Abstract
Pathologic TAR-DNA-binding protein 43 (TDP-43) is a disease protein in frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U) and amyotrophic lateral sclerosis. We studied the presence, frequency, and distribution of TDP-43 pathology by immunohistochemistry and biochemistry in a series of clinically well-characterized tauopathy patient brains, including 182 Alzheimer disease (AD), 39 corticobasal degeneration, 77 progressive supranuclear palsy, and 12 Pick disease cases and investigated the clinical impact of concomitant TDP-43 pathology in these cases. TAR-DNA-binding protein 43 pathology was found in 25.8% of AD cases. It was restricted to the dentate gyrus and entorhinal cortex in approximately 75% of cases; approximately 25% showed more widespread TDP-43 pathology in frontal and temporal cortices, resembling the FTLD-U subtype associated with progranulin mutations. TAR-DNA-binding protein 43 pathology in AD was associated with significantly longer disease duration, but there was no association with the clinical presentation (148 cases diagnosed as AD and 34 cases diagnosed as frontotemporal lobar degeneration). Progressive supranuclear palsy and Pick disease cases showed no TDP-43 inclusions and no biochemical alterations of TDP-43. There was, however, a unique, predominantly glial TDP-43 pathology with staining of astrocytic plaque-like structures and coiled bodies in 15.4% of corticobasal degeneration cases; this was associated with biochemical TDP-43 changes similar to those in FTLD-U. These findings provide further insight into the burden and clinical significance of TDP-43 pathology in disorders other than FTLD-U and amyotrophic lateral sclerosis.
Keywords: Alzheimer disease, Corticobasal degeneration, Frontotemporal dementia, Tauopathy, TDP-43
INTRODUCTION
Frontotemporal lobar degeneration (FTLD) is a heterogeneous group of progressive neurodegenerative diseases that manifest as language dysfunction or as a disorder of social comportment and executive functioning (1). Ubiquitin-positive, tau-, and α-synuclein-negative inclusions found predominantly in neurons in the dentate gyrus and frontotemporal cortex are the characteristic lesions in the most common neuropathologic subtype of FTLD, that is, FTLD with ubiquitin-positive inclusions (FTLD-U) (2–4). The TAR-DNA-binding protein 43 (TDP-43) has recently been identified as a major disease protein in the ubiquitinated inclusions in FTLD-U and amyotrophic lateral sclerosis (ALS) (5). This is consistent with the hypothesis that these diseases represent a clinicopathologic spectrum of disorders that can be subsumed under the term TDP-43 proteinopathies (6, 7). The initial findings of TDP-43 as the most specific and sensitive marker for detection of neuronal inclusions and neurites in FTLD-U and ALS have been rapidly confirmed by others in an extensive series of postmortem cases, including more than 370 sporadic and familial FTLD-U and ALS cases (8–13). Moreover, antibodies to TDP-43 facilitated the detection of previously unrecognized pathology in oligodendroglial cells in these conditions (9, 14). TAR-DNA-binding protein 43 immunohistochemistry (IHC) now also enables the investigation of the co-occurrence of FTLD-U-type TDP-43 pathology in other common neurodegenerative disorders such as Alzheimer disease (AD) and other tauopathies and Lewy body (LB) disorders. Because ubiquitin immunoreactivity is found in most abnormal protein aggregates characteristic of these neurodegenerative disorders (15), the identification of TDP-43 pathology as the hallmark of FTLD-U now allows separation of FTLD-U-related inclusions from other types of ubiquitin-positive inclusions such as neurofibrillary tangles in AD and LBs in dementia with LBs (DLB). Indeed, recent reports have demonstrated that 20% to 30% of AD and DLB cases (especially those DLB cases with a high burden of tau pathology) demonstrate concomitant TDP-43 pathology similar to that found in FTLD-U (16, 17). The co-occurrence of TDP-43 pathology in another tauopathy, the parkinsonism-dementia complex of Guam (18, 19), further suggests that TDP-43 pathology might be triggered by tau pathology in general. The clinical relevance of concomitant TDP-43 pathology in other neurodegenerative disorders has not been examined in any substantive detail. Because clinicopathologic studies have shown that neuropathologically confirmed AD and DLB cases may present with clinical features that meet all current criteria for the clinical diagnosis of FTLD (20) such as progressive social and behavioral changes and/or changes in language functions (21, 22) we speculate that concomitant TDP-43 pathology contributes to the unusual clinical presentation in these clinical FTLD variants of AD.
Therefore, the aims of this study were to 1) analyze the presence and frequency of concurrent TDP-43 pathology in a large series (n = 310) of clinically well-characterized tauopathies, including AD, corticobasal degeneration (CBD), progressive supranuclear palsy (PSP), and Pick disease (PiD) cases; and 2) determine whether concomitant TDP-43 pathology influences the clinical presentation in these diseases.
MATERIALS AND METHODS
Case Selection
The databases of the Center for Neurodegenerative Disease Research Brain Bank at the University of Pennsylvania and the Center for Neuropathology and Prion Research Brain Bank at the University of Munich, Germany, were searched for cases using the following inclusion criteria for the different cohorts.
AD Group
All cases included in this group were clinically diagnosed with a progressive dementia and showed typical AD pathology (CERAD C and Braak and Braak Stages V–VI) by neuropathologic examination, thereby fulfilling the criteria for a high likelihood of AD according to the National Institute on Aging criteria (23). From the 182 included cases, 5 had familial AD with mutations in the presenilin 1 gene (n = 4) and the amyloid precursor protein gene (n = 1). Additional LBs in the amygdala were found in 18 cases, and 4 cases had limbic LB pathology according to the Newcastle criteria for DLB (24).
PSP Group
All 77 cases identified showed tau pathology with globoid tangles and tufted astrocytes in the brainstem and basal ganglia, fulfilling the neuropathologic criteria for typical PSP (25). Additional brainstem-type LBs were present in 4 cases, and 6 cases had additional tau pathology typical of argyrophilic grain disease.
CBD Group
The neuropathologic diagnosis of CBD was based on the presence of abundant gray and white matter tau pathology with tangles, numerous threads, astrocytic plaques, and coiled bodies in the cortex and brainstem according to published criteria (26). We studied 39 cases.
PiD Group
We selected 12 cases with characteristic pathologic features of PiD, that is, numerous tau-positive round inclusions in the dentate gyrus and temporal and frontal cortices (Pick bodies), as well as ballooned neurons (Pick cells) (20).
IHC and Semiquantitative Assessment of TDP-43 Pathology
Immunohistochemistry for TDP-43 (rabbit polyclonal antibody; ProteinTech Group, Chicago, IL; dilution, 1:2000–1:4500) was performed on formalin- or ethanol-fixed, paraffin-embedded 5-µm sections. In selected cases with TDP-43-positive inclusions, IHC was performed with FTLD-U Subtype-1 (monoclonal antibody [mAb] 182)- and Subtype-2 (mAb 137)-specific antibodies (5, 27). Immunohistochemistry was carried out using the avidin-biotin complex detection system (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine as chromogen. Antigen retrieval was performed by boiling the sections in 10 mmol/L citrate buffer (pH 6.0) in a microwave oven (3 times for 5 minutes). Double labeling immunofluorescence for TDP-43 and phosphorylated tau (clone AT8; Innogenetics, Gent, Belgium) was performed using Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary antibodies (Molecular Probes, Eugene, OR). For all cases, sections of the hippocampal formation, including the dentate gyrus, the subiculum, entorhinal cortex and parahippocampal gyrus, fusiform gyrus, and adjacent temporal cortex, were screened for TDP-43 pathology. In all cases with any TDP-43-positive inclusions in these sections, additional IHC was performed on sections from the temporal cortex, frontal cortex, and medulla.
The postmortem interval of cases studied ranged from 4 to 72 hours. The fixation times ranged from 1 day up to 5 years. No obvious changes were observed with respect to the physiologic nuclear staining, which served as an internal quality control for TDP-43 staining in all cases.
Sequential Biochemical Fractionation and Immunoblot Analysis
Frozen tissues from frontal and temporal cortex as well as hippocampus were used for the sequential extraction of TDP-43 with buffers of increasing stringency as previously described (5, 27). Briefly, gray matter was extracted at 5 mL/g (vol/w) with low-salt buffer (10 mmol/L Tris, pH 7.5; 5 mmol/L EDTA; 1 mmol/L dithiothreitol; 10% sucrose; and a cocktail of protease inhibitors), high-salt Triton X buffer (low-salt buffer + 1% Triton X-100 + 0.5 mol/L NaCl), myelin flotation buffer (Triton X buffer containing 30% sucrose), and sarkosyl buffer (low-salt buffer + 1% N-lauroyl-sarcosine + 0.5 mol/L NaCl). The detergent-insoluble materials were extracted in 0.25 mL/g urea buffer (7 mol/L urea, 2 mol/L thiourea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 30 mmol/L Tris, pH 8.5). For Western blot analysis, protein extracts were resolved by Trisglycine 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene diflouride membranes (Millipore, Billerica, MA), and probed with antibodies to TDP-43. Primary antibodies were detected with alkaline phosphatase-conjugated anti-mouse or anti-rabbit immunoglobulin G (DAKO, Hamburg, Germany) and visualized by incubation with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche Molecular Biochemicals, Mannheim, Germany). Protein extracts from FTLD-U cases described in previous articles (5) were used as positive controls.
Statistical Analysis
Unpaired Student t-tests were used to analyze differences between groups for age at death and disease duration. The Fisher exact test was used to compare categoric neuropathologic and clinical variables. Significance level was set as p < 0.05.
RESULTS
Frequency and Distribution of TDP-43 Pathology in AD
We studied 182 neuropathologically confirmed AD cases (Table 1). TAR-DNA-binding protein 43-positive inclusions, predominantly neuronal cytoplasmic inclusions (NCIs) and dystrophic neurites (DNs), were found in 25.8% (n = 47) of AD cases with highly variable frequency and regional distribution among these cases (Table 2). All 47 AD cases with TDP-43 pathology showed NCIs and DNs in the entorhinal cortex with numbers ranging from few (n = 15), to moderate (n = 19), to numerous (n = 13) inclusions (Fig. 1B). Neuronal cytoplasmic inclusions in dentate granule neurons were found in 70.2% (n = 33) of TDP-43-positive AD cases with the additional presence of neuronal intranuclear inclusions (NIIs) in the dentate gyrus in a single case (Fig. 1A). In contrast to FTLD-U cases, in most AD cases with TDP-43 pathology (n = 35; 74.5%), the TDP-43 pathology was restricted to the entorhinal cortex and dentate gyrus (“limbic” distribution pattern in Table 2 according to Amador-Ortiz et al [16]). The other 12 AD cases with TDP-43 pathology (25.5%) showed more widespread lesions with numerous NCIs and DNs in the upper cortical layers in temporal and frontal cortex (Figs. 1C, D; “diffuse” distribution pattern in Table 2 according to Amador-Ortiz et al [16]). Together with the presence of NIIs detected in approximately 50% of cases, the morphology and distribution of TDP-43 pathology in AD cases with a diffuse TDP-43 distribution pattern are very similar to the recently described histologic FTLD-U Subtype 3 according to the classification scheme by Sampathu et al (27). This is further supported by the observation that mAbs 182 and 137 (which are specific for the detection of TDP-43-positive inclusions in FTLD-U Subtypes 1 and 2 cases, respectively) did not label the TDP-43-positive inclusions in the TDP-43-positive AD case (data not shown).
TABLE 1.
Demographics of Cases Included in the Study
| AD | CBD | PSP | PiD | |
|---|---|---|---|---|
| No. cases | 182 | 39 | 77 | 12 |
| Male/female | 74/108 | 17/22 | 48/29 | 8/4 |
| Mean age at death (SD), years | 77.5 (10.4) | 67.6 (7.4) | 73.6 (7.1) | 67.1 (8.3) |
| Mean disease duration (SD), years | 8.9 (4.2) | 4.5 (2.1) | 6.5 (2.9) | 7.8 (3.5) |
AD, Alzheimer disease; CBD, corticobasal degeneration; PiD, Pick disease; PSP, progressive supranuclear palsy; SD, standard deviation.
TABLE 2.
Presence and Distribution of TDP-43 Pathology in AD Cases
| AD Cases Independent of Clinical Diagnosis (n = 182) |
AD Cases With clinAD (n = 148) | AD Cases With clinFTLD (n = 34) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TDP-43 Positive | TDP-43 Positive | TDP-43 Positive | ||||||||||
| TDP-43 Negative |
Total | TDP-43 Distribution | TDP-43 Negative |
Total | TDP-43 Distribution | TDP-43 Negative |
Total | TDP-43 Distribution | ||||
| Limbic | Diffuse | Limbic | Diffuse | Limbic | Diffuse | |||||||
| No. cases | 135 | 47 | 35 | 12 | 107 | 41 | 31 | 10 | 28 | 6 | 4 | 2 |
| Percentage | 74.2 | 25.8 | 74.5* | 25.5* | 72.3 | 27.7 | 75.6* | 24.4* | 78.6 | 21.4 | 66.7* | 33.3* |
| Male/female | 58/77 | 16/31 | 11/24 | 5/7 | 46/61 | 16/25 | 11/20 | 5/5 | 12/16 | 0/6 | 0/4 | 0/2 |
| Mean age at death (SD), years | 76.7 (10.8) | 79.8 (8.7) | 79.8 (8.6) | 80.1 (9.4) | 78.5 (9.9) | 80.9 (7.8) | 80.5 (8.3) | 82.0 (6.0) | 69.9 (11.6) | 72.8 (12.1) | 74.0 (9.8) | 70.5 (20.5) |
| Mean disease duration (SD), years | 8.5 (3.9) | 10.4† (4.9) | 10.1 (4.1) | 11.5 (7.3) | 8.6 (3.9) | 9.7 (3.9) | 9.6 (3.7) | 10.3 (5.2) | 8.1 (4.0) | 16.0‡ (8.6) | 17.0 (4.2) | 15.0 (14.1) |
| Details on pathology | ||||||||||||
| Severe hippocampus sclerosis | 6 | 15 | 11 | 4 | 4 | 12 | 9 | 3 | 2 | 3 | 2 | 1 |
| TDP-43 pathology | ||||||||||||
| Dentate granule cells | 0 | 33 | 21 | 12 | 0 | 29 | 19 | 10 | 0 | 4 | 2 | 2 |
| Entorhinal cortex | 0 | 47 | 35 | 12 | 0 | 41 | 31 | 10 | 0 | 6 | 4 | 2 |
| Temporal cortex | 0 | 12 | 0 | 12 | 0 | 10 | 0 | 10 | 0 | 2 | 0 | 2 |
| Frontal cortex | 0 | 6 | 0 | 6 | 0 | 4 | 0 | 4 | 0 | 1 | 0 | 1 |
| Glial inclusions in white matter | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Neuronal intranuclear inclusions | 0 | 7 | 2 | 5 | 0 | 5 | 1 | 4 | 0 | 2 | 1 | 1 |
, Percentages for cases with limbic and diffuse TDP-43 distribution are calculated based on the total number of cases with TDP-43 pathology in the respective groups.
, p = 0.0167 (TDP-43 negative vs TDP-43 positive for AD cases independent of clinical diagnosis).
, p = 0.005 (TDP-43 negative vs TDP-43 positive for AD cases with clinical diagnosis of FTLD).
AD, Alzheimer disease; clinAD, clinical diagnosis of AD; clinFTLD, clinical diagnosis of FTLD; FTLD, frontotemporal lobar degeneration; SD, standard deviation; TDP-43, TAR-DNA/binding protein 43.
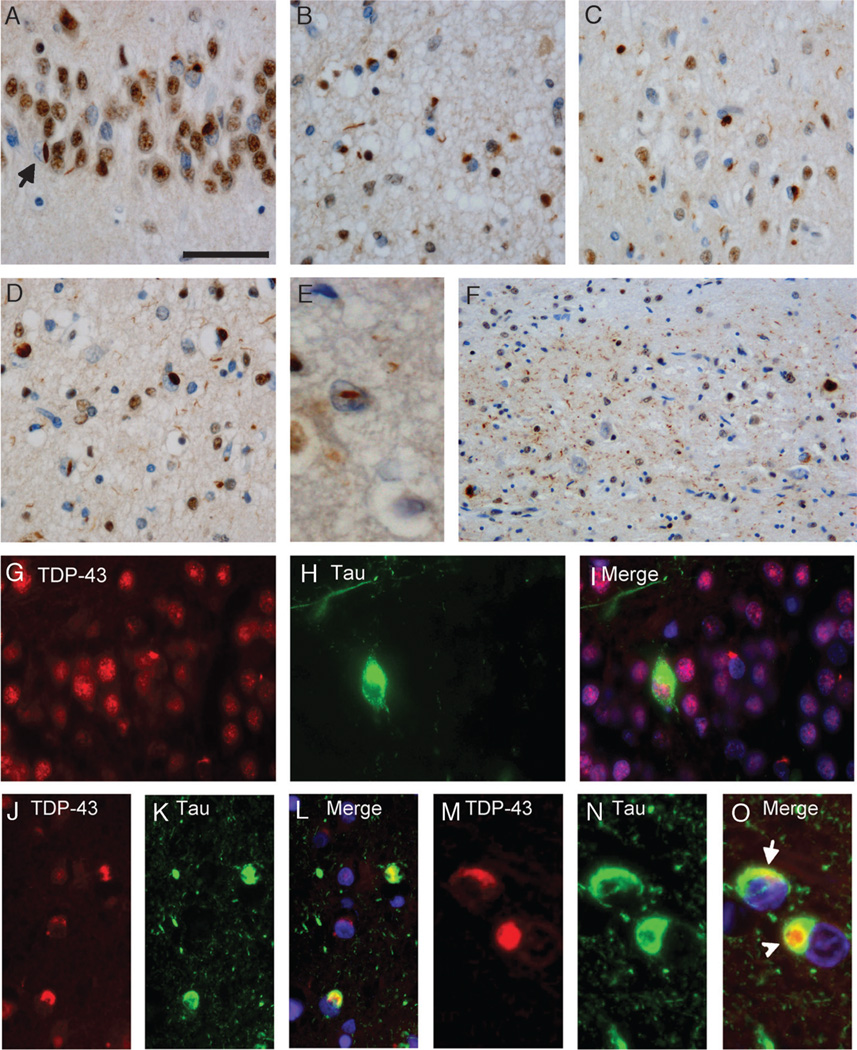
FIGURE 1.
TAR-DNA-binding protein 43 (TDP-43) pathology in Alzheimer disease (AD). Immunohistochemistry with anti-TDP-43 showing neuronal cytoplasmic inclusions in dentate granule cells (A). Note the neuronal intranuclear inclusion (NII) (A; arrow). Cytoplasmic inclusions and small neuritic profiles in entorhinal cortex (B), temporal cortex (C), and frontal cortex (D) in a case with diffuse TDP-43 pathology. Higher magnification of an NII in the frontal cortex is shown (E). An AD case with hippocampal sclerosis shows abundant neuritic pathology in the CA1 region (F). (G–O) Double label immunofluorescence with anti-TDP-43 (G, J, M) and anti-tau (H, K, N). Merged images show cell nuclei stained with 4′6-diamidino-2-phenylindole (I, L, O). Inclusions in the dentate granule cells are either labeled by anti-TDP-43 or anti-tau (G–I). Partial colocalization of tau and TDP-43 signals is observed in neurons in the entorhinal cortex, whereas the neuritic tau pathology is not stained by anti-TDP-43, and 2 TDP-43-positive neuronal inclusions are not labeled by anti-tau (J–L). (M–O) Higher magnification of neurons with partially overlapping (O; arrow) or separate TDP-43- and tau-positive inclusions (O; arrowhead). Scale bars = (A) 50 (A–D, G–L); (E) 25; and (M–O) 20 µm.
Severe neuron loss in the hippocampus and subiculum (“hippocampal sclerosis”) was found in 11.5% (n = 21) of AD cases and was associated with TDP-43 pathology in 71.4% (n = 15) of these cases, confirming previous results (16). Most cases with hippocampal sclerosis also showed a dense network of TDP-43 immunoreactivity in cell processes in the CA1 and subiculum region (Fig. 1F). The ratio of cases with limbic and diffuse TDP-43 distribution pattern was similar between TDP-43-positive cases with and without hippocampal sclerosis (24:8 vs 11:4).
Double labeling immunofluorescence experiments for phosphorylated tau and TDP-43 showed that inclusions in the dentate granule neurons were either labeled by TDP-43 or tau (Figs. 1G–I). In the entorhinal and temporal cortices, most inclusions (>80%) were also labeled either for TDP-43 or tau. Some neurons showed cytoplasmic inclusions immunoreactive for both markers. There was, however, only partial overlap between tau and TDP-43 immunoreactivity in most of these structures (Figs. 1J–L); sometimes, clearly discrete inclusions were labeled in the same neurons (Figs. 1M–O).
Correlation of Concomitant TDP-43 Pathology With Clinical Presentation in AD Cases
Results of statistical analysis of AD cases are summarized in Table 2. There were no differences in age at death or sex distribution between AD cases with and without TDP-43 pathology (Table 2). There was, however, a statistically significant difference in disease duration, that is, AD cases with TDP-43 pathology had longer durations than AD cases without TDP-43 pathology (8.5 ± 3.9 vs 10.4 ± 4.9; p = 0.0167).
Because AD cases may present with clinical features that lead to the clinical diagnosis of FTLD, we determined whether concomitant TDP-43 pathology in AD patients is more common in these clinical FTLD variants of AD. Of the included 182 AD cases in this study, 148 cases had a clinical diagnosis of AD (clin AD), while a clinical diagnosis of frontotemporal dementia, progressive aphasia, or CBD (collectively termed “clinFTLD”) was made in 34 cases (Table 2). Detailed clinical information on 19 cases of the clinFTLD group has been previously published (22). The percentage of cases with TDP-43 pathology was, however, similar in the clinAD and clinFTLD groups (27.7% vs 21.4%). Furthermore, the frequencies of the limbic and diffuse patterns of TDP-43 pathology were similar in the 2 groups (31:10 in clinAD vs 4:2 in clinFTLD).
Concomitant TDP-43 Pathology Is Present in CBD but Not in PSP and PiD
To examine whether tau pathology in other tauopathies is associated with TDP-43 accumulation, we studied a large series (n = 128) of other tauopathy cases, including CBD (n = 39), PSP (n = 77), and PiD (n = 12) by TDP-43 IHC (Table 1).
A hitherto unappreciated burden of TDP-43 pathology was revealed in 15.4% (n = 6) of 39 CBD cases. All 6 of these cases showed TDP-43-positive NCIs in the dentate granule cells and entorhinal cortex, with the additional presence of NIIs in 50% of cases (Fig. 2C). In 2 of these cases, TDP-43 pathology was restricted to these regions, resembling the limbic distribution pattern seen in AD cases. In the other 4 cases, a unique, more widespread TDP-43 staining pattern was observed in the temporal and frontal cortex and basal ganglia. Numerous threadlike TDP-43-positive inclusions that were often arranged in annular clusters resembling astrocytic plaques were seen in these gray matter areas (Fig. 2D), whereas NCIs were rare. In contrast, the white matter contained abundant TDP-43-immunoreactive cell processes and oligodendroglial inclusions resembling coiled bodies (Fig. 2E).
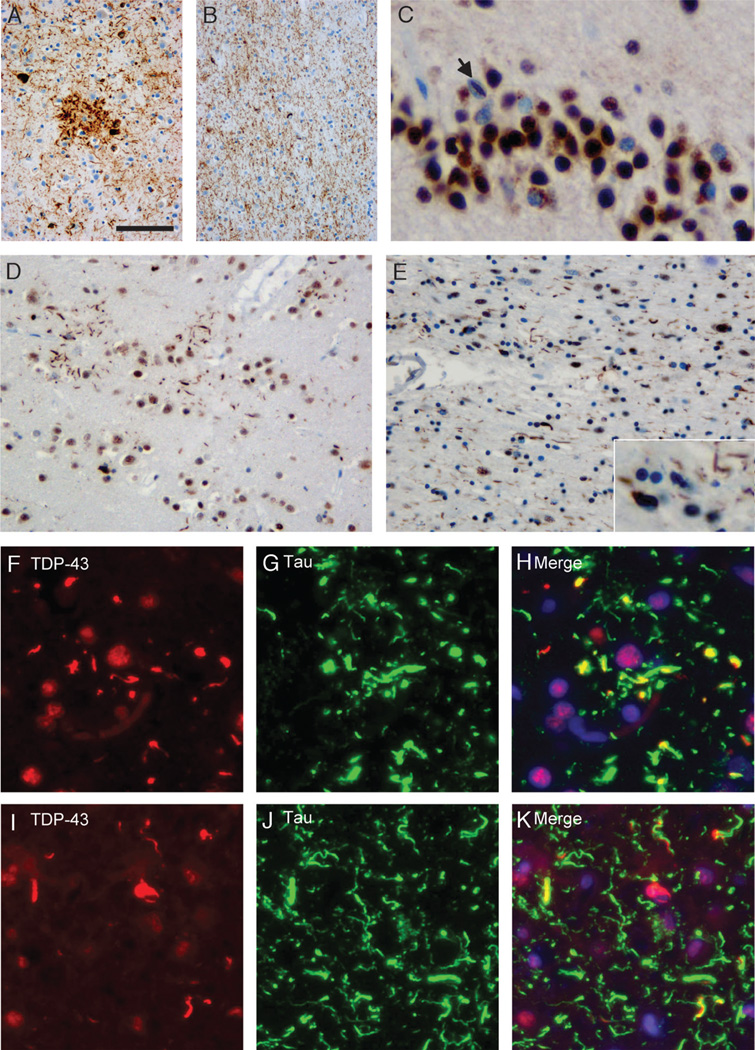
FIGURE 2.
Pattern of tau and TAR-DNA-binding protein 43 (TDP-43) pathology in CBD. Anti-tau immunohistochemistry reveals characteristic features of corticobasal degeneration with astrocytic plaques, neurofibrillary tangles, and numerous dystrophic neurites in the frontal gray matter (A) and numerous threadlike processes and coiled bodies in the white matter (B). (C) Immunohistochemistry of the same case with anti-TDP-43 showed neuronal cytoplasmic inclusions in dentate granule cells with a single neuronal intranuclear inclusion (C; arrow). In the frontal gray matter, there are several TDP-43-positive threadlike inclusions that are often arranged in annular clusters resembling astrocytic plaques (D). The frontal white matter shows TDP-43-positive threadlike processes and oligodendroglial inclusions (E). Double label immunofluorescence for TDP-43 (F, I) and tau (G, J) with merged images (H, K) shows partial colocalization of most TDP-43-positive threadlike inclusions with tau in the frontal gray matter. A small subset of inclusions is labeled by TDP-43 alone, whereas numerous tau-inclusions are TDP-43 negative (F–H). In the frontal white matter, TDP-43-positive threadlike inclusions partially colocalize with tau. In addition, there are inclusions that are single labeled for either tau or TDP-43 (I–K). Scale bars = (A) 100 (A, B); 50 (D, E); 25 (C; insert in E); 20 (I–K); and 15 µm (F–H).
Double labeling immunofluorescence studies revealed that most of the TDP-43-positive threadlike inclusions in the gray and white matters showed partial but not complete overlap with tau immunoreactivity (Figs. 3F–K). Most oligodendroglial cytoplasmic inclusions were either labeled for TDP-43 or tau but not both (Figs. 3I–K).
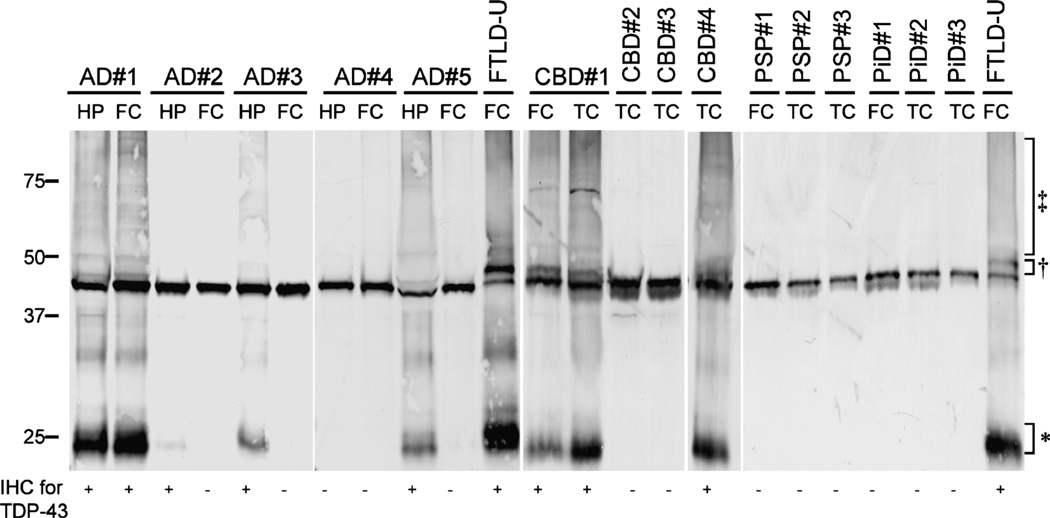
FIGURE 3.
Biochemical analysis of TAR-DNA-binding protein 43 (TDP-43) in Alzheimer disease (AD), corticobasal degeneration (CBD), progressive supalsy (PSP) Pick disease (PiD), and frontotemporal lobar degeneration with ubiquitin inclusions (FTLD-U) cases. Proteins were sequentially extracted from hippocampus (HP) and frontal cortex (FC) of AD cases with diffuse (AD 1), limbic (AD 2, 3, and 5), or no TDP-43 pathology (AD 4), from FC and/or temporal cortex (TC) of CBD cases with (CBD 1 and 4) and without (CBD 2 and 3) TDP-43 pathology. Variable amounts of pathologic TDP-43 bands approximately 25 (*), 45 (†), and high molecular smears (‡) are present in urea samples from AD and CBD cases with TDP-43-positive inclusions by immunohistochemistry (IHC) in the respective brain regions, similar to the distinct pathologic profile of TDP-43 obtained in FTLD-U cases used as positive controls. Pathologic TDP-43 species were not detected in progressive PSP cases 1 to 3 or PiD cases 1 to 3, or in AD (AD 4) and CBD (CBD 2 and 3) cases without detectable TDP-43 pathology by IHC.
There were no significant differences for age at death or disease duration between the TDP-43-positive and TDP-43-negative CBD groups (Table 3). None of the 77 PSP or 12 PiD cases studied had any TDP-43 pathology in the hippocampus sections.
TABLE 3.
Presence and Distribution of TDP-43 Pathology in CBD
| CBD (n = 39) | ||
|---|---|---|
| TDP-43 Negative | TDP-43 Positive | |
| No. cases | 33 | 6 |
| Percentage | 84.6 | 15.4 |
| Male/female | 14/19 | 3/3 |
| Mean age at death (SD), years | 67.8 (6.8) | 66.0 (10.5) |
| Mean disease duration (SD), years | 4.4 (2.1) | 5.2 (1.7) |
| TDP-43 pathology | ||
| Dentate granule cells | 0 | 6 |
| Entorhinal cortex | 0 | 6 |
| Temporal cortex | 0 | 4 |
| Frontal cortex | 0 | 4 |
| NIIs | 0 | 3 |
CBD, corticobasal degeneration; NII, neuronal intranuclear inclusion; SD, standard deviation; TDP-43, TAR-DNA/binding protein 43.
Biochemical Alterations of TDP-43 in TDP-43-Positive AD and CBD Cases Are Similar in FTLD-U
To investigate whether TDP-43 protein in AD and CBD cases with immunohistochemical evidence of TDP-43-positive inclusions is biochemically modified in a manner similar to that described in FTLD-U and ALS, samples of AD and CBD cases were sequentially extracted with buffers of increasing strength and analyzed by TDP-43 immunoblot. Pathologic alterations of TDP-43 similar to those detected in FTLD-U were found in AD cases and CBD cases with TDP-43-positive inclusions by IHC. The alterations consisted of additional TDP-43 immunobands with Mrs of approximately 25 and 45 kd and a high Mr smear (Fig. 3). Progressive supranuclear palsy, CBD, PiD, and AD cases without detectable TDP-43 pathology by IHC only showed full-length TDP-43 migrating as an approximately 43-kd band similar to normal control brains. There was a good correlation between presence of pathologic TDP-43 species by immunoblot and presence of TDP-43-positive inclusions obtained by IHC. Thus, AD cases with TDP-43 pathology restricted to limbic regions showed pathologic TDP-43 species only in the hippocampus sample but not in the frontal sample, whereas AD cases with a diffuse distribution of TDP-43 pathology by IHC showed the specific biochemical signature in both samples (Fig. 3).
DISCUSSION
TAR-DNA-binding protein 43 was recently identified as a disease protein in the characteristic lesions in FTLD-U and ALS (5); it has been rapidly confirmed as the most sensitive and specific marker to detect pathologic inclusions in these conditions (8–13). The purpose of this study was to determine the frequency and distribution of TDP-43 pathology in the spectrum of neurodegenerative diseases characterized by tau pathology, including large numbers of AD, CBD, PSP, and PiD cases, and to investigate the clinical relevance of concomitant TDP-43 pathology in these conditions.
Although most of the characteristic tau-positive lesions (neurofibrillary tangles, neuropil threads, and DNs) in AD were uniformly negative for TDP-43, thereby confirming our previous results (5, 8), additional TDP-43 pathology was detected in approximately 25% of cases in our AD cohort of 182 cases. This percentage is in accordance with a recent report by Amador-Ortiz et al (16) describing TDP-43 pathology in 20% to 30% of AD cases. In contrast to FTLD-U cases in which TDP-43 pathology is widespread in the hippocampus and frontotemporal cortices, however, the distribution of TDP-43 pathology in AD cases was limited to the entorhinal cortex and dentate gyrus in approximately 75% of AD cases (present study; 16).
Several recent studies have shown that FTLD-U pathology in FTLD-U cases is heterogeneous based on the morphology and laminar distribution of ubiquitin- and TDP-43-positive inclusions, leading to the description of at least 4 distinct subtypes (8, 27, 28). Because these classification schemes are predominantly based on pathologic findings in cortical regions, the FTLD-U pathology in the AD cases with a limbic distribution pattern is difficult to subtype. In the remaining 25% of TDP-43-positive AD cases with diffuse TDP-43 pathology, however, the NCIs and small DNs were predominantly found in the upper cortical regions. Together with the presence of characteristic lentiform NIIs in several of these cases, the TDP-43 pathology reflects characteristic features of the described FTLD-U Subtype 3 according to the classification scheme by Sampathu et al (27) or FTLD-U subtype 1 according to the classification scheme by Mackenzie et al (28). This was further supported by the absence of immunoreactivity in these inclusions for FTLD-U Subtype-1- and FTLD-U Subtype-2-specific antibodies (numbering according to classification scheme by Neumann et al [5] and Sampathu et al [27]). Interestingly, this subtype of TDP- 43 pathology in FTLD-U cases has been recently shown to be associated with familial forms of FTLD-U due to mutations in the progranulin gene (8); this raises the possibility that mutations or variants in the progranulin gene might contribute to the additional TDP-43 pathology in AD. To date, the role of the progranulin gene in AD has only been studied in 1 recent report by Brouwers et al (29). Although progranulin null mutations seem to be infrequent in clinical AD cases, these authors identified 7 missense mutations in 11 AD and PD patients, although the functional consequences of these changes have not yet been demonstrated.
Documentation of combinations of distinct neurodegenerative disease lesions in the brains of patients with progressive neurologic impairments is an emerging theme in research on these disorders. This is exemplified by the high frequency of LBs detectable in AD in distinct brain regions that was only recognized after the development of α-synuclein antibodies to detect LBs in routine diagnostic studies. The effects of additional LB pathology in the setting of advanced AD pathology on the clinical presentation of the patient are, however, presently uncertain (24). Because AD cases can present with clinical signs that meet current criteria for the clinical diagnosis of FTLD (21, 22), we speculated that this atypical clinical presentation might be due to concomitant TDP-43 pathology. By comparing the frequency and distribution pattern of TDP-43 pathology in AD cases with a clinical diagnosis of AD to cases with a clinical diagnosis of FTLD, however, we found no association between TDP-43 pathology and clinical manifestations of FTLD in the AD cohort. Thus, the present data argue against a strong impact of TDP-43 pathology on the clinical presentation in AD patients. It should be noted, however, that in the present study, only a limited set of clinical manifestations of AD were examined, and more detailed correlations with larger data sets on behavioral impairments in AD have to be performed in future studies to detect possible subtle differences among TDP-43-positive and TDP-43-negative AD cases.
Most interestingly, by screening this large series of different tauopathies, we detected a previously unrecognized burden of TDP-43 pathology in a subset of CBD cases. This had not been observed in the smaller numbers of CBD cases included in previous studies (5, 8). The inclusions in the dentate gyrus in CBD were similar to FTLD-U inclusions, but a unique, predominantly glial TDP-43 pathology with labeling of threadlike structures in the gray matter that resembles astrocytic plaques and oligodendroglial white matter pathology were identified as predominant lesions in CBD. No obvious clinical correlate was found for additional TDP-43 pathology in CBD cases, however, and the significance of these findings is presently unclear. Biochemical analysis of TDP-43 extracted from AD and CBD cases with TDP-43-positive inclusions showed the same pathologic alterations of TDP-43 in insoluble protein fractions, that is, N-terminal truncation, hyperphosphorylation, and ubiquitination, as described in FTLD-U and ALS (5). There was also a clear correlation between immunohistochemical and biochemical findings among different brain regions.
In accordance with previous results on small numbers of cases (5, 8, 12), no TDP-43 pathology was detected by IHC in the large series of PSP and PiD cases in the present study. Moreover, we confirmed our negative IHC findings by demonstrating the absence of biochemical alterations of TDP-43 in protein fractions extracted from PiD and PSP tissue in a consistent manner. In addition, 1 recent publication from our group described exceptionally well-preserved RNA among our autopsy cases, which indicates excellent tissue integrity (30). The lack of TDP-43 pathology in our PiD cohort is, however, in contrast with 2 reports describing TDP-43 immunoreactivity in a subset of PiD cases (13, 31). Several reasons might explain the conflicting results, including technical differences of IHC or tissue handling, interpretation of staining, and case selection. In this context, it is important to mention that we noticed no differences in TDP-43 immunoreactivity with respect to physiologic nuclear TDP-43 labeling among our cases that were fixed either in ethanol or formalin, with fixation times ranging from 1 day up to 5 years. Moreover, Arai et al (13) were only able to detect TDP-43 labeling of Pick bodies with a polyclonal antibody but not with a monoclonal antibody to TDP-43. Unfortunately, no biochemical analyses of TDP-43 are shown in the study by Arai et al (13) and/or in the study by Freeman et al (31) in their PiD cases.
The data presented here and in previous reports, including the findings of TDP-43 pathology in another tauopathy, the Parkinson-dementia complex of Guam, clearly demonstrate the co-occurrence of TDP-43 pathology in different tauopathies (16, 18, 19, 32). The relationship between the deposition of hyperphosphorylated tau and the accumulation of TDP-43 is currently unclear. Although TDP-43 and tau immunoreactivity can be found in the same neurons, the signals only partially colocalize in most labeled structures, or separate inclusions are labeled as shown in this and previous reports (16–19). Therefore, a direct interaction between tau and TDP-43 seems unlikely but needs to be investigated in more biochemical detail. In addition, the absence of TDP-43 pathology in PSP, a tauopathy that shares clinical, biochemical, pathologic, and genetic similarities with CBD (33, 34), also argues against a simple relationship between tau dysfunction and TDP-43 accumulation.
In conclusion, our study provides further insight into the burden of TDP-43 pathology in the spectrum of tauopathies but does not demonstrate obvious clinical significance of concomitant TDP-43 pathology in these conditions.
ACKNOWLEDGMENTS
The authors thank the families of patients who made this research possible.
Supported by Grant Nos. AG10124 and AG17586 from the National Institutes of Health and by Grant No. 01GI0505 from the German Federal Ministry of Education and Research.
REFERENCES
- 1.Grossman M. Frontotemporal dementia: A review. J Int Neuropsychol Soc. 2002;8:566–583. doi: 10.1017/s1355617702814357. [DOI] [PubMed] [Google Scholar]
- 2.Shi J, Shaw CL, Du Plessis D, et al. Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol (Berl) 2005;110:501–512. doi: 10.1007/s00401-005-1079-4. [DOI] [PubMed] [Google Scholar]
- 3.Lipton AM, White CL, 3rd, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol (Berl) 2004;108:379–385. doi: 10.1007/s00401-004-0900-9. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: Demographic characteristics of 353 patients. Arch Neurol. 2005;62:925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- 5.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 6.Neumann M, Kwong LK, Sampathu DM, et al. TDP-43 Proteinopathy in frontotemporal lobar degeneration and amyotrophic lateral sclerosis: Protein misfolding diseases without amyloidosis. Arch Neurol. 2007;64:1388–1394. doi: 10.1001/archneur.64.10.1388. [DOI] [PubMed] [Google Scholar]
- 7.Kwong LK, Neumann M, Sampathu DM, et al. TDP-43 proteinopathy: The neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol (Berl) 2007;114:63–70. doi: 10.1007/s00401-007-0226-5. [DOI] [PubMed] [Google Scholar]
- 8.Cairns NJ, Neumann M, Bigio EH, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackenzie IR, Bigio EH, Ince PG, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 10.Dickson DW, Josephs KA, Amador-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol (Berl) 2007;114:71–79. doi: 10.1007/s00401-007-0234-5. [DOI] [PubMed] [Google Scholar]
- 11.Tan CF, Eguchi H, Tagawa A, et al. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol (Berl) 2007;113:535–542. doi: 10.1007/s00401-007-0206-9. [DOI] [PubMed] [Google Scholar]
- 12.Davidson Y, Kelley T, Mackenzie IR, et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol (Berl) 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- 13.Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 14.Neumann M, Kwong LK, Truax AC, et al. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol. 2007;66:177–183. doi: 10.1097/01.jnen.0000248554.45456.58. [DOI] [PubMed] [Google Scholar]
- 15.Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: A decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 16.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashima-Yasuda H, Uryu K, Robinson J, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol (Berl) 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, Arai T, Akiyama H, et al. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007;130:1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- 19.Geser F, Winton MJ, Kwong LK, et al. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol (Berl) 2008;115:133–145. doi: 10.1007/s00401-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 20.McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: Report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 21.Kertesz A, McMonagle P, Blair M, et al. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 22.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: Clinicopathological correlations. Ann Neurol. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyman BT, Trojanowski JQ. Editorial on consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 24.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 25.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP International Workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- 27.Sampathu DM, Neumann M, Kwong LK, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie IR, Baborie A, Pickering-Brown S, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: Classification and relation to clinical phenotype. Acta Neuropathol (Berl) 2006;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouwers N, Nuytemans K, van der Zee J, et al. Alzheimer and Parkinson diagnoses in progranulin null mutation carriers in an extended founder family. Arch Neurol. 2007;64:1436–1446. doi: 10.1001/archneur.64.10.1436. [DOI] [PubMed] [Google Scholar]
- 30.Chen-Plotkin AS, Geser F, Plotkin JB, et al. Variations in the progranulin gene affect global gene expression in frontotemporal lobar degeneration. Hum Mol Genet. 2008 doi: 10.1093/hmg/ddn023. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman SH, Spires-Jones T, Hyman BT, et al. TAR-DNA binding protein 43 in Pick disease. J Neuropathol Exp Neurol. 2008;67:62–67. doi: 10.1097/nen.0b013e3181609361. [DOI] [PubMed] [Google Scholar]
- 32.Higashi S, Iseki E, Yamamoto R, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 33.Mott RT, Dickson DW, Trojanowski JQ, et al. Neuropathologic, biochemical, and molecular characterization of the frontotemporal dementias. J Neuropathol Exp Neurol. 2005;64:420–428. doi: 10.1093/jnen/64.5.420. [DOI] [PubMed] [Google Scholar]
- 34.Houlden H, Baker M, Morris HR, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56:1702–1706. doi: 10.1212/wnl.56.12.1702. [DOI] [PubMed] [Google Scholar]