girard J.P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J., Francis S., Brookes Z., Shaw G., Graham D., Alp N.J., Dower S., Crossman D.C. Interleukin-1 regulates multiple atherogenic mechanisms in response to fat feeding. PLoS ONE. 2009;4:e5073. doi: 10.1371/journal.pone.0005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.Y., Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.J., Kono H., Golenbock D., Reed G., Akira S., Rock K.L. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Chi H., Messas E., Levine R.A., Graves D.T., Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- Clarke M.C., Talib S., Figg N.L., Bennett M.R. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ. Res. 2010;106:363–372. doi: 10.1161/CIRCRESAHA.109.208389. [DOI] [PubMed] [Google Scholar]
- Cohen I., Rider P., Carmi Y., Braiman A., Dotan S., White M.R., Voronov E., Martin M.U., Dinarello C.A., Apte R.N. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc. Natl. Acad. Sci. USA. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F., Re F., Muzio M., Bertini R., Polentarutti N., Sironi M., Giri J.G., Dower S.K., Sims J.E., Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Davis M.J., Hanson K.A., Clark F., Fink J.L., Zhang F., Kasukawa T., Kai C., Kawai J., Carninci P., Hayashizaki Y., Teasdale R.D. Differential use of signal peptides and membrane domains is a common occurrence in the protein output of transcriptional units. PLoS Genet. 2006;2:e46. doi: 10.1371/journal.pgen.0020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., Abela G.S., Franchi L., Nuñez G., Schnurr M. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrod T., Park J.H., Harder J., Iwakura Y., Núñez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J. Immunol. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettelschoss A., Kistowska M., LeibundGut-Landmann S., Beer H.D., Johansen P., Senti G., Contassot E., Bachmann M.F., French L.E., Oxenius A., Kündig T.M. Inflammasome activation and IL-1β target IL-1α for secretion as opposed to surface expression. Proc. Natl. Acad. Sci. USA. 2011;108:18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F., Apetoh L., Tesniere A., Aymeric L., Ma Y., Ortiz C., Vermaelen K., Panaretakis T., Mignot G., Ullrich E. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Gross O., Yazdi A.S., Thomas C.J., Masin M., Heinz L.X., Guarda G., Quadroni M., Drexler S.K., Tschopp J. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Kamari Y., Werman-Venkert R., Shaish A., Werman A., Harari A., Gonen A., Voronov E., Grosskopf I., Sharabi Y., Grossman E. Differential role and tissue specificity of interleukin-1alpha gene expression in atherogenesis and lipid metabolism. Atherosclerosis. 2007;195:31–38. doi: 10.1016/j.atherosclerosis.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Keller M., Rüegg A., Werner S., Beer H.D. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Yamamoto K., Saido T., Kawasaki H., Oppenheim J.J., Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc. Natl. Acad. Sci. USA. 1990;87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H., Rock K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H., Karmarkar D., Iwakura Y., Rock K.L. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J. Immunol. 2010;184:4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuida K., Lippke J.A., Ku G., Harding M.W., Livingston D.J., Su M.S., Flavell R.A. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Laios A., O’Toole S.A., Flavin R., Martin C., Ring M., Gleeson N., D’Arcy T., McGuinness E.P., Sheils O., Sheppard B.L., O’ Leary J.J. An integrative model for recurrence in ovarian cancer. Mol. Cancer. 2008;7:8. doi: 10.1186/1476-4598-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine C.G., Mitra D., Sharma A., Smith C.L., Hegde R.S. The efficiency of protein compartmentalization into the secretory pathway. Mol. Biol. Cell. 2005;16:279–291. doi: 10.1091/mbc.E04-06-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Liu C., Hart R.P., Liu X.J., Clevenger W., Maki R.A., De Souza E.B. Cloning and characterization of an alternatively processed human type II interleukin-1 receptor mRNA. J. Biol. Chem. 1996;271:20965–20972. doi: 10.1074/jbc.271.34.20965. [DOI] [PubMed] [Google Scholar]
- Luheshi N.M., Kovács K.J., Lopez-Castejon G., Brough D., Denes A. Interleukin-1α expression precedes IL-1β after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J. Neuroinflammation. 2011;8:186. doi: 10.1186/1742-2094-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A.U., Cullen S.P., McNeela E.A., Duriez P.J., Afonina I.S., Sheridan C., Brumatti G., Taylor R.C., Kersse K., Vandenabeele P. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- March C.J., Mosley B., Larsen A., Cerretti D.P., Braedt G., Price V., Gillis S., Henney C.S., Kronheim S.R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985;315:641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Melcher A., Todryk S., Hardwick N., Ford M., Jacobson M., Vile R.G. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat. Med. 1998;4:581–587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- Menu P., Pellegrin M., Aubert J.F., Bouzourene K., Tardivel A., Mazzolai L., Tschopp J. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M., Martins I., Sukkurwala A.Q., Adjemian S., Ma Y., Pellegatti P., Shen S., Kepp O., Scoazec M., Mignot G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- Mosley B., Urdal D.L., Prickett K.S., Larsen A., Cosman D., Conlon P.J., Gillis S., Dower S.K. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J. Biol. Chem. 1987;262:2941–2944. [PubMed] [Google Scholar]
- Orlando S., Sironi M., Bianchi G., Drummond A.H., Boraschi D., Yabes D., Mantovani A. Role of metalloproteases in the release of the IL-1 type II decoy receptor. J. Biol. Chem. 1997;272:31764–31769. doi: 10.1074/jbc.272.50.31764. [DOI] [PubMed] [Google Scholar]
- Prudovsky I., Mandinova A., Soldi R., Bagala C., Graziani I., Landriscina M., Tarantini F., Duarte M., Bellum S., Doherty H., Maciag T. The non-classical export routes: FGF1 and IL-1alpha point the way. J. Cell Sci. 2003;116:4871–4881. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- Rajamäki K., Lappalainen J., Oörni K., Välimäki E., Matikainen S., Kovanen P.T., Eklund K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D.A., Tracey K.J., Pober J.S. IL-1alpha and IL-1beta are endogenous mediators linking cell injury to the adaptive alloimmune response. J. Immunol. 2007;179:6536–6546. doi: 10.4049/jimmunol.179.10.6536. [DOI] [PubMed] [Google Scholar]
- Rao D.A., Eid R.E., Qin L., Yi T., Kirkiles-Smith N.C., Tellides G., Pober J.S. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J. Exp. Med. 2008;205:3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K.L., Latz E., Ontiveros F., Kono H. The sterile inflammatory response. Annu. Rev. Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückert F., Dawelbait G., Winter C., Hartmann A., Denz A., Ammerpohl O., Schroeder M., Schackert H.K., Sipos B., Klöppel G. Examination of apoptosis signaling in pancreatic cancer by computational signal transduction analysis. PLoS ONE. 2010;5:e12243. doi: 10.1371/journal.pone.0012243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., He G., Matsuzawa A., Yu G.Y., Maeda S., Hardiman G., Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter B., Albert M.L., Francisco L., Larsson M., Somersan S., Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P., Misteli T., Bianchi M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Sims J.E., Smith D.E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Stewart C.R., Stuart L.M., Wilkinson K., van Gils J.M., Deng J., Halle A., Rayner K.J., Boyer L., Zhong R., Frazier W.A. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga H., Saito S., Sakai K., Yamaguchi R., Katsuyama I., Arakawa T., Onozaki K., Arakawa T., Tokunaga M. Halophilic beta-lactamase as a new solubility- and folding-enhancing tag protein: production of native human interleukin 1alpha and human neutrophil alpha-defensin. Appl. Microbiol. Biotechnol. 2010;86:649–658. doi: 10.1007/s00253-009-2325-9. [DOI] [PubMed] [Google Scholar]
- Wang K.K. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- Wang D., Zhang S., Li L., Liu X., Mei K., Wang X. Structural insights into the assembly and activation of IL-1β with its receptors. Nat. Immunol. 2010;11:905–911. doi: 10.1038/ni.1925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.