SYNOPSIS
Chronic lung diseases, including chronic obstructive pulmonary disease (COPD) and pulmonary hypertension (PH) are unusually prevalent among persons infected with HIV. In many cases these disease states are identified at younger ages than would be expected in the general population. Recent epidemiologic, basic science, and cross-sectional clinical data have implicated immune dysfunction and cellular senescence as potential drivers of advanced presentations of age-related diseases in HIV-infected persons. This article describes how HIV-associated COPD and PH may fit into a paradigm of immunosenescence and outlines the hypothesized associations among chronic HIV infection, immune dysfunction and senescence, and cardiopulmonary outcomes.
Keywords: HIV, chronic obstructive pulmonary disease, pulmonary hypertension, immune activation, immune senescence, inflammation
Background/Introduction
Due to the success of combination antiretroviral therapy (ART) in restoring immune function, there have been marked declines in mortality from HIV infection in individuals with access to treatment1. As a result, over 30% of the HIV-infected population in the United States is currently over the age of 50, with 50% expected to be over the age of 50 by the year 20202. Unfortunately, these gains in longevity have been accompanied by a growing burden of age-related diseases, attributable both to natural aging and to a recently described potential for HIV to cause accelerated cellular senescence3,4.
Diseases traditionally associated with aging have been identified in several organ systems (including renal, neurologic, and cardiac) at unexpected prevalence in cohorts of midlife HIV-infected individuals4–8, and similar findings are being investigated in the pulmonary system. Because of its unique exposure to airborne pathogens and toxins, the damage from which may be enhanced by HIV infection, the lung and its circulation may be particularly sensitive to early presentation of chronic disease. Two lung diseases in particular, chronic obstructive pulmonary disease (COPD) and pulmonary hypertension (PH), are accelerated in HIV and may be linked to senescence9–21.
The prevalence of COPD increases with age and is associated with senescence in both the HIV-uninfected and HIV-infected populations11,22–27. The hypothesis that HIV-associated COPD occurs as a consequence of accelerated cellular aging is particularly compelling because inflammatory dysfunction, a hallmark of HIV-associated senescence, plays a central role in the pathogenesis of COPD28.
PH is also a possible senescence-related complication of HIV. Although PH is not typically considered an age-dependent phenomenon in the general population, subsets of PH, including those secondary to left ventricular dysfunction29 or COPD, are associated with increasing age; furthermore, a recent study suggests that COPD-associated PH is related to leukocyte and smooth muscle cellular senescence30. While the pathogenesis of HIV-associated PH is poorly understood, current studies are evaluating the possible relationships among immune activation, aging, and the development of HIV-associated PH.
This article reviews the features of cellular senescence and its relationship to HIV and immune activation and examines the foundation of the hypothesis that senescence is associated with COPD and PH in persons with HIV. We address recent advances in the understanding of the complex interplay between antigenic exposures, host immune response, and inflammation in both chronic HIV infection and chronic cardiopulmonary dysfunction. Ongoing studies continue to investigate links between these disease states; these relationships may be instrumental in developing effective prevention and treatment of pulmonary complications associated with chronic HIV infection.
HIV, Immune Activation, and Aging: The Immunosenescence Hypothesis
Cellular senescence is one of the central features of aging organ systems and is defined by the inability of a cell to undergo further division. Senescence can be induced either by direct insult to cells (e.g. oxidative stress) or via replicative senescence, a phenomenon by which repeated cell division results in proliferative arrest. The cells of the immune system, which are subject to frequent rounds of division via clonal expansion following antigenic exposures31, are particularly susceptible to replicative senescence. In the case of HIV infection, either HIV itself or the co-infections commonly associated with HIV (including cytomegalovirus (CMV)32 and hepatitis C33,34) drive repeated cycles of immune activation and T-cell proliferation. Due to repeated triggering and expansion, the immune cells reach a replicative limit, characterized by loss of the costimulatory surface receptor CD28 (i.e. CD28null)35. These senescent CD28null T-cells are dysfunctional; they are less able to clear infections, but contribute to a persistent upregulation of the inflammatory response with increases in peripheral inflammatory cytokines36–38. This smoldering inflammation resulting from persistent immune activation and immune senescence characterizes the systemic milieu of chronic HIV infection.
During acute and chronic HIV infection, T-cells demonstrate elements of immune cell aging, with features of both chronic senescence and activation39–41. An early study of T-cell response to HIV found that during acute infection, both HIV-specific CD8+ cells and the overall CD8+ cell population are highly activated (expressing the cellular activation marker CD38 and the proliferative marker Ki67). Although the percentage of these activated populations dropped during chronic infection, paralleling the fall in the viral load, the terminally differentiated “senescent” T-cell population (CD28null) is markedly enriched in chronic infection, a phenomenon that is even more pronounced in the overall CD8+ population than in HIV-specific CD8+ cells41. Further studies of CD4+ and CD8+ cell phenotypes in persons with chronic HIV infection have demonstrated that abnormal immune activation persists even in individuals with sustained viral suppression on ART42,43.
This state of immune activation and senescence, provoked by chronic HIV and by the chronic viral and recurrent bacterial infections that frequently accompany it, has been linked to features of aging and disease in the cardiovascular system44 and is suspected to be at play in other disease states. It has been hypothesized that non-immune organs sustain collateral damage due to the systemic inflammatory milieu, either via circulating inflammatory cytokines or from more direct insults, when activation and senescent T-cells are recruited to these organs at sites of injury or infection. Because of its vulnerability to repeated exposures to tobacco smoke, other environmental toxins and microbes (via infection or colonization), the lung and its circulation may be at heightened risk to sustain damage from senescent and activated circulating immune cells. COPD and PH, which are seen at higher than expected prevalence in mid-life HIV-infected persons, have been associated with systemic immune activation and inflammation in the non-HIV population, and are therefore of particular interest.
COPD: Relationships to Immune Activation and Senescence
COPD is common in the HIV-uninfected population and is the fourth leading cause of mortality in the US45. This disease typically presents in the sixth decade of life or later, but in the HIV population, it is often diagnosed at a younger age. Investigators have reported severe emphysema in HIV-infected persons in their thirties13, and studies of COPD in HIV-infected cohorts have found a mean age of those with COPD to range from 40 to 50 years46. Studies have found a prevalence of 20–60% of physiologic measures of COPD in HIV-infected persons12,14,17,46 versus 7% reported in the general population47. In general, most ART-era studies of HIV-infected persons show a high overall prevalence of airflow obstruction, ranging from 8–21%12,14,15,17, despite the relatively young age of the HIV-infected participants. While HIV-infected persons do have particularly high exposure to pulmonary risk factors (most notably cigarette smoking12,48) that likely interact with other factors to contribute to chronic respiratory illness, these data provide epidemiologic support for the hypothesis that the lung may be another end-organ affected by senescence in HIV.
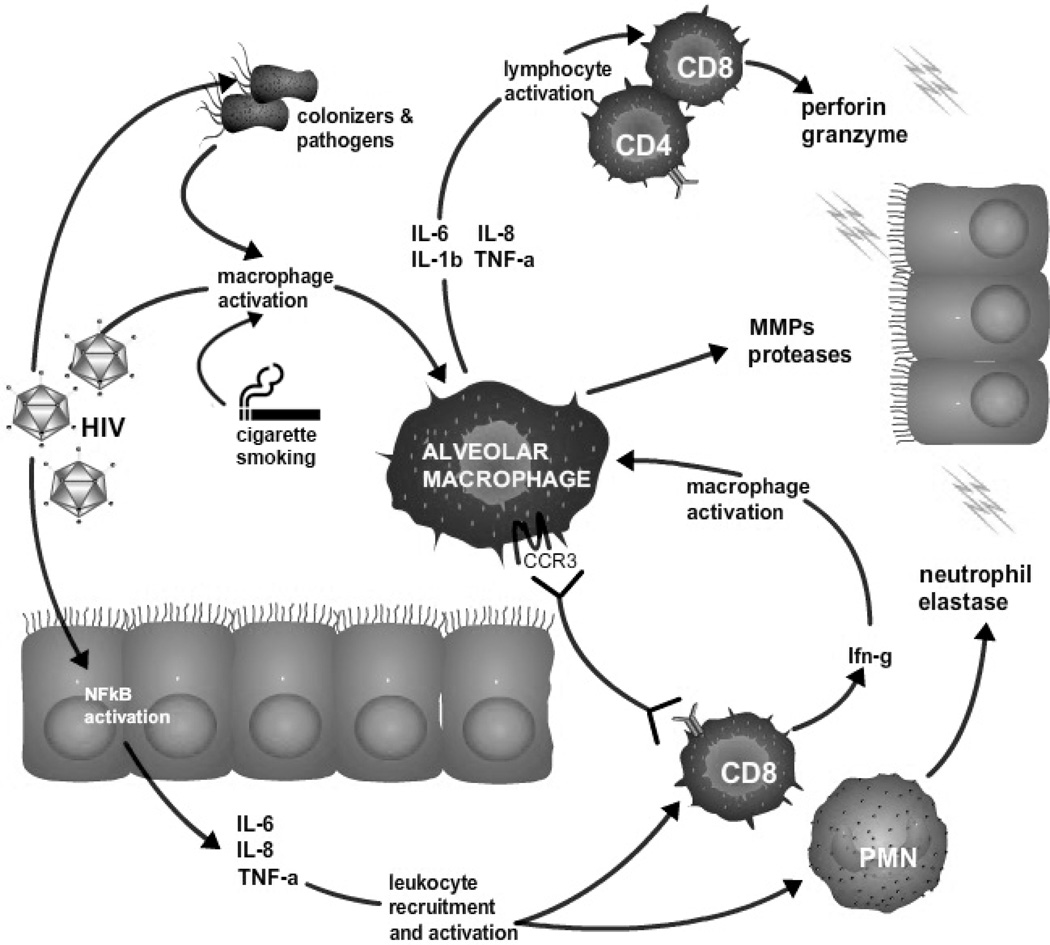
Molecular data examining immune-mediated inflammatory pulmonary cell damage and resultant accelerated alveolar epithelial cell senescence also support the role of aging in HIV COPD (Figure 1). Recent advances in COPD have shown that the disease is not driven by one mechanism, but is a syndrome that is precipitated by multiple insults, which may act individually or in concert to cause irreversible damage to the airways or alveoli49,50. Immune-mediated inflammatory pulmonary cell damage and resultant accelerated alveolar epithelial cell senescence may contribute to HIV COPD. Immune activation and senescence have not been directly investigated in HIV-associated COPD, but indirect links from studies of aging and inflammation in COPD in the HIV-uninfected population support a role for aging in HIV-associated COPD as well. The presence and severity of obstruction in COPD in the general population has been associated with increased systemic inflammatory cytokines including high sensitivity C-reactive protein (hsCRP), interleukin (IL)-6, and fibrinogen. Persistence of this systemic inflammatory phenotype predicts both COPD exacerbation rate and all-cause mortality51. A study specifically examining intracellular cytokine levels in circulating leukocytes and bronchoalveolar lavage cells found elevated interferon (IFN)-γ and tumor necrosis factor (TNF)-α within circulating CD8+ cells and bronchoalveolar lavage CD8+ and CD4+ cells in persons with COPD52.
Figure 1. Theoretical framework of development of HIV-associated COPD.
HIV-associated immune deficiency allows for high burden of microbial infection and colonization – both HIV virions and other microbes lead to macrophage activation. In addition to releasing matrix metalloproteases, activated alveolar macrophages express inflammatory cytokines that activate local CD4+ and CD8+ lymphocytes. Additionally, activated macrophages express the cytokine receptor CCR3, encouraging trafficking of CD8+ T-cells from the circulation. Activated CD8+ cells (which are likely to be senescent in the setting of chronic HIV infections) elaborate interferon-gamma, which leads to amplification of macrophage activation. Additionally, HIV directly activates NFκB in alveolar epithelial cells, leading to expression of inflammatory cytokines, further driving leukocyte recruitment and activation. Recruited and activated immune cells may cause local pulmonary damage (i.e. COPD) and epithelial cell senescence via the expression of proteases, perforin, granzyme, and neutrophil elastase. Abbreviations: CCR, chemokine receptor; IFN, interferon; IL, interleukin; MMP, matrix metoalloprotease; NF, nuclear factor; PMN, polymorphonuclear cells; TNF, tumor necrosis factor.
While human studies of inflammation and COPD have been largely associative, animal and in vitro studies are able to investigate the directionality of the relationship. For example, researchers have determined that chronic systemic inflammation in a murine model results in pulmonary inflammation and senescent lung changes53. A recent murine study that induced chronic systemic inflammation via subcutaneous lipopolysaccharide (LPS) implant found that LPS-exposed mice developed pulmonary inflammatory changes (increased alveolar macrophages) and evidence for pulmonary cell DNA double-strand breaks, which are precursors of cellular apoptosis and senescence53. In turn, senescence of pulmonary epithelial cells may also lead to regional lung inflammation, creating a vicious cycle of local damage. Induction of an in vitro senescent phenotype in lung epithelial cells in culture leads to higher levels of pro-inflammatory NF-kB activation and also results in higher pulmonary epithelial production of the inflammatory cytokines IL-6, IL-8, and TNF-α. Ex vivo studies of lung tissue explants established that type II epithelial cells from COPD patients demonstrated higher expression of the cellular senescence marker p16, and that senescent cells more frequently demonstrated a pro-inflammatory phenotype as measured by presence of phosphorylated NF-kB54. These findings further bolster the supposition that chronic systemic inflammation may lead to the inflammatory and senescent changes of COPD.
In addition to data supporting inflammation and pulmonary cell senescence as possible drivers of COPD, several investigations have specifically addressed the contributions of immune senescence. A cross-sectional study examining the T-cell repertoire and inflammatory response in association with COPD found that higher senescent (CD28null) circulating CD4+ cell percentage correlated with lower forced expiratory volume in one second (FEV1) percent-predicted and greater midflow obstruction25. Although some circulating inflammatory markers (IFN-γ, TNF-α, and IL-1β) were associated with better FEV1 percent-predicted, when T-cells in culture were activated, the cells from early-stage COPD secreted increased levels of IFN-γ and TNF-α, suggesting that local stimulation (e.g., at the alveolar-capillary interface) of primed senescent cells may lead to enhanced release of these pro-inflammatory cytokines25. A cross-sectional assessment of the relationship between immune cell telomere length (where shorter telomeres may identify a more senescent cell phenotype) and lung function found that participants with COPD have shorter telomeres in circulating leukocytes telomeres than do healthy controls. Additionally, telomere shortening was correlated with reduced activity of superoxide dismutase (a free radical scavenger that may provide protection from COPD)55. A similar study found that circulating leukocyte telomere shortening was associated with COPD and that higher circulating levels of the inflammatory cytokine IL-6 were associated both with telomere attrition and with the presence of COPD56. Given the immune dysfunction described in the previous section, any or all of these mechanisms are likely to play a role in the pathogenesis of HIV-associated COPD.
Pulmonary Hypertension: Relationships to Immune Activation and Senescence
Pulmonary hypertension, like COPD, is described at increased frequency among persons with HIV. Both before and after the availability of combination antiretroviral therapy, PH has been found at a prevalence of 0.5%20,57 among HIV-infected persons. Further directed investigations assessing pulmonary artery pressures in current-era HIV-infected cohorts have found prevalence of echocardiographic markers of pulmonary hypertension ranging from 15.5–35%18,21,58, with potential risk factors including male sex20, injection drug use20, and CD4+ count < 200 cells/uL18,20. Animal models have supported the direct pathogenic role of HIV in the development of PH. For example, in a recently published study, simian immunodeficiency virus (SIV) and simian-human immunodeficiency virus (SHIV)-infected macaques developed echocardiographic and right heart catheterization findings of PH that were significantly worse when compared to uninfected controls59. Mechanisms underlying HIV-associated PH are a subject of ongoing investigation – potential contributors have been covered in another article in this issue and in several previous review articles16,60,61. These potential contributors include enhanced production of growth factors also implicated in non-HIV PH (including platelet-derived growth factor and vascular endothelial growth factor) and virus-specific factors, namely nef and the viral envelope glycoprotein-120.
As with COPD, immune dysfunction, immune senescence, and constitutive cell (in this case, endothelial and pulmonary artery smooth muscle cell) senescence may play a role in development of HIV-associated PH. Studies of non-HIV PH have demonstrated mixed associations with inflammatory markers, but the data generally suggest that inflammation of some variety contributes to disease pathogenesis. Inflammatory infiltrates of mononuclear cells have been identified in the characteristic plexiform lesions of PH, and a recent study has demonstrated organized lymphoid structures in idiopathic PH62. Multiple markers of inflammation and immune dysfunction have been described in association with non-HIV PH, and are summarized in depth in recent review articles60,63. Additionally, there is potential evidence for inflammation-mediated senescence in non-HIV PH – a study investigating markers of cell senescence in patients with COPD found that higher circulating IL-6 and shorter telomeres in circulating leukocytes correlate with increasing pulmonary artery pressures30.
The possible inflammatory and immunosenescent features of HIV-associated PH have only recently been investigated, but early data are promising. One study identified that circulating IL-8, IFN-γ, and activated T-cells (CD8+CD69+) were associated with elevated pulmonary artery systolic pressure (PASP) and tricuspid regurgitant jet velocity (TRV), as was sputum IL-818. Interestingly, increasing PASP and TRV were independently associated with pulmonary function abnormalities, including worse spirometry and lower diffusing capacity for carbon monoxide. Of note, none of the participants required oxygen, and none had severe COPD, arguing against pre-existing hypoxemic lung disease as the etiology of the elevated pulmonary artery (PA) pressures. Additionally, elevated TRV was associated with serologic markers of poorly-controlled HIV (CD4 < 200 cells/µL or elevated HIV RNA levels), irrespective of ART use18. These findings suggest that systemic and/or pulmonary immune activation and resultant inflammation, which is worse in the setting of more advanced HIV, may underlie both pulmonary dysfunction and elevated PA pressures in HIV-infected persons.
The Complex Interplay among HIV, Immune Activation, and Pulmonary Dysfunction: Future Directions
While the accelerated pulmonary disease seen in HIV is in part related to traditional risk factors, the early and unusually prevalent presentations of disorders such as COPD and PH suggest a distinctive contribution of HIV that may involve unique mechanistic pathways (Figure 1). Senescent and activated T-cells, which are commonly elevated even among virally-suppressed persons, may contribute to lung and pulmonary circulatory damage either indirectly (via inflammatory cytokines expressed in the circulation) or directly, when they are recruited to the lung or its circulation in response to stimuli (including cigarette smoke, other inhaled toxins, or pulmonary microbes). There is also potential for cellular aging in the resident immune cells or constitutive cells of the lung and pulmonary artery, either as a direct effect of HIV or as a result of inflammation due to trafficking of dysregulated systemic immune cells. While these relationships are speculative, current research directed at describing the associations between indicators of HIV-associated immune activation, immune senescence, epithelial and endothelial cell aging, and outcomes of pulmonary dysfunction are currently underway. Determining the roles of accelerated immune aging and chronic inflammation in HIV may eventually allow for effective directed interventions in this population64.
KEY POINTS.
HIV is now a chronic disease among persons treated with highly active combination antiretroviral therapy, and it is frequently complicated by comorbid age-related conditions. Pulmonary comorbidities of chronic HIV are increasingly recognized.
Chronic obstructive pulmonary disease (COPD) and pulmonary hypertension (PH) are both present at increased frequency among persons infected with HIV.
Sustained HIV-associated systemic inflammation may result in accelerated cellular senescence with cardiopulmonary end-organ injury, thus contributing to COPD and pulmonary vascular disease.
Acknowledgments
DISCLOSURES
Funding sources:
Fitzpatrick: F32HL114426
Crothers: R01HL090342
Morris: R01HL090339, U01HL098962, P01HL103455
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Fitzpatrick: n/a
Crothers: n/a
Morris: n/a
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 4.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008;16:94–98. [PubMed] [Google Scholar]
- 6.Wand H, Calmy A, Carey DL, et al. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. Aids. 2007;21:2445–2453. doi: 10.1097/QAD.0b013e3282efad32. [DOI] [PubMed] [Google Scholar]
- 7.Bozzette SA, Ake CF, Tam HK, et al. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–710. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 8.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crothers K. Chronic obstructive pulmonary disease in patients who have HIV infection. Clin Chest Med. 2007;28:575–587. doi: 10.1016/j.ccm.2007.06.004. vi. [DOI] [PubMed] [Google Scholar]
- 10.Crothers K, Butt AA, Gibert CL, et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 11.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Q, Carruthers S, McIvor A, et al. Effect of smoking on lung function, respiratory symptoms and respiratory diseases amongst HIV-positive subjects: a cross-sectional study. AIDS Res Ther. 2010;7:6. doi: 10.1186/1742-6405-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132:369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 14.George MP, Kannass M, Huang L, et al. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4:e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gingo MR, Morris A. Pathogenesis of HIV and the Lung. Curr HIV/AIDS Rep. 2012 doi: 10.1007/s11904-012-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirani A, Cavallazzi R, Vasu T, et al. Prevalence of obstructive lung disease in HIV population: a cross sectional study. Respir Med. 2011;105:1655–1661. doi: 10.1016/j.rmed.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Morris A, Gingo MR, George MP, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS. 2012;26:731–740. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrache I, Diab K, Knox KS, et al. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax. 2008;63:463–469. doi: 10.1136/thx.2007.079111. [DOI] [PubMed] [Google Scholar]
- 20.Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 21.Mondy KE, Gottdiener J, Overton ET, et al. High Prevalence of Echocardiographic Abnormalities among HIV-infected Persons in the Era of Highly Active Antiretroviral Therapy. Clin Infect Dis. 2011;52:378–386. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 22.Aoshiba K, Nagai A. Senescence hypothesis for the pathogenetic mechanism of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:596–601. doi: 10.1513/pats.200904-017RM. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 24.Karrasch S, Holz O, Jorres RA. Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir Med. 2008;102:1215–1230. doi: 10.1016/j.rmed.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Lambers C, Hacker S, Posch M, et al. T cell senescence and contraction of T cell repertoire diversity in patients with chronic obstructive pulmonary disease. Clin Exp Immunol. 2009;155:466–475. doi: 10.1111/j.1365-2249.2008.03835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD) Biochem Soc Trans. 2009;37:819–823. doi: 10.1042/BST0370819. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886–893. doi: 10.1164/rccm.200509-1374OC. [DOI] [PubMed] [Google Scholar]
- 28.Gadgil A, Duncan SR. Role of T-lymphocytes and pro-inflammatory mediators in the pathogenesis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:531–541. doi: 10.2147/copd.s1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam CS, Borlaug BA, Kane GC, et al. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–2670. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noureddine H, Gary-Bobo G, Alifano M, et al. Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease. Circ Res. 2011;109:543–553. doi: 10.1161/CIRCRESAHA.111.241299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karrer U, Sierro S, Wagner M, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 32.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacs A, Karim R, Mack WJ, et al. Activation of CD8 T cells predicts progression of HIV infection in women coinfected with hepatitis C virus. J Infect Dis. 2010;201:823–834. doi: 10.1086/650997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sajadi MM, Pulijala R, Redfield RR, et al. Chronic immune activation and decreased CD4 cell counts associated with hepatitis C infection in HIV-1 natural viral suppressors. AIDS. 2012;26:1879–1884. doi: 10.1097/QAD.0b013e328357f5d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Effros RB. Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Dev Comp Immunol. 1997;21:471–478. doi: 10.1016/s0145-305x(97)00027-x. [DOI] [PubMed] [Google Scholar]
- 36.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Sansoni P, Vescovini R, Fagnoni F, et al. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Cao W, Jamieson BD, Hultin LE, et al. Premature aging of T cells is associated with faster HIV-1 disease progression. J Acquir Immune Defic Syndr. 2009;50:137–147. doi: 10.1097/QAI.0b013e3181926c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep. 7:4–10. doi: 10.1007/s11904-009-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appay V, Papagno L, Spina CA, et al. Dynamics of T cell responses in HIV infection. J Immunol. 2002;168:3660–3666. doi: 10.4049/jimmunol.168.7.3660. [DOI] [PubMed] [Google Scholar]
- 42.Valdez H, Connick E, Smith KY, et al. Limited immune restoration after 3 years' suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–1866. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 43.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu JQKK, Tejada-Vera B. Deaths: Preliminary data for 2007. National vital statistics reports. National Center for Health Statistics. 2009 [Google Scholar]
- 46.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160:1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 48.Niaura R, Shadel WG, Morrow K, et al. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis. 2000;31:808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- 49.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 50.Casanova C, de Torres JP, Aguirre-Jaime A, et al. The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med. 2011;184:1015–1021. doi: 10.1164/rccm.201105-0831OC. [DOI] [PubMed] [Google Scholar]
- 51.Agusti A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7:e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hodge G, Nairn J, Holmes M, et al. Increased intracellular T helper 1 proinflammatory cytokine production in peripheral blood, bronchoalveolar lavage and intraepithelial T cells of COPD subjects. Clin Exp Immunol. 2007;150:22–29. doi: 10.1111/j.1365-2249.2007.03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arimura K, Aoshiba K, Tsuji T, et al. Chronic Low-Grade Systemic Inflammation Causes DNA Damage in the Lungs of Mice. Lung. 2012 doi: 10.1007/s00408-012-9414-8. [DOI] [PubMed] [Google Scholar]
- 54.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration. 2010;80:59–70. doi: 10.1159/000268287. [DOI] [PubMed] [Google Scholar]
- 55.Houben JM, Mercken EM, Ketelslegers HB, et al. Telomere shortening in chronic obstructive pulmonary disease. Respir Med. 2009;103:230–236. doi: 10.1016/j.rmed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Savale L, Chaouat A, Bastuji-Garin S, et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:566–571. doi: 10.1164/rccm.200809-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Speich R, Jenni R, Opravil M, et al. Primary pulmonary hypertension in HIV infection. Chest. 1991;100:1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 58.Hsue PY, Deeks SG, Farah HH, et al. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS. 2008;22:825–833. doi: 10.1097/QAD.0b013e3282f7cd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.George MP, Champion HC, Simon M, et al. Physiologic Changes in a Non-Human Primate Model of HIV-Associated Pulmonary Arterial Hypertension. Am J Respir Cell Mol Biol. 2012 doi: 10.1165/rcmb.2011-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price LC, Wort SJ, Perros F, et al. Inflammation in pulmonary arterial hypertension. Chest. 2012;141:210–221. doi: 10.1378/chest.11-0793. [DOI] [PubMed] [Google Scholar]
- 61.Cicalini S, Almodovar S, Grilli E, et al. Pulmonary hypertension and human immunodeficiency virus infection: epidemiology, pathogenesis, and clinical approach. Clin Microbiol Infect. 2011;17:25–33. doi: 10.1111/j.1469-0691.2010.03286.x. [DOI] [PubMed] [Google Scholar]
- 62.Perros F, Dorfmuller P, Montani D, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:311–321. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- 63.El Chami H, Hassoun PM. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog Cardiovasc Dis. 2012;55:218–228. doi: 10.1016/j.pcad.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plaeger SF, Collins BS, Musib R, et al. Immune activation in the pathogenesis of treated chronic HIV disease: a workshop summary. AIDS Res Hum Retroviruses. 2012;28:469–477. doi: 10.1089/aid.2011.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]