Summary
Cell diversity and organization in the neural tube depend on the integration of extrinsic signals acting along orthogonal axes. These are believed to specify distinct cellular identities by triggering all-or-none changes in expression of combinations of transcription factors [1]. Under the influence of a common dorsoventral signal, sonic hedgehog, and distinct anterior-posterior (A-P) inductive signals [2, 3], two topographically related progenitor pools that share a common transcriptional code produce serotonergic and V3 neurons in the hindbrain and spinal cord, respectively [4–7]. These neurons have different physiological properties, functions, and connectivity [8, 9]. Serotonergic involvement in neuropsychiatric diseases has prompted greater characterization of their postmitotic repertoire of fate determinants, which include Gata2, Lmx1b, and Pet1 [10], whereas V3 neurons express Sim1 [4]. How distinct serotonergic and V3 neuronal identities emerge from progenitors that share a common transcriptional code is not understood. Here, we show that changes in retinoid activity in these two progenitor pools determine their fates. Retinoids, via Notch signaling, control the expression level in progenitors of the transcription factor Ascl1, which selects serotonergic and V3 neuronal identities in a dose-dependent manner. Therefore, quantitative differences in the expression of a single component of a transcriptional code can select distinct cell fates.
Highlights
► Graded Ascl1 expression in the neural tube specifies distinct neuronal subtypes ► Retinoid activity and Ascl1 expression are inversely correlated ► Binary differences in retinoid signaling regulate graded Ascl1 expression via Notch ► Dose-dependent effects of Ascl1 explains regional segregation of neuronal subtypes
Results
Through the inductive effects of signaling molecules such as sonic hedgehog [4] and fibroblast growth factors [3], hindbrain serotonergic (5HT) progenitors acquire a Foxa2+Nkx2.2+Ascl1+ transcriptional code. Topographically related V3 neural progenitors in the spinal cord express the same three factors (see Figure S1A available online). All three factors have been shown to be necessary for the generation of 5HT neurons, and Nkx2.2 and Ascl1 are critical for V3 neurogenesis [4, 5, 11, 12]. Consistent with its expression in spinal cord p3 progenitors, Foxa2 is also required for the generation of V3 neurons (Figures S1B, S1D, and S1E) [13]. This raises the question of how hindbrain and spinal p3 progenitors, designated p3[5HT] and p3[V3], respectively, generate different cell types.
Retinoids Regulate p3 Progenitor Identity
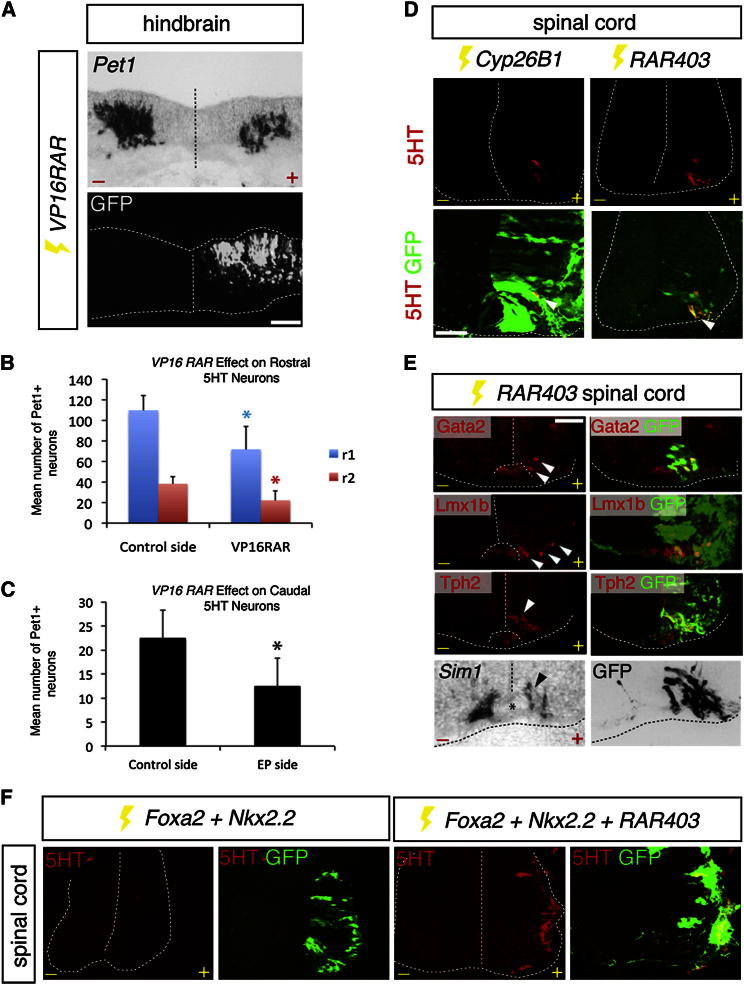
We analyzed β-gal expression in the hindbrain and spinal cord of RARE-LacZ transgenic reporter mice [14]. The hindbrain has a segmental organization and is composed of eight compartments termed rhombomeres [15]. β-gal immunostaining revealed low or absent RA signaling in p3[5HT] progenitors at multiple rhombomeric levels, but high levels of activity in p3[V3] cells (Figure S2A). This is consistent with other assays of retinoid signaling in amniotes (for example, see [16, 17]) and suggested that retinoid signaling could be involved in p3 progenitor fate determination. To test this, we took advantage of a constitutively active retinoid receptor α derivative, VP16RAR, which has previously been shown to activate retinoic acid receptor target genes independently of RA [18]. Forced expression of VP16RAR by in ovo electroporation reduced 5HT neuronal differentiation by 40%–50% throughout the A-P extent of the hindbrain (Figures 1A–1C). Concomitantly, occasional ectopic V3 neurons, marked by expression of the postmitotically expressed transcription factor Sim1, were produced in the caudal hindbrain (data not shown) [4]. Conversely, blockade of retinoid signaling in p3[V3] progenitors, either by misexpression of Cyp26B1, a member of the cytochrome P450 class of enzymes that degrades retinoic acid, or RAR403, a dominant negative version of the human RA receptor α [19], resulted in ectopic 5HT neuronal generation (Figure 1D). These neurons expressed the postmitotic serotonergic determinants Gata2 [20] and Lmx1b [21, 22], but not Pet1 [23], and they also expressed the terminal differentiation marker Tph2 [24] (Figure 1E). Therefore, ectopic spinal 5HT neurons share the same differentiation pathway as hindbrain 5HT neurons, of which approximately one-third do not depend on Pet1 for their generation [25, 26].
Figure 1.
Retinoid Signaling Determines p3 Progenitor Fate
(A) Upregulation of RA signaling in hindbrain p3[5HT] progenitors by misexpression of VP16RAR-IRES GFP reduces Pet1 expression. Scale bar represents 25 μm.
(B and C) Effect of VP16RAR misexpression on Pet1+ neurons in the rostral (B) or caudal (C) hindbrain at E5. ∗p = 0.025 (B, blue bar), ∗p = 0.026 (B, red bar), ∗p = 8.11 × 10−6 (C, black bar) (unpaired Student’s t tests). Error bars represent SD in all graphs. r, rhombomere.
(D) Forced expression of Cyp26B1 or RAR403 in p3[V3] generates ectopic 5HT neurons (arrowheads) by E5. Scale bar represents 14 μm.
(E) Ectopic spinal 5HT neurons express the indicated markers (arrowheads), and Sim1 expression is reduced. Asterisk marks the floor plate. Scale bar represents 16 μm.
(F) Nkx2.2 and Foxa2 misexpression only induce ectopic spinal 5HT neurons when RA signaling is blocked by RAR403. –, control side; +, electroporated side. See also Figure S2.
The ventral restriction of ectopic spinal 5HT neurons suggested their derivation from presumptive p3[V3] progenitors, and blockade of Nkx2.2 or Foxa2 activity using a dominant interfering derivative of Nkx2.2 (Nkx2.2-VP16) [27] or Foxa2 (FkhA2-EnR) [5], respectively, prevented RAR403 induction of ectopic 5HT neurons (Figure S2B). Consistent with these data, forced expression of Foxa2, Nkx2.2, and RAR403 in the dorsal neural tube led to extensive ectopic generation of 5HT neurons, whereas the omission of RAR403 led to a failure to induce ectopic 5HT neurons (Figure 1F). This result also suggested that the small number of ectopic 5HT neurons generated by p3[V3] in ovo electroporation with RAR403 is likely to be a consequence of the restricted overlap of endogenous Foxa2 and Nkx2.2 expression and mosaic misexpression of RAR403. Furthermore, as quantified below, at brachial level the p3 domain normally only generates relatively small numbers of V3 neurons, suggesting that low neurogenic potential might further limit ectopic 5HT neurogenesis.
V3 neuronal differentiation was suppressed by 60% upon RAR403 misexpression (V3 neurons in control = 10.7 [mean] ± 4.1 [SD]; V3 neurons following RAR403 misexpression = 4.7 ± 2.2; p = 7.18 × 10−9, unpaired Student’s t test) (Figure 1E). This was not due to cell death or to a fate transformation to other ventral neuronal subtypes (Figure S2C). Furthermore, ectopic visceral motor (VM) neurons that precede the generation of 5HT neurons in the hindbrain from p3 progenitors [6] (Figure S1A) were not detected (Figure S2C). Altogether, these data indicate that high-level, versus low-level or absent, retinoid signaling in p3[V3] and p3[5HT], respectively, determines the corresponding neuronal identities.
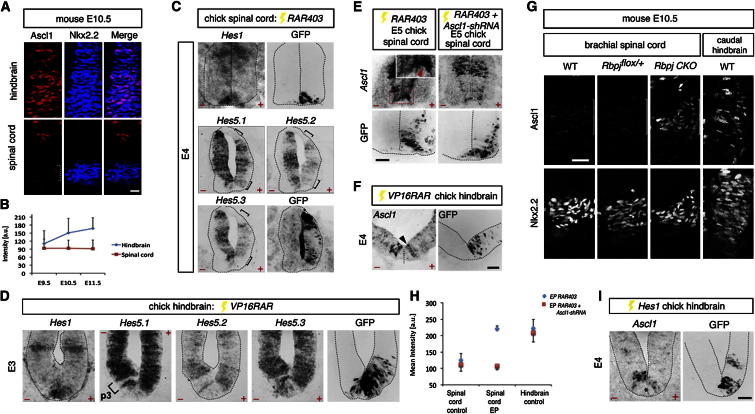
Retinoids Regulate Ascl1 Expression in p3 Progenitors via the Notch Pathway
The profile of RA signaling in the ventral neural tube correlates with the longitudinal expression of Ascl1 in p3 progenitors (Figures S1C and S2A). The significance of this observation lies in the critical subtype-specification function of Ascl1 in p3[5HT] and p3[V3] progenitors, which is separable from its neurogenic properties [11, 12]. In the p3[5HT] domain where RA signaling is weak or absent, the expression level of chick Ascl1 appeared markedly higher than in the p3[V3] domain where RA signaling is strong (Figure S1C). This observation prompted closer scrutiny of the expression of Ascl1 in p3 progenitors in the spinal cord and hindbrain (Figures 2A and 2B). Indeed, the mean level of Ascl1 expression was consistently greater in p3[5HT] than in p3[V3] progenitors between E9.5 and E11.5. Moreover, Ascl1 expression level was enhanced in hindbrain p3 progenitors after E9.5, corresponding to the time 5HT neurogenesis commences [5].
Figure 2.
Ascl1 Expression Level in p3 Progenitors Is Negatively Correlated with Notch Activity, which in Turn Is Regulated by Retinoids
(A and B) Ascl1 expression in mouse Nkx2.2+ p3 progenitors (A), with quantification (mean ± SD) shown in (B). Scale bar represents 9 μm. a.u., arbitrary units. n = 3 sections from each of three embryos per developmental stage.
(C) Spinal RAR403 misexpression, marked by anti-GFP immunofluorescence, downregulates Hes gene expression (bracketed).
(D) Misexpression of VP16RAR upregulates hindbrain p3 Hes gene expression.
(E) Misexpression of RAR403 in p3[V3] progenitors upregulates Ascl1 expression (arrowhead, inset) at E5, and this is prevented by cAscl1-shRNA. Inset shows a high-power image of the boxed region. Scale bar represents 18 μm.
(F) VP16RAR misexpression attenuates Ascl1 expression (arrowhead) in p3[5HT] progenitors. Scale bar represents 18 μm.
(G) Increased Ascl1 expression in brachial Nkx2.2+ p3[V3] cells (white brackets) of RbpjCKO mouse embryos is similar to Ascl1 expression in WT p3[5HT] cells. Scale bar represents 14 μm.
(H) Quantification of Ascl1 expression in presumptive p3[V3] cells misexpressing RAR403 or RAR403 and cAscl1-shRNA.
(I) Misexpression of Hes1-IRES GFP in caudal hindbrain p3[5HT] reduces Ascl1 expression. Scale bar represents 18 μm. See also Figure S1.
The Notch pathway is known to inhibit proneural gene expression, and, consistent with this regulatory relationship, in chick p3 progenitors the Hes family of Notch target genes showed reciprocal patterns of expression to Ascl1 (Figures S2D and S2E). Moreover, the Notch pathway lies downstream of retinoid signaling. Misexpression of RAR403 in the p3[V3] domain led to the downregulation of the Notch pathway by E4 (Figure 2C). Conversely, forced expression of VP16RAR in the hindbrain p3[5HT] domain upregulated Notch activity (Figure 2D). Retinoid regulation of the Notch pathway suggested Notch signaling might serve to link the A-P pattern of retinoid activity and Ascl1 expression in p3 progenitors.
To test whether the expression level of Ascl1 in p3[5HT] and p3[V3] is regulated by retinoid signaling, we analyzed Ascl1 expression in chick p3 progenitors following cell-autonomous blockade or activation of RA signaling. RAR403 markedly upregulated Ascl1 expression in the p3[V3] domain by E5 24 hr after the downregulation of the Notch pathway (control p3[V3] expression = 126 [mean] ± 19.3 [SD] [arbitrary units]; RAR403 p3[V3] expression = 221.6 ± 7.8; control p3[5HT] expression = 223.1 ± 26.3). (Figures 2E and 2H; Figure S2F). Moreover, the upregulation of Ascl1 could be prevented by the comisexpression of a short hairpin RNA against chick Ascl1 (cAscl1-shRNA) (control p3[V3] expression = 110.9 [mean] ± 19.4 [SD]; RAR403 + cAscl1-shRNA p3[V3] expression = 107.1 ± 11.2; control p3[5HT] expression = 208.4 ± 27.5) (Figures 2E and 2H). By contrast, misexpression of VP16RAR in p3[5HT] progenitors downregulated Ascl1 by E4 1 day after the upregulation of the Notch pathway in these progenitors (control p3[5HT] expression = 193.1 [mean] ± 27.1 [SD]; VP16RAR p3[5HT] expression = 94.4 ± 16.9; control p3[V3] expression = 105.1 ± 16.9) (Figure 2F). In neither condition were there detectable changes in expression of the other proneural genes, Neurog1 and Neurog2, or of other ventral progenitor markers or sonic hedgehog. Moreover, there was no increase in cell death (Figures S2F–S2H).
To determine whether Notch signaling regulates Ascl1 expression in p3 progenitors, we conditionally deleted recombination signal binding protein for immunoglobulin kappa j region (Rbpj), an obligate component of the Notch signaling pathway [28], using Olig2-cre [29]. At E10.5, mice lacking Rbpj (RbpjCKO) expressed higher levels of Ascl1 in the spinal cord p3 domain, compared to either mice that have only one conditional allele or mice that lacked Cre (WT p3[V3] expression: 50.5 [mean Ascl1 expression] ± 28.0 [SD]; Rbpjflox/+ p3[V3] expression: 49.3 ± 29.0; RbpjCKO p3[V3] expression: 78.1 ± 50.4; WT p3[5HT] expression: 90.3 ± 56.8) (Figure 2G). Conversely, misexpression of Hes1, an effector of Notch signaling, in the caudal hindbrain of the chick lowered the expression of Ascl1 in p3[5HT] progenitors to levels comparable to the expression of Ascl1 in spinal cord p3 progenitors (control p3[5HT] = 221.9 [mean] ± 20.9 [SD]; Hes1 electroporation p3[5HT] = 123.4 ± 8.7; control p3[V3] = 119.8 ± 22.1) (Figure 2I). The expression of Foxa2 and Nkx2.2 were unaffected (Figure S2I). Together, these gain- and loss-of-function data suggested that differential expression of Ascl1 in the two p3 progenitor pools is determined by retinoid signaling acting via the Notch pathway. Furthermore, our findings raised the possibility that differences in the expression level of Ascl1 might determine p3 progenitor fate.
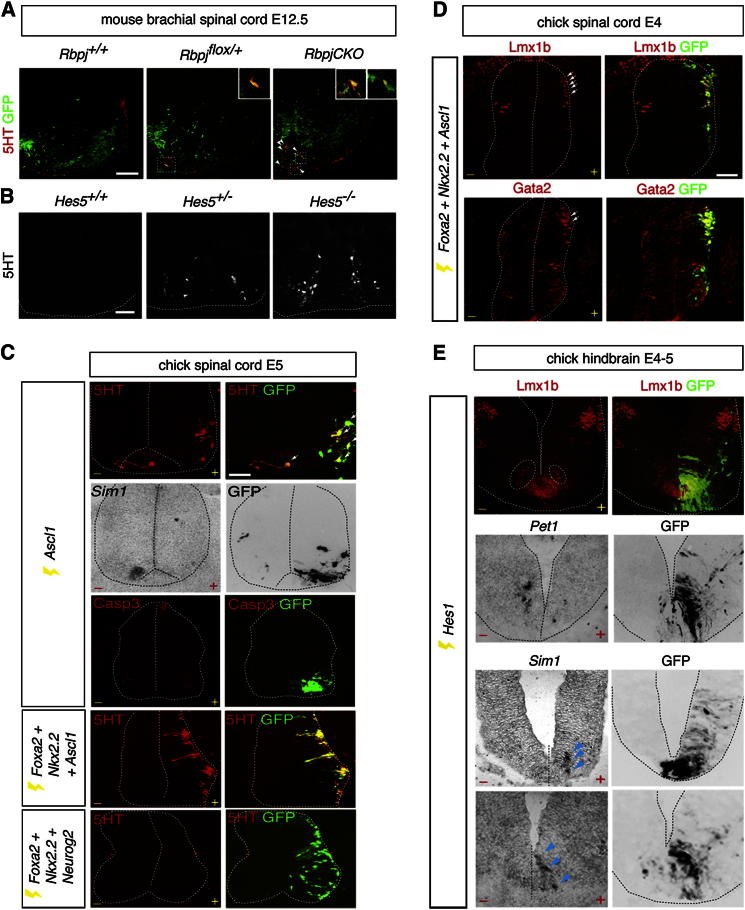
Ascl1 Expression Level Discriminates p3[V3] from p3[5HT] Progenitors
We exploited the regulatory relationship between Notch signaling and proneural gene expression to test the effect of boosting Ascl1 expression in the spinal cord on p3 progenitor fate. Strikingly, although 5HT neurons are normally never found in this region of the neural tube, ectopic spinal 5HT+GFP+ neuronal cell bodies were observed in the cervical cords of nine out of nine Rbpj conditional null mice and two out of five Cre/+; Rbpjflox/+ mice (Figure 3A). Moreover, analysis of embryos lacking Hairy and Enhancer-of-Split (Hes) family member Hes5, which is a target of Notch signaling broadly expressed in the spinal cord [30] (Figure 3B), revealed ectopic 5HT neurons in the ventral spinal cord at forelimb levels in three out of four Hes5+/− embryos and four out of four Hes5 null embryos. These neurons were more abundant than in Rbpj mutants: the later deletion of Rbpj in neural cells by Olig2-cre (∼E9.5) might explain the smaller number of 5HT neurons.
Figure 3.
Altering Ascl1 Expression Level in p3 Progenitors Produces Corresponding Changes in p3 Progenitor Identity
(A and B) Ectopic 5HT+GFP+ neurons (yellow cells, arrowheads) in the cervical cord of Rbpj mutants (A) and 5HT+ cells in the brachial cord of Hes5 mutants (B). Insets show high-power images of 5HT+GFP+ neurons in boxed regions. Scale bar represents 25 μm in (A) and 13 μm in (B).
(C) Forced Ascl1 expression in cervicobrachial p3[V3] cells induces ectopic 5HT neurons (arrows) and reduces V3 differentiation. Cell death, assayed by activated Caspase3 expression, is not detected in the ventral spinal cord. Ascl1, but not Neurog2, substitutes for RAR403 in combined electroporation with Foxa2 and Nkx2.2 to induce ectopic spinal 5HT neurons. Scale bar represents 13 μm.
(D) Ectopic Lmx1b and Gata2 expression (arrows) upon combined Foxa2, Nkx2.2, and Ascl1 electroporation at brachial level. Scale bar represents 14 μm.
(E) Hes1-IRES GFP misexpression in caudal p3[5HT] cells reduces 5HT neuronal differentiation marked by Lmx1b (circled) and Pet1 and induces ectopic V3 neurons (arrowheads).
To further explore the role of Ascl1 expression level in regulating p3 progenitor fate, we first increased Ascl1 levels in the p3[V3] domain by direct overexpression in ovo (Figure 3C). Boosting the level of Ascl1 in the p3[V3] domain was sufficient to generate ectopic 5HT neurons cell autonomously and suppress V3 neuronal differentiation (the number of Sim1+ cells on the electroporated side was 1.59 [mean] ± 1.6 [SD], and on the control side there were 8.12 ± 2.3, p = 2.80 × 10−10; n = 18 sections from five embryos), indicating that upregulation of Ascl1 reprograms the p3[V3] domain to a p3[5HT] identity. Moreover, there was no evidence that cell death, as revealed by activated Caspase3 immunostaining, could account for the reduced number of V3 neurons (Figure 3C). Next, we misexpressed the three p3-defining transcription factors, Nkx2.2, Foxa2, and Ascl1, in the dorsal spinal cord. The widespread and cell-autonomous generation of ectopic 5HT neurons that resulted shows that Ascl1 can substitute for RAR403 and supports the view that high-level Ascl1 expression is sufficient to select a p3[5HT] identity over a p3[V3] identity (Figure 3C). Ectopic 5HT neurons expressed postmitotic markers of hindbrain 5HT neurons Lmx1b and Gata2, but not Pet1 (Figure 3D and data not shown), suggesting that the differentiation of these neurons largely recapitulates the serotonergic differentiation pathway. Importantly, neither overexpression of Foxa2 and Nkx2.2 alone nor replacement of Ascl1 by another bHLH gene, Neurog2, led to ectopic 5HT neuronal generation (Figures 1F and 3C). Therefore, increasing the expression of Ascl1 converts p3[V3] to a p3[5HT] identity.
We then asked whether reducing the expression of Ascl1 in presumptive p3[5HT] progenitors would cause a reciprocal switch to a p3[V3] identity. Following Hes1 in ovo electroporation into presumptive p3[5HT] progenitors, significant reductions in 5HT neuronal differentiation were observed, using Lmx1b and Pet1 as markers (control side 5HT neurons = 8.2 [mean] ± 2.8 [SD]; Hes1-electroporated side = 2.5 ± 2.1, p = 8.2 × 10−8), and small numbers of Sim1-expressing ectopic V3 neurons were consistently detected in the caudal hindbrain (Figure 3E). This is consistent with the low neurogenic potential of the caudal hindbrain p3[5HT] domain. The block in generation of ectopic V3 neurons by comisexpression of Nkx2.2-VP16 confirmed the derivation of these Hes1-induced ectopic V3 neurons from the presumptive p3[5HT] domain (Figure S3A). To further test the idea that Ascl1 expression level is important for p3[5HT] identity, we measured the expression of Ascl1 in Ascl1 heterozygote mice, in which 5HT neuronal differentiation is intact [11]. No significant change in Ascl1 expression was detected (WT p3[5HT] expression: 151.1 [mean] ± 53.2 [SD]; Ascl1+/− p3[5HT] expression: 149.3 ± 39.7), which implies that allele number is not the main determinant of the level of Ascl1 expression (Figure S3B). Together, these data support the case for the critical role of Ascl1 expression level in the selection of 5HT over V3 neuronal fate.
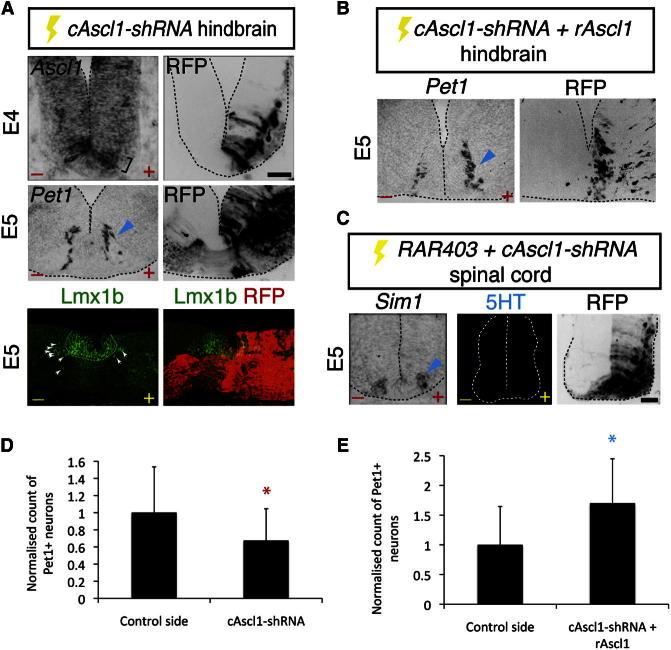
Finally, to test directly whether the level of Ascl1 determines p3 progenitor fate, we used cAscl1-shRNA. Forced expression of cAscl1-shRNA in the ventral region of the caudal hindbrain reduced the expression of Ascl1 in hindbrain p3 progenitors (control p3[5HT] expression: 213.6 [mean] ± 22.8 [SD]; cAscl1-shRNA p3[5HT] expression: 123.9 ± 13.3; control p3[V3] expression: 119.5 ± 19.5), and this was accompanied by a ∼40% reduction in 5HT neuronal differentiation (Figures 4A and 4D). Consistent with the retinoid and Notch pathway gain-of-function experiments in the caudal hindbrain in ovo, occasional ectopic V3 neurons were also detected (Figure S3C). We then attempted to rescue these neural patterning defects by comisexpression of rat Ascl1 (rAscl1), which is predicted to be resistant to this shRNA (Figure 4B). rAscl1 restored serotonergic neurogenesis and prevented the ectopic generation of any V3 neurons by cAscl1-shRNA (Figures 4B and 4E; Figure S3D). Transfection of a scrambled version of the chick Ascl1 shRNA (cAscl1-shRNA-SCR) did not alter the expression of Ascl1 in p3[5HT] progenitors (control p3[5HT] expression = 185.9 [mean] ± 30.1 [SD]; cAscl1-shRNA-SCR p3[5HT] expression = 176.0 ± 38.5), nor did it alter 5HT neurogenesis or induce any ectopic V3 neurons (Figures S3E and S3F). Furthermore, when the increased expression of Ascl1 in p3[V3] progenitors elicited by RAR403 was prevented by comisexpression of cAscl1-shRNA (Figure 2E), V3 neuronal differentiation was rescued (V3 neurons in control = 11 [mean] ± 4.6 [SD]; V3 neurons following RAR403 + cAscl1-shRNA misexpression = 10.8 ± 5.1; p = 0.92, not significant), and ectopic 5HT neuronal generation was blocked (Figure 4C). Taken together, these data indicate that Ascl1 recapitulates the effect of retinoids on p3 progenitor identity and that retinoids regulate the neuronal fates of p3 progenitors in an Ascl1-dependent manner.
Figure 4.
Knockdown of Chick Ascl1 Alters p3 Progenitor Identity
(A) Misexpression of cAscl1-shRNA IRES RFP decreases Ascl1 expression in hindbrain p3 progenitors (bracket) and reduces Pet1 (arrowhead) and Lmx1b expression (short arrows). Dotted white line demarcates Lmx1b expression corresponding to the floor plate. RFP expression on the control side is confined to decussating axons. Scale bar represents 18 μm.
(B) Coelectroporation of cAscl1-shRNA and rAscl1 rescues Pet1 expression (arrowhead).
(C) cAscl1-shRNA restores Sim1+ V3 neurons (arrowhead) and blocks ectopic spinal 5HT expression induced by RAR403. Scale bar represents 13 μm.
(D and E) Quantification of Pet1+ neurons in the caudal hindbrain at E5 following forced expression of cAscl1-shRNA (D) or cAscl1-shRNA and rAscl1 (E). Red asterisk, p = 0.028; blue asterisk, p = 0.002. See also Figure S3.
Discussion
We have shown how different profiles of retinoid signaling in p3 progenitors that share a common transcriptional code generate distinct neuronal identities at spinal cord and hindbrain levels. Binary differences in retinoid activity result in graded shifts in expression of a pivotal transcription factor, Ascl1, which is critical for the selection of alternate progenitor identities. This mechanism of progenitor fate specification is distinct from the conventional combinatorial model, because allocation to V3 or 5HT neuronal identity is determined not by qualitative but by quantitative differences in the transcriptional code [1]. Moreover, this model does not exclude the possibility that other, as yet uncharacterized, intrinsic determinants function in the same pathway as Ascl1 and could discriminate p3[5HT] from p3[V3].
In invertebrates, graded activity of transcription factors has been shown to control differential gene expression and thereby diversify cell fates [31]. By contrast in vertebrates, in general, transcription factor gradients appear to refine neural identity rather than instruct distinct cell identities in a dose-dependent manner [32–36]. Consistent with their closely similar genetic identities, the manipulations of retinoid signaling and Ascl1 expression demonstrate that spinal p3 progenitors possess a cryptic bipotency. The induction of ectopic V3 neurons in the hindbrain is less robust, which implies that hindbrain p3 progenitors are not fully interconvertible by graded Ascl1 expression alone. Nevertheless, it is clear that Ascl1 expression must be tightly regulated to regionally constrain 5HT neurogenesis. Importantly, failure to appropriately regulate the number of 5HT neurons is associated with neuropsychiatric disease states, for example Smith-Lemli-Opitz syndrome, in which hypermorphic serotonergic differentiation occurs [37].
Acknowledgments
We thank F. Guillemot and E. Kutejova for comments on the manuscript and P. Dollé and V. Ribes for RARE-LacZ mice. This work was funded by the Medical Research Council (MRC) (UK). J.K. and B.G.N. were supported by the Broad Center for Regenerative Medicine and Stem Cell Research and by grants to B.N. from the National Institute of Neurological Disorders and Stroke (NS053976) and the March of Dimes Foundation (06-FY10-296). Olig2-Cre; ROSA26-GFP; Rbpjflox/+ mice were maintained in accordance with UCLA Chancellor’s Animal Research Committee husbandry guidelines. Ascl1+/− mice were maintained in the animal husbandry facility at the MRC National Institute for Medical Research in accordance with standards set by the Animals (Scientific Procedures) Act, 1986. Hes5+/− mice were handled in accordance with the Kyoto University Guide for the Care and Use of Laboratory Animals.
Published: February 14, 2013
Footnotes
Supplemental Information includes three figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.01.046.
Contributor Information
John Jacob, Email: jjacob@nimr.mrc.ac.uk.
James Briscoe, Email: jbrisco@nimr.mrc.ac.uk.
Supplemental Information
References
- 1.Jessell T.M. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 2.Briscoe J., Ericson J. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 2001;11:43–49. doi: 10.1016/s0959-4388(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 3.Ye W., Shimamura K., Rubenstein J.L., Hynes M.A., Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- 4.Briscoe J., Sussel L., Serup P., Hartigan-O’Connor D., Jessell T.M., Rubenstein J.L., Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- 5.Jacob J., Ferri A.L., Milton C., Prin F., Pla P., Lin W., Gavalas A., Ang S.L., Briscoe J. Transcriptional repression coordinates the temporal switch from motor to serotonergic neurogenesis. Nat. Neurosci. 2007;10:1433–1439. doi: 10.1038/nn1985. [DOI] [PubMed] [Google Scholar]
- 6.Pattyn A., Vallstedt A., Dias J.M., Samad O.A., Krumlauf R., Rijli F.M., Brunet J.F., Ericson J. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 2003;17:729–737. doi: 10.1101/gad.255803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordes S.P. Molecular genetics of cranial nerve development in mouse. Nat. Rev. Neurosci. 2001;2:611–623. doi: 10.1038/35090039. [DOI] [PubMed] [Google Scholar]
- 8.Ray R.S., Corcoran A.E., Brust R.D., Kim J.C., Richerson G.B., Nattie E., Dymecki S.M. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Narayan S., Geiman E., Lanuza G.M., Velasquez T., Shanks B., Akay T., Dyck J., Pearson K., Gosgnach S. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60:84–96. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deneris E.S., Wyler S.C. Serotonergic transcriptional networks and potential importance to mental health. Nat. Neurosci. 2012;15:519–527. doi: 10.1038/nn.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pattyn A., Simplicio N., van Doorninck J.H., Goridis C., Guillemot F., Brunet J.F. Ascl1/Mash1 is required for the development of central serotonergic neurons. Nat. Neurosci. 2004;7:589–595. doi: 10.1038/nn1247. [DOI] [PubMed] [Google Scholar]
- 12.Sugimori M., Nagao M., Bertrand N., Parras C.M., Guillemot F., Nakafuku M. Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development. 2007;134:1617–1629. doi: 10.1242/dev.001255. [DOI] [PubMed] [Google Scholar]
- 13.Ribes V., Balaskas N., Sasai N., Cruz C., Dessaud E., Cayuso J., Tozer S., Yang L.L., Novitch B., Marti E., Briscoe J. Distinct Sonic Hedgehog signaling dynamics specify floor plate and ventral neuronal progenitors in the vertebrate neural tube. Genes Dev. 2010;24:1186–1200. doi: 10.1101/gad.559910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossant J., Zirngibl R., Cado D., Shago M., Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- 15.Lumsden A., Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- 16.MacLean G., Abu-Abed S., Dollé P., Tahayato A., Chambon P., Petkovich M. Cloning of a novel retinoic-acid metabolizing cytochrome P450, Cyp26B1, and comparative expression analysis with Cyp26A1 during early murine development. Mech. Dev. 2001;107:195–201. doi: 10.1016/s0925-4773(01)00463-4. [DOI] [PubMed] [Google Scholar]
- 17.Wilson L.J., Myat A., Sharma A., Maden M., Wingate R.J. Retinoic acid is a potential dorsalising signal in the late embryonic chick hindbrain. BMC Dev. Biol. 2007;7:138. doi: 10.1186/1471-213X-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro D.S., Arvidsson M., Bondesson Bolin M., Perlmann T. Activity of the Nurr1 carboxyl-terminal domain depends on cell type and integrity of the activation function 2. J. Biol. Chem. 1999;274:37483–37490. doi: 10.1074/jbc.274.52.37483. [DOI] [PubMed] [Google Scholar]
- 19.Damm K., Heyman R.A., Umesono K., Evans R.M. Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc. Natl. Acad. Sci. USA. 1993;90:2989–2993. doi: 10.1073/pnas.90.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craven S.E., Lim K.C., Ye W., Engel J.D., de Sauvage F., Rosenthal A. Gata2 specifies serotonergic neurons downstream of sonic hedgehog. Development. 2004;131:1165–1173. doi: 10.1242/dev.01024. [DOI] [PubMed] [Google Scholar]
- 21.Cheng L., Chen C.L., Luo P., Tan M., Qiu M., Johnson R., Ma Q. Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J. Neurosci. 2003;23:9961–9967. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Y.Q., Marklund U., Yuan W., Yin J., Wegman L., Ericson J., Deneris E., Johnson R.L., Chen Z.F. Lmx1b is essential for the development of serotonergic neurons. Nat. Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- 23.Hendricks T., Francis N., Fyodorov D., Deneris E.S. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J. Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Beaulieu J.M., Sotnikova T.D., Gainetdinov R.R., Caron M.G. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- 25.Hendricks T.J., Fyodorov D.V., Wegman L.J., Lelutiu N.B., Pehek E.A., Yamamoto B., Silver J., Weeber E.J., Sweatt J.D., Deneris E.S. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 26.Kiyasova V., Fernandez S.P., Laine J., Stankovski L., Muzerelle A., Doly S., Gaspar P. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J. Neurosci. 2011;31:2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muhr J., Andersson E., Persson M., Jessell T.M., Ericson J. Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell. 2001;104:861–873. doi: 10.1016/s0092-8674(01)00283-5. [DOI] [PubMed] [Google Scholar]
- 28.Kopan R., Ilagan M.X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dessaud E., Yang L.L., Hill K., Cox B., Ulloa F., Ribeiro A., Mynett A., Novitch B.G., Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- 30.Hatakeyama J., Bessho Y., Katoh K., Ookawara S., Fujioka M., Guillemot F., Kageyama R. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004;131:5539–5550. doi: 10.1242/dev.01436. [DOI] [PubMed] [Google Scholar]
- 31.Lewis J. From signals to patterns: space, time, and mathematics in developmental biology. Science. 2008;322:399–403. doi: 10.1126/science.1166154. [DOI] [PubMed] [Google Scholar]
- 32.Chen A.I., de Nooij J.C., Jessell T.M. Graded activity of transcription factor Runx3 specifies the laminar termination pattern of sensory axons in the developing spinal cord. Neuron. 2006;49:395–408. doi: 10.1016/j.neuron.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 33.Sansom S.N., Livesey F.J. Gradients in the brain: the control of the development of form and function in the cerebral cortex. Cold Spring Harb. Perspect. Biol. 2009;1:a002519. doi: 10.1101/cshperspect.a002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamasaki T., Leingärtner A., Ringstedt T., O’Leary D.D. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Dasen J.S., De Camilli A., Wang B., Tucker P.W., Jessell T.M. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Rousso D.L., Gaber Z.B., Wellik D., Morrisey E.E., Novitch B.G. Coordinated actions of the forkhead protein Foxp1 and Hox proteins in the columnar organization of spinal motor neurons. Neuron. 2008;59:226–240. doi: 10.1016/j.neuron.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waage-Baudet H., Lauder J.M., Dehart D.B., Kluckman K., Hiller S., Tint G.S., Sulik K.K. Abnormal serotonergic development in a mouse model for the Smith-Lemli-Opitz syndrome: implications for autism. Int. J. Dev. Neurosci. 2003;21:451–459. doi: 10.1016/j.ijdevneu.2003.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.