Abstract
Burn injury causes a major systemic catabolic response that is associated with mitochondrial dysfunction in skeletal muscle. We investigated the effects of the mitochondria-targeted peptide antioxidant Szeto-Schiller 31 (SS-31) on skeletal muscle in a mouse burn model using in vivo phosphorus-31 nuclear magnetic resonance (31P NMR) spectroscopy to noninvasively measure high-energy phosphate levels; mitochondrial aconitase activity measurements that directly correlate with TCA cycle flux, as measured by gas chromatography mass spectrometry (GC-MS); and electron paramagnetic resonance (EPR) to assess oxidative stress. At 6 h postburn, the oxidative ATP synthesis rate was increased 5-fold in burned mice given a single dose of SS-31 relative to untreated burned mice (P=0.002). Furthermore, SS-31 administration in burned animals decreased mitochondrial aconitase activity back to control levels. EPR revealed a recovery in redox status of the SS-31-treated burn group compared to the untreated burn group (P<0.05). Our multidisciplinary convergent results suggest that SS-31 promotes recovery of mitochondrial function after burn injury by increasing ATP synthesis rate, improving mitochondrial redox status, and restoring mitochondrial coupling. These findings suggest use of noninvasive in vivo NMR and complementary EPR offers an approach to monitor the effectiveness of mitochondrial protective agents in alleviating burn injury symptoms.—Righi, V., Constantinou, C., Mintzopoulos, D., Khan, N., Mupparaju, S. P., Rahme, L. G., Swartz, H. M., Szeto, H. H., Tompkins, R. G., and Tzika, A. A. Mitochondria-targeted antioxidant promotes recovery of skeletal muscle mitochondrial function after burn trauma assessed by in vivo 31P nuclear magnetic resonance and electron paramagnetic resonance spectroscopy.
Keywords: physiology, molecular medicine, mice, mitochondria, oxidative stress
Severe burn injury is associated with anatomical, physiological, endocrinological, and immunological changes, which lead to generalized cachexia, as well as metabolic alterations that play a central role in pathophysiological progression (1). The major systemic catabolic response to burn injury is associated with mitochondrial dysfunction in skeletal muscle, heart, and liver (2). Early burn wound excision has been shown to reduce catabolism (3), but many burn patients still experience extensive catabolism following excision. Thus, agents that buffer postburn physiological responses could be developed into powerful medicines for ameliorating cachexia.
Burn-induced mitochondrial dysfunction is associated with increased mitochondrial reactive oxygen species (ROS) and decreased oxidative phosphorylation (4, 5). Mitochondria are the major source of intracellular ROS and are particularly vulnerable to oxidative stress. Mitochondrial damage has been shown to further impair function, leading to cell death via apoptosis and necrosis. Overproduction of ROS instigates a feed-forward loop, whereby ROS-mediated oxidative damage to mitochondria leads to even more ROS generation, resulting in a vicious cycle. A number of studies have provided evidence for a link between postburn mitochondrial dysfunction and skeletal muscle wasting (4–12). Indeed, skeletal muscle wasting caused by mechanical ventilation and immobilization is also associated with mitochondrial oxidative stress and dysfunction (13, 14).
It was reported recently that treatment with the mitochondria-targeted peptide antioxidant Szeto-Schiller 31 (SS-31) can attenuate mitochondrial ROS production, normalize mitochondrial respiration, and prevent myofiber atrophy following mechanical ventilation and immobilization (13, 14). SS-31 (D-Arg-2′6′-dimethyl-Tyr-Lys-Phe-NH2) is a synthetic aromatic-cationic tetrapeptide that targets and concentrates ∼1000-fold in the inner mitochondrial membrane, where it has been shown to selectively reduce mitochondrial ROS, protect mitochondrial function, and prevent cell death by apoptosis and necrosis (15, 16). Treatment with SS-31 can also attenuate excessive mitochondrial ROS emission in skeletal muscles associated with insulin resistance and in cardiac muscle in multiple models of heart failure (17, 18). Furthermore, the protection provided by SS-31 in both skeletal and cardiac muscle was similar to that achieved by overexpression of catalase targeted to mitochondria (17, 18). These findings support the notion that reduction of mitochondrial ROS may be a therapeutic target for burn-induced muscle wasting. Indeed, it was reported recently that treatment with SS-31 after burn injury reduced oxidized proteins and prevented increase in caspase-3 activity and apoptosis in skeletal muscles (19). SS-31 also ameliorated burn-induced insulin resistance (20).
It remains to be demonstrated, however, whether the aforementioned protective actions are related to SS-31's ability to reduce mitochondrial oxidative stress and protect mitochondrial function. Therefore, here, we investigated the effects of SS-31 on oxidative stress and adenosine triphosphate (ATP) production in skeletal muscle in a mouse model of burn injury using phosphorus-31 nuclear magnetic resonance (31P NMR) and complementary electron paramagnetic resonance (EPR) spectroscopy experiments. In addition, we analyzed mitochondrial aconitase activity, a correlate of tricarboxylic acid (TCA) cycle flux and index of oxidative stress.
MATERIALS AND METHODS
Experimental animals and study design
Male 6-wk-old CD1 mice (20–25 g) were maintained on a regular light-dark cycle (lights on from 8:00 AM to 8:00 PM) at an ambient temperature of 22 ± 1°C and had free access to food and water. The animals were anesthetized by intraperitoneal (i.p.) injection of 40 mg/kg pentobarbital sodium and the left hind limb of all mice was shaved. Burn injury was produced by a nonlethal scald injury of 3–5% total body surface area by immersing the left hind limb in 90°C water for 3 s (21). Control animals were prepared in the same way, with the exception that limb immersion was performed with room temperature water. After burn or sham treatment, the mice were randomized into 4 groups: burn (B), burn + SS-31 peptide (B+P), control (C), and control + SS-31 (C+P). Two types of experiments were performed: 1) SS-31 (3 mg/kg) was administered by i.p. injection immediately after burn and 2) SS-31 (3 mg/kg) was administered by i.p. injection at 30 min preburn and again immediately postburn. The EPR experiments were performed prior to burn, and at 0, 3, 6, 24, and 48 h postburn. The NMR experiments were performed at 6 h postburn, as EPR showed that the SS-31 effect is maximal at this time point. NMR analysis was also performed at 7 d postburn. The contralateral hind limb served as the control, as its NMR values were not significantly different from those found for the nonburned controls and were in agreement with the control values previously observed (4, 10). All animal experiments were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital (Boston, MA, USA), and by the Institutional Animal Care and Use Committee of Dartmouth Medical School (Hanover, NH, USA).
EPR
For assessing oxidative damage after burn trauma, in vivo EPR using nitroxides was adopted as a complementary approach to NMR since NMR cannot measure redox status and ROS, while EPR can. Mice were randomized into B, B+P, C, and C+P groups (n=6–8). SS-31 (3 mg/kg) was administered by i.p. injection at 0, 3, 6, 24, and 48 h postburn. EPR measurements were carried out with a 1.2-GHz EPR spectrometer equipped with a microwave bridge and surface loop resonator specially designed for in vivo experiments. Typical spectrometer parameters were incident microwave power, 8 mW; magnetic field center, 400 G; and modulation frequency, 27 kHz. The decay kinetics of intravenously injected 3-carbamoyl-2,2,5,5-tetramethylpyrrolidin-1-yloxyl nitroxide (CPA; 150 mg/kg) over time was used to assess mitochondrial redox status of the muscle (5).
31P NMR
In vivo NMR spectroscopy permits quantification of intracellular physiological variables without removal or destruction of the tissue to be examined (22, 23). In vivo NMR represents a significant advance in the study of mitochondrial function by permitting measurements under physiological conditions in intact skeletal muscle without the artifacts of in vitro studies. This methodology enables the measurement of the rate of skeletal muscle ATP synthesis catalyzed by mitochondrial ATPase (24, 25), which is proportional to oxygen consumption via the P/O ratio (ratio of the net rate of ATP synthesis by oxidative phosphorylation to the rate of oxygen consumption; refs. 26, 27).
In vivo NMR spectroscopy was performed preburn and at 6 h and 7 d postburn
The mice were anesthetized transiently with a mixture of isoflurane (3.0%) and O2 (2.0 L/min) delivered through a nose cone, while the animals were held in a customized restraining tube. Each animal's left hind limb was placed into a solenoid coil (4 turns; length, 2 cm; diameter, 1 cm) tuned to 31P frequency (162.1 MHz). Body temperature was maintained at 37 ± 1°C using heated water blankets. All in vivo 31P NMR experiments were performed in a horizontal bore magnet (proton frequency, 400 MHz; diameter, 21 cm; Magnex Scientific; Varian, Palo Alto, CA, USA) using a Bruker Advance console (Bruker Corp., Billerica, MA, USA) and a custom 31P coil designed to fit the mouse hind limb. Field homogeneity was adjusted using the 1H signal of tissue water. A 90° pulse was optimized for detection of phosphorus spectra (repetition time 2 s, 192 averages, 4000 data points). Saturation 90° selective pulse trains (duration, 36.534 ms; bandwidth, 75 Hz) followed by crushing gradients were used to saturate the γATP peak. The same saturation pulse train was applied downfield of the inorganic phosphate (Pi) resonance, symmetrically to the γATP resonance. Relaxation time (T1) values for Pi and phosphocreatine (PCr) were measured using an inversion recovery pulse sequence in the presence of γATP saturation. An adiabatic pulse (400 scans; sweep width, 10 kHz; 4000 data points) was used to invert Pi and PCr, with an inversion time between 152 and 7651 ms.
Following the induction of anesthesia and for the duration of the MR imaging, the mice were kept anesthetized with a mixture of isoflurane (1.5%) and O2 (0.6 L/min). Each mouse's body temperature was maintained at 37°C with a warm water blanket. All of the experiments were run in the same way for all groups.
NMR data analysis
The theoretical basis of saturation transfer experiments has been described previously by Forsen and Hoffman (28). 31P NMR spectra were analyzed using the MestRe-C NMR software package (Mestrelab Research, Santiago de Compostela, Spain; http://www.mestrec.com). Free induction decays were zero-filled to 8000 points and apodized with exponential multiplication (30 Hz) before Fourier transformation. The spectra were then manually phased and corrected for baseline broad features. The Levenberg-Marquardt algorithm was used to least-squares fit a model of mixed gaussian/lorentzian functions to the data. Similarly, the observed relaxation time T1obs for Pi and PCr was calculated by fitting the function y = A1[1 − A2e−(t/T1obs)] to the inversion recovery data, where y is the z magnetization, and t is the inversion time. The fractional change in ΔM/M0 and the observed spin lattice relaxation time, T1obs, were used to calculate the flux constant κf using the equation (1/T1obs) × (ΔM/M0). The ATP synthesis rate was then obtained as the product of κf and Pi concentration (for supporting calculations, see ref. 4).
In vivo intramyocellular pH from NMR data
Intramyocellular pH was calculated using the formula pH = 6.75 + log [(s − 3.27)/(5.69 − s)], where s is the chemical shift difference (in ppm) between the Pi and the PCr peaks (29).
Mitochondrial aconitase activity
Mitochondrial aconitase activity correlates with TCA flux and is an index of oxidative stress because it is inhibited by ROS. Mitochondrial aconitase activity was assessed using the Bioxytech Aconitase-340 kit (catalog no. 21041) from OxiResearch (OXIS Health Products, Portland, OR, USA) with small modifications. Briefly, immediately after the in vivo NMR procedure, all animals were anesthetized by i.p. injection of 40 mg/kg pentobarbital sodium, and both gastrocnemius muscles (burned and contralateral) were excised. All mice were then administered a lethal dose of pentobarbital sodium (200 mg/kg, i.p.). Each isolated gastrocnemius muscle was minced and then homogenized in 2 ml of ice-cold 50 mM Tris (pH 7.4; assay buffer, provided with the kit) using a Brinkmann Polytron PT 3000 homogenizer (Brinkmann Instruments, Westbury, NY, USA). The homogenates were then centrifuged at 800 g for 10 min at 4°C. The supernatants were collected and recentrifuged at 20,000 g for 10 min at 4°C, and the resultant pellets were resuspended in 500 μl of ice-cold assay buffer. Samples were then stored at −80°C until further analysis.
To assess mitochondrial aconitase activity, all samples were thawed and sonicated at low power for 20 s on ice. Then, 50-μl aliquots of each sample or assay buffer (used as a blank) were added to the wells of an in ice-cold 96-well plate in triplicate. Then, 50 μl of 0.2 mM sodium citrate (substrate buffer, provided with kit) was added to the assay buffer in each well together with 50 μl of enzyme (isocitrate dehydrogenase provided with the kit) and 50 μl of NADP (freshly reconstituted according to manufacturer's instructions). All samples were then incubated at 37°C for 10 min, and absorbance changes at 340 nm over a period of 5 min at 37°C were then recorded. Final calculations of mitochondrial aconitase activity were performed according to manufacturer's instructions, and activity was expressed in milliunits.
TCA flux assessment
TCA cycle flux was calculated from the time course of 13C mass isotopomers of glutamate (mass isotopomers M+1 and M+2 of glutamate) during an infusion of 2-13C acetate. Plasma acetate concentration and 13C enrichment of glutamate in the gastrocnemius muscle were determined by GC/MS, as described previously (30, 31).
A 1-compartment dynamic metabolic model was used to fit 13C time courses of mass isotopomers of glutamate, to determine gastrocnemius metabolic flux values. In this model, the infused labeled glucose, or the unlabeled plasma glucose, is transported from the extracellular medium to muscle cells, assuming reversible nonsteady-state Michaelis-Menten transport kinetics through the GLUT4 glucose transporter. Labeled acetate molecules are transported from the plasma to cells and consequently to mitochondria, obeying Michaelis-Menten kinetics through the monocarboxylate transporter. This metabolic network includes glycolysis, the TCA cycle, α-ketoglutarate–glutamate and oxaloacetate-aspartate exchange, pyruvate carboxylase activity, anaplerosis at the succinyl-CoA level, pyruvate recycling through malic enzyme, and acetyl-CoA synthetase activity. Once acetyl-CoA is formed, either through pyruvate dehydrogenase complex or acetyl-CoA synthetase, it is used by the TCA cycle to produce energy and electron carriers.
This 1-compartment dynamic metabolic model is mathematically expressed using 2 types of mass balance equations: mass balance for total metabolite concentration and 13C mass isotopomer mass balance for labeled metabolites and their fragments, based on assumed bionetwork and atom distribution matrices (fragmented mass isotopomer framework).
Mass isotopomer balance equations were derived for bonded cumulative isotopomers, e.g., glutamate, glutamine, and aspartate, as described by Shestov et al. (32), to produce a set of 75 nonlinear mass isotopomer ordinary differential equations. The use of fragmented mass isotopomers, bonded cumulative isotopomers (32), and bonded cumomers produces fewer and simpler equations, vs. a model that includes all possible isotopomers, while retaining all mass spectrometry-measurable mass isotopomer data. For ordinary differential equations, the model describes the rates of loss and creation of particular labeled and unlabeled metabolite forms (mass isotopomers) after infusion of a labeled substrate. These Equations 1, derived from the flux balance of metabolites and for parallel unimolecular reactions, take the form:
where metabolite M is downstream of another metabolite Sj. The total outflux ∑k Fk balances total influx ∑ Fj. [M] represents the total pool size of metabolite M, while μ(i) and σj(i) represent the I mass isotopomer fraction of metabolite M (M+I mass isotopomer) and metabolite Sj (S+I mass isotopomer), respectively. I is the number of labeled C atoms in molecule changes between 0 and N, where N is the total number of C atoms in the metabolites.
For labeled [2-13C]-acetate infusion, the fitted time courses were Glu M + 1 and Glu M + 2, with total 2 curves. Two fluxes were determined: gastrocnemius TCA cycle, FTCA, and exchange between glutamate and 2-oxoglutarate, FX. Solving a system of nonlinear differential equations for whole/fragmented mass isotopomers with the Runge-Kutta fourth-order procedure for stiff systems, yields time courses for all possible 13C mass isotopomers, e.g., glutamate, glutamine, and aspartate. The cost function is used to quantify differences between measurements and computational results for labeled dynamic data. Minimization was performed with BFGS or Simplex algorithms. Proper mean-square convergence was confirmed by verifying that goodness-of-fit values were close to expected theoretical values. Data errors for the Monte Carlo simulations were determined using experimental noise levels. All numerical procedures were carried out in MatLab (MathWorks, Natick, MA, USA).
RESULTS
The concentrations of high-energy-related phosphates calculated from the relative peak areas in NMR spectra and the intramyocellular pH for all groups are reported in Table 1. Mean ATP concentration was significantly lower in B group mice than in C group mice, by ∼50% (Tables 1 and 2). Although both the PCr concentration and the PCr/ATP concentration ratio differed between groups across several group comparisons (Tables 1 and 2), the Pi/PCr concentration ratio and the intramyocellular pH were similar across all groups of mice.
Table 1.
Concentrations of high-energy phosphates and pH in skeletal muscle of mice by group
| Metabolite or variable | Concentration (%) |
||||
|---|---|---|---|---|---|
| C, n = 6 | C+P, n = 8 | B, n = 8 | B+Pa, n = 8 | B+Pb, n = 8 | |
| Pi | 4.61 ± 0.71 | 4.90 ± 0.72 | 3.11 ± 0.60 | 3.20 ± 0.59 | 3.97 ± 0.65 |
| PCr | 60.75 ± 2.32 | 82.45 ± 10.68 | 27.98 ± 1.63 | 39.55 ± 3.02 | 36.88 ± 3.85 |
| βATP | 18.50 ± 1.51 | 19.35 ± 2.02 | 6.02 ± 0.53 | 8.46 ± 0.97 | 7.71 ± 0.81 |
| αATP | 23.80 ± 2.60 | 26.46 ± 2.65 | 7.39 ± 0.59 | 12.56 ± 1.03 | 10.43 ± 1.46 |
| γATP | 20.36 ± 2.18 | 23.05 ± 2.66 | 7.51 ± 0.58 | 11.22 ± 1.15 | 9.7 ± 1.04 |
| Mean ATP | 20.88 ± 2.08 | 22.96 ± 2.42 | 6.98 ± 0.55 | 10.74 ± 0.92 | 9.3 ± 0.96 |
| PCr/ATP | 2.97 ± 0.23 | 3.56 ± 0.10 | 4.05 ± 0.15 | 3.71 ± 0.10 | 4.01 ± 0.22 |
| Pi/PCr | 0.075 ± 0.010 | 0.06 ± 0.01 | 0.12 ± 0.029 | 0.08 ± 0.014 | 0.12 ± 0.026 |
| pH | 7.22 ± 0.02 | 7.37 ± 0.15 | 7.13 ± 0.07 | 7.24 ± 0.01 | 7.27 ± 0.27 |
Concentrations are calculated from relative peak areas (fraction of total integral) in NMR spectra. NMR spectral values are indicated as mean ± se percentage of the total integral. B+P
B+P immediately after burn; B+P
B+P before and immediately after burn. Mean ATP represents mean of 3 peaks. pH is calculated as 6.75 + log [(s − 3.27)/(5.69 − s)], where s is the chemical shift difference (in ppm) between the Pi and the PCr peaks (29).
Table 2.
Post hoc multiple group comparisons of high-energy phosphate concentrations and pH in mouse skeletal muscle
| Metabolite or variable | Difference in concentraton [% (P)] |
||||||
|---|---|---|---|---|---|---|---|
| C vs. B | C vs. C+P | C vs. B+Pa | C vs. B+Pb | B vs. B+Pa | B vs. B+Pb | B+Pa vs. B+Pb | |
| Pi | −32.54 | 6.29 | −30.58 | −13.88 | 2.89 | 27.65 | 24.06 |
| (0.15) | (0.78) | (0.181) | (0.78) | (0.91) | (0.0.24) | (0.18) | |
| PCr | −53.94 | 35.72 | −33.66 | −39.29 | 41.35 | 31.81 | −6.75 |
| (0.000002) | (0.12) | (0.001) | (0.54) | (0.01) | (0.03) | (0.59) | |
| βATP | −67.46 | 4.59 | −54.27 | −58.32 | 40.53 | 28.07 | −8.87 |
| (0.00002) | (0.75) | (0.0002) | (0.0018) | (0.06) | (0.04) | (0.58) | |
| αATP | −68.94 | 11.18 | −47.23 | −56.17 | 69.96 | 41.12 | −16.96 |
| (0.00007) | (0.50) | (0.0006) | (0.78) | (0.002) | (0.03) | (0.19) | |
| γATP | −63.11 | 13.21 | −44.89 | −52.35 | 49.40 | 29.16 | −13.54 |
| (0.0001) | (0.47) | (0.002) | (0.0004) | (0.02) | (0.07) | (0.36) | |
| Mean ATP | −66.59 | 9.96 | −48.55 | −55.46 | 53.87 | 33.24 | −13.41 |
| (0.00005) | (0.55) | (0.0004) | (0.0011) | (0.007) | (0.03) | (0.30) | |
| PCr/ATP | 36.36 | 19.86 | 24.91 | 35.01 | 8.39 | −0.98 | 8.08 |
| (0.0032) | (0.03) | (0.06) | (0.0003) | (0.07) | (0.96) | (0.21) | |
| Pi/PCr | 60.0 | −20.0 | 6.66 | 60.0 | −33.3 | 0.0 | 50.0 |
| (0.286) | (0.2108) | (0.80) | (0.014) | (0.23) | (0.90) | (0.22) | |
| pH | 1.24 | 2.07 | 0.27 | 0.69 | 1.54 | 1.96 | 0.41 |
| (0.315) | (0.39) | (0.943) | (0.81) | (0.46) | (0.78) | (0.86) | |
Values represent difference from first to second group in each comparison pair. P values were calculated using ANOVA with the Bonferroni correction to account for multiple comparisons. B+P
B+P immediately after burn; B+P
B+P before and immediately after burn.
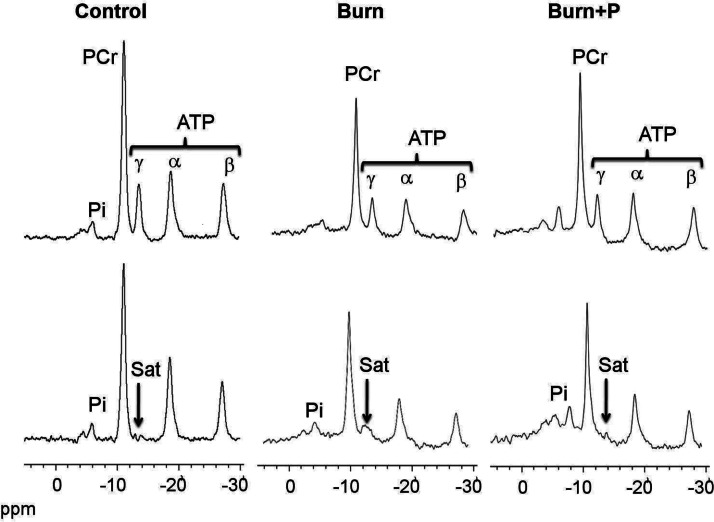
Representative spectra from 31P NMR saturation-transfer experiments conducted to determine the rate of mitochondrial ATP synthesis before and after saturation of the γ-ATP resonance are shown in Fig. 1 (top and bottom panels, respectively). On irradiation of the γATP resonance, the signal intensities of PCr, Pi, αATP, and βATP resonances all decreased, either by magnetization transfer or direct off-resonance saturation. The B group showed a decrease in PCr peak and ATP resonances compared to the C group. The increased PCr and ATP peaks in SS-31-treated groups may be due to partial recovery of ATP synthesis rate.
Figure 1.
NMR spectra from 31P NMR saturation-transfer on the hindlimb skeletal muscle of live mice. Representative summed 31P NMR spectra acquired from control, burned (burn), and burned after SS-31 peptide injection (burn+P) mice before (top spectra) and after (bottom spectra) saturation of the γ-ATP resonance. Arrow indicates the position of saturation (Sat) by RF irradiation (−13.2 ppm, γ-ATP). ppm, chemical shift in parts per million.
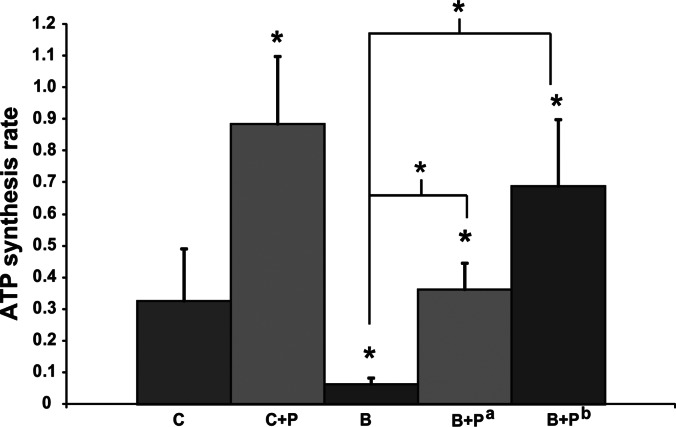
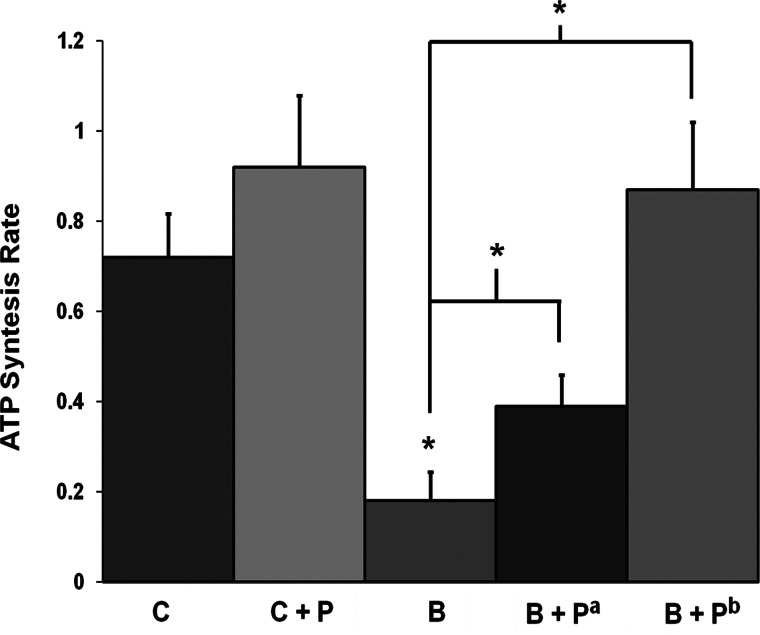
The group mean ATP synthesis rate results, estimated from the 31P NMR spectra from all four groups, are presented in Table 3; ATP synthesis rate calculations were made based on both the NMR data (the flux constant κf) and from a biochemical assay (the ATP concentration measurement used to estimate [Pi]). Both κf and [Pi] were decreased in the burned mice. Relative to the C group (percentage change in ΔM/M0, Table 4), the NMR-measured fractional change ΔM/M0 was decreased by 7.9% in the B group, increased by 39.9% in the C+P group, increased by 56.7% in the B+Pa group (1 dose of SS-31 immediately after burn), and increased by 24% in the B+Pb group (1 dose of SS-31 before and 1 dose immediately after burn). The final calculated rates for oxidative and anaerobic ATP synthesis reactions are shown in Figs. 2 and 3.
Table 3.
Results of in vivo 31P-NMR saturation transfer experiments performed on mouse skeletal muscle
| Variable | Group |
||||
|---|---|---|---|---|---|
| C | B | C+P | B+Pa | B+Pb | |
| n | 5 | 8 | 5 | 8 | 8 |
| Oxidative reaction: Pi → gATP | |||||
| ΔM/M0 | 0.24 ± 0.05 | 0.23 ± 0.05 | 0.15 ± 0.02 | 0.39 ± 0.07 | 0.31 ± 0.06 |
| T1obs (s) | 1.16 ± 0.1 | 1.33 ± 0.27 | 1.16 ± 0.4 | 1.33 ± 0.27 | 1.33 ± 0.27 |
| κf (s−1) | 0.21 ± 0.04 | 0.17 ± 0.04 | 0.14 ± 0.02 | 0.29 ± 0.05 | 0.24 ± 0.05 |
| ATP (μmol/g) | 1.21 ± 1.156 | 1.1 ± 0.0473 | 1.405 ± 0.346 | 1.18 ± 0.131 | 1.23 ± 0.158 |
| Pi (μmol/g) | 1.01 ± 0.28 | 1.41 ± .26 | 5.49 ± 0.28 | 1.41 ± 0.26 | 2.93 ± 0.56 |
| ATP synthesis rate (μmol/g/s) | 0.25 ± 0.09 | 0.06 ± 0.06 | 0.74 ± 0.09 | 0.36 ± 0.08 | 0.63 ± 0.11 |
| Anerobic reaction: PCr → gATP | |||||
| ΔM/M0 | 0.36 ± 0.018 | 0.19 ± 0.04 | 0.29 ± 0.04 | 0.24 ± 0.04 | 0.37 ± 0.04 |
| T1obs (s) | 1.16 ± 0.10 | 1.33 ± 0.27 | 1.16 ± 0.4 | 1.33 ± 0.27 | 1.33 ± 0.27 |
| κf (s−1) | 0.31 ± 0.016 | 0.08 ± 0.03 | 0.26 ± 0.04 | 0.18 ± 0.03 | 0.27 ± 0.04 |
| ATP (μmol/g) | 1.21 ± 0.156 | 1.1 ± 0.0473 | 1.405 ± 0.346 | 1.18 ± 0.131 | 1.23 ± 0.158 |
| PCr (μmol/g) | 2.28 ± 0.23 | 1.16 ± 0.232 | 3.76 ± 0.51 | 2.04 ± 0.09 | 3.25 ± 0.29 |
| ATP synthesis rate (μmol/g/s) | 0.72 ± 0.097 | 0.18 ± 0.065 | 0.92 ± 0.16 | 0.60 ± 0.11 | 0.87 ± 0.15 |
Values are means ± se; ΔM/M0 is the fractional change in Pi magnetization as a result of saturation transfer; T1obs is the observed spin lattice relaxation time of Pi during γATP saturation; κf is the rate constant for the reaction Pi → γATP, calculated as (1/T1obs) × (ΔM/M0). ATP synthesis is calculated as [Pi] × κf. B+P
B+P immediately after burn; B+P
B+P before and immediately after burn.
Table 4.
Post hocmultiple group comparisons of ATP synthesis variables in mouse skeletal muscle
| Variable | Difference in concentraton [% (P)] |
||||||
|---|---|---|---|---|---|---|---|
| C vs. B | C vs. C+P | C vs. B+Pa | C vs. B+Pb | B vs. B+Pa | B vs. B+Pb | B+Pa vs. B+Pb | |
| Oxidative reaction: Pi → gATP | |||||||
| ΔΜ/Μ0 | −4.16 | −37.5 | 62.5 | 29.1 | 69.5 | 34.7 | −20.5 |
| (0.902) | (0.09) | (0.072) | (0.27) | (0.06) | (0.18) | (0.18) | |
| κf (s−1) | −19.04 | −33.3 | 38.09 | 14.2 | 70.5 | 41.17 | −17.24 |
| (0.605) | (0.096) | (0.15) | (0.771) | (0.063) | (0.11) | (0.22) | |
| Pi (μmol/g) | 39.6 | 443.5 | 39.6 | 190.09 | 0.0 | 107.8 | 107.8 |
| (0.006) | (0.0008) | (0.166) | (0.035) | (0.008) | (0.001) | (0.015) | |
| ATP synthesis (μmol/g/s) | −76.0 | 196 | 44.0 | 152.0 | 500.0 | 950 | 75 |
| (0.026) | (0.008) | (0.21) | (0.046) | (0.002) | (0.00006) | (0.040) | |
| Anerobic reaction: PCr → gATP | |||||||
| ΔΜ/Μ0 | −47.2 | −19.4 | −33.3 | 2.77 | 26.3 | 94.7 | 54.1 |
| (0.004) | (0.08) | (0.11) | (0.24) | (0.08) | (0.04) | (0.21) | |
| κf (s−1) | −74.1 | −16.1 | −41.9 | −12.9 | 125.0 | 237.5 | 50.0 |
| (0.0009) | (0.082) | (0.17) | (0.38) | (0.01) | (0.03) | (0.21) | |
| PCr (μmol/g) | −49.1 | 64.9 | −10.5 | 42.5 | 75.8 | 180.1 | 59.3 |
| (0.008) | (0.02) | (0.008) | (0.08) | (0.002) | (0.00004) | (0.001) | |
| ATP synthesis (μmol/g/s) | −75.0 | 27.7 | −16.7 | 20.8 | 233 | 383 | 45.0 |
| (0.004) | (0.48) | (0.28) | (0.11) | (0.02) | (0.0001) | (0.14) | |
Values represent difference from first to second group in each comparison pair. P values were calculated using ANOVA with the Bonferroni correction to account for multiple comparisons. B+P
B+P immediately after burn; B+P
B+P before and immediately after burn.
Figure 2.
Oxidative ATP synthesis rate (μmol/g/s) from Pi concentration in C, C+P, B, and B+P groups (B+Pa, 1 dose immediately after burn; B+Pb, 1 dose before and 1 dose immediately after burn) by 31P NMR 6 h after burn. *P < 0.05.
Figure 3.
Anaerobic ATP synthesis rate (μmol/g/s) from PCr concentration in C, C+P, B, and B+P groups (B+Pa, 1 dose immediately after burn; B+Pb, 1 dose before and 1 dose immediately after burn) by 31P NMR 6 h after burn. *P < 0.05.
Our ATP synthesis rate comparisons (percentage difference and P values) are reported in detail in Table 4. Briefly, we found that the oxidative ATP synthesis rate (Pi → γATP) at 6 h postburn was markedly lower in burned mice than in control mice (P=0.026; Table 4 and Fig. 2); SS-31 injection resulted in an increased oxidative ATP synthesis rate in control mice (P=0.008; Table 4 and Fig. 2). Notably, the oxidative ATP synthesis rate was increased in burned mice injected with SS-31 compared to untreated burned mice (P=0.002 for B vs. B+Pa and P=0.00006 for B vs. B+Pb; Table 4 and Fig. 2). A single injection of SS-31 in burned mice normalized the oxidative ATP synthesis rate, while 1 dose of SS-31 before and 1 dose immediately postburn increased the oxidative ATP synthesis rate (P=0.046, C vs. B+Pb; Table 4 and Fig. 2). Moreover, SS-31 increased the anaerobic ATP synthesis rate (reaction PCr → γATP) in burned mice (P=0.002 for B vs. B+Pa and P=0.0001 for B vs. B+Pb; Table 4 and Fig. 3). In addition, the oxidative ATP synthesis rate was not significantly different for mice at 7 d postburn.
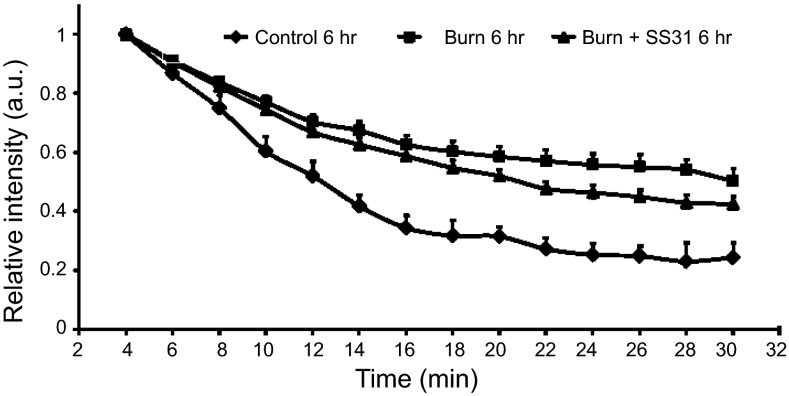
Meanwhile, the EPR results revealed a significant decrease at 6 h in the redox status (i.e., nitroxide decay rate) of the B (0.00043±0.00004 s−1) and B+P (0.00058±0.00003 s−1) groups compared to that of the C group (0.0011±0.0002 s−1; P<0.05; see Supplemental Table S1). Also, a significant increase (recovery) in the redox status of the burn + SS-31 group compared to burn group was observed (P<0.05; Fig. 4).
Figure 4.
Decay kinetics of nitroxide (CPA) in the gastrocnemius muscle of control (n=6), burn (n=6), and burn + SS-31 (n=9) mice. Redox status was measured 6 h after burn. Values are expressed as means ± se. Burn and burn + SS-31 groups had a significantly lower redox status than the control group (P<0.05). Burn + SS-31 group had an increased redox status compared to burn group, indicative of recovery (P<0.05).
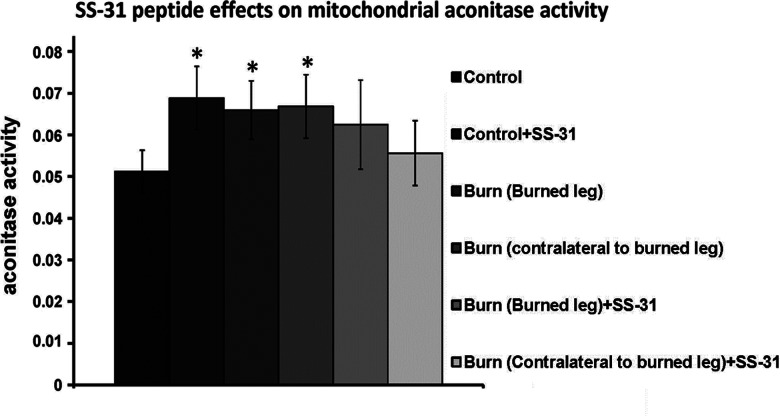
The effects of SS-31 on mitochondrial aconitase activity are summarized in Fig. 5. SS-31 increased mitochondrial aconitase activity in control animals. In the absence of SS-31, we observed elevated mitochondrial aconitase activity, relative to untreated control animals, in both burned muscle (local burn effect) and contralateral to the burned leg (systemic burn effect) in B group animals. Meanwhile, the mean mitochondrial aconitase activity levels in burned muscle and contralateral muscle of B+P group animals did not differ significantly from the mean level observed in muscle specimens from mice in the C group.
Figure 5.
SS-31 affected mitochondrial aconitase activity. Mitochondrial aconitase activity is shown for the following specimen groups: control, control + SS31, burned leg, contralateral to burned leg, burned leg + SS31, and contralateral to burned leg + SS31. *P < 0.05 vs. control.
GC-MS analysis showed an 82.4% increase (P<0.003) for the TCA flux in control limbs (0.017±0.004; n=18), vs. burned limbs (0.031±0.005; n=18), and SS-31 injection normalized this futile TCA flux. The ATP synthesis rate was 0.25 ± 0.09 and 0.06 ± 0.06 for the control and burned mice, respectively (Table 3). The ratio of this rate to TCA flux, an index of mitochondrial coupling, was 14.7 and 1.94 for the control and burn mice, respectively. As such, burn produced an 87% decrease (P<0.03) in the mitochondrial coupling index. SS-31 injection restored this index, as the difference between the SS-31-injected vs. control mice was insignificant.
DISCUSSION
The results of this study provide proof of the concept that SS-31 administration increases ATP synthesis rate in murine skeletal muscle. Although the initial demonstration was accomplished by injecting mice both before and immediately after burn, this increase in ATP synthesis rate remained even if only a single injection of SS-31 was administered immediately after burn, a period of critical importance for clinical intervention. We also observed that SS-31 lowered mitochondrial aconitase activity levels in burned animals to levels that were not significantly different from control levels, indicating that SS-31 lowers TCA cycle flux, which is elevated in burn victims due to a futile TCA cycle, in agreement with the TCA flux results. This is consistent with the data from an independent recent report by Yo et al. (33), demonstrating that SS31 attenuates burn injury-induced hypermetabolism.
Given that the ratio of ATP synthesis rate to the TCA cycle flux is an index of mitochondrial coupling (34), our results suggest that SS-31 administration increases mitochondrial coupling, which is suppressed following burn injury. Furthermore, our complementary EPR results showed that SS-31 facilitates recovery of the diminished mitochondrial redox status that is observed after burn injury. This positive effect of SS-31 on redox status can be attributed to SS-31's ability to act as a ROS scavenger, while also decreasing ROS production (15, 16). These findings suggest that SS-31 administration can be used as a mitochondrial protective agent to alleviate the symptoms of severe burn trauma.
The present findings provide confirmation that burn injury slows ATP synthesis, without altering Pi/PCr concentration ratio or pH, suggesting that there is a significant reduction in the rate of mitochondrial phosphorylation postburn (4, 10). Notably, the in vivo 31P NMR saturation-transfer technique used here (24, 25) enabled us to measure fast enzyme reaction exchange rates noninvasively (35) and thus provided an index of the net skeletal muscle rate of oxidative ATP synthesis catalyzed by mitochondrial ATPase, which by definition is proportional to oxygen consumption rate by the P/O ratio (the ratio of the net rate of ATP synthesis by oxidative phosphorylation to the rate of oxygen consumption; refs. 26, 27). Meanwhile, unidirectional ATP synthesis flux, as measured by NMR, is thought to primarily reflect flux through F1-F0–ATP synthase, with negligible coupled glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase reactions (36). Although the net glycolytic contribution via glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase to ATP production is small, compared with that via oxidative phosphorylation, these enzymes occur at near equilibrium, and thus unidirectional production of ATP can be high. Because burn injury down-regulates glycolytic enzyme gene expression (4), the contribution of glycolytic reactions to the unidirectional ATP synthesis flux postburn is likely to be negligible. We conducted direct measurements of tissue redox status by EPR to complement NMR measurements in the assessment of tissue dysfunction and in the evaluation of therapeutic effectiveness of mitochondrial protective agents in severe burn trauma.
The novel principal finding of our experiments is that animals treated with a single injection of SS-31 postburn showed an improved ATP synthesis rate relative to untreated burned animals. That the same ATP synthesis rate was seen at 7 d suggests that SS-31 protection persists over time. While repeated injections of SS-31 might further improve healing and recovery from burn injury, these experiments are beyond the scope of the present study. Our results extend previous work demonstrating that SS-31 treatment reduces the rate of hydrogen peroxide release from mitochondria (14, 17). In light of the recent demonstration that reduced oxidative phosphorylation coupling precedes electron transport chain defects due to mild oxidative stress in mice (37), we suggest that SS-31 may yield improvement in oxidative phosphorylation via direct effects. Interestingly, SS-31 also significantly increased ATP synthesis rate in the control mice.
Another major finding of this study is that mitochondrial aconitase activity was elevated both ipsilateral (local burn effect) and contralateral to the burned leg (systemic burn effect). We believe this is most probably the result of generalized hypermetabolism induced by the burn injury, despite the expectation for decreased mitochondrial aconitase activity due to increased ROS production. Thus, the increased ROS production that occurs postburn could inhibit mitochondrial aconitase activity and may not overcome the induced hypermetabolism in terms of mitochondrial aconitase activity and thus TCA flux. Similar observations were made following exercise (repeated contractions) in human subjects and in isolated mouse skeletal muscle, when ROS levels are also high (36). Given that burn injury slowed net oxidative ATP synthesis, we can deduce that the TCA cycle was futile in the postburn hypermetabolic state of our mice. Furthermore, reduced ATP synthesis and elevated TCA flux (inferred from increased aconitase activity) points to decreased mitochondrial coupling index (ATP synthesis:TCA cycle flux) after burn injury (25). Also, because SS-31 can reduce H2O2 production, SS-31 treatment may dampen fundamental changes in the governance of electron leakage from the electron transport system (i.e., increased membrane potential of the electron transport system) while increasing regulation of the redox state within myofibers (17). Our finding here that SS-31 decreased mitochondrial aconitase activity in burned animals to control levels indicates that SS-31 can promote recovery of TCA flux, possibly in response to more effective aerobic respiration, as suggested by the increased ATP synthesis rate observed in treated burned animals compared with that in untreated burned animals.
Finally, our complementary EPR results showed that SS-31 facilitates the recovery of mitochondrial redox status—a measure of oxidative stress that is increased by burn injury. This effect can be attributed to SS-31 acting as a ROS scavenger while decreasing ROS production (13, 14). Notably, our mitochondrial redox status and aconitase results are in agreement. Aconitase activity is redox dependent (38) and inhibited by ROS scavengers (including SS-31); thus, SS-31 may reduce oxidative stress inasmuch as aconitase activity is an index of oxidative stress (39–42). Recovery of mitochondrial redox status could also occur via regulation of the expression of mitochondrial uncoupling protein 3 and its upstream key metabolic regulator peroxisome proliferator activated receptor-γ coactivator-1β, both of which have been shown to be dysregulated as early as 6 h after burn (1, 4, 10, 12). Indeed, this mechanism may underlie, at least in part, the restoration of mitochondrial redox status that we observed in our EPR experiments.
While SS-31 is a proven antioxidant and lowers ROS, it remains unknown whether it induces mitochondrial SOD and catalase. Glutathione S-transferase a 4 (hGSTA4) is down-regulated in muscle tissue of thermally injured patients (43), and whole blood glutathione concentrations and absolute synthesis rates in the basal state are lower in burn vs. control subjects (44). Also, SS-31 prevents glutathione depletion in ischemic brain injury (45). As such, we assume these same effects occur in burn injury. Future experiments, which are beyond the scope of this study, are needed to substantiate this assumption.
Overall, this study demonstrates the utility of using EPR to obtain direct measurements of tissue parameters (i.e., redox status) to complement NMR measurements in studies examining tissue dysfunction and the therapeutic effectiveness of mitochondrial protective agents following burn trauma. Our multimodality NMR/EPR approach advances the development of novel nondestructive therapeutic approaches in murine models of mitochondrial pathologies, provides biomarkers for investigation of mitochondrial paradigms, and thus contributes to the development of novel therapeutic agents.
In summary, the present results demonstrate that treatment with SS-31 promotes recovery of mitochondrial function after burn injury by increasing ATP synthesis rate, improving mitochondrial redox status, and restoring mitochondrial coupling. Our in vivo NMR findings, which were cross-validated and supplemented by EPR measurements and mitochondrial aconitase activity measurements, suggest that SS-31 may be an effective mitochondrial protective agent for alleviation of symptoms of severe burn trauma.
Supplementary Material
Acknowledgments
This work was supported in part by a U.S. National Institute Institutes of Health (NIH) center grant (P50GM021700) to R.G.T. (A.A.T., director of the NMR core), Shriner's Hospital for Children research grants to A.A.T. (8893) and L.G.R. (8892), and a research support grant (DM103014) from the U.S. Defense Medical Research and Development Program (DMRDP).
The authors thank Dr. Alan J. Fischman for his advice. The authors also thank Dr. Ann Power Smith (Write Science Right, Las Vegas, NV, USA) for editorial assistance. Disclosure: the SS peptides technology has been licensed for commercial development to Stealth Peptides Inc. (Newton Centre, MA, USA) by the Cornell Research Foundation (CRF), and both CRF and H.H.S. have financial interests.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 31P NMR
- phosphorus-31 nuclear magnetic resonance
- κf
- flux constant
- ATP
- adenosine triphosphate
- B group
- burn group
- B+P group
- burn + Szeto-Schiller 31 peptide group
- C group
- control group
- CPA
- 3-carbamoyl-2,2,5,5-tetramethylpyrrolidin-1-yloxyl nitroxide
- C+P group
- control + Szeto-Schiller 31 peptide group
- EPR
- electron paramagnetic resonance
- i.p.
- intraperitoneal
- PCr
- phosphocreatine
- Pi
- inorganic phosphate
- ROS
- reactive oxygen species
- SS-31
- Szeto-Schiller 31
- T1
- relaxation time
- T1obs
- observed relaxation time
- TCA
- tricarboxylic acid
REFERENCES
- 1. Yu Y. M., Tompkins R. G., Ryan C. M., Young V. R. (1999) The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J. Parenter. Enteral. Nutr. 23, 160–168 [DOI] [PubMed] [Google Scholar]
- 2. Sheridan R. L., Tompkins R. G. (2004) What's new in burns and metabolism. J. Am. Coll. Surg. 198, 243–263 [DOI] [PubMed] [Google Scholar]
- 3. Barret J. P., Herndon D. N. (2003) Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch. Surg. 138, 127–132 [DOI] [PubMed] [Google Scholar]
- 4. Padfield K. E., Astrakas L. G., Zhang Q., Gopalan S., Dai G., Mindrinos M. N., Tompkins R. G., Rahme L. G., Tzika A. A. (2005) Burn injury causes mitochondrial dysfunction in skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 102, 5368–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan N., Mupparaju S. P., Mintzopoulos D., Kesarwani M., Righi V., Rahme L. G., Swartz H. M., Tzika A. A. (2008) Burn trauma in skeletal muscle results in oxidative stress as assessed by in vivo electron paramagnetic resonance. Mol. Med. Rep. 1, 813–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Astrakas L. G., Goljer I., Yasuhara S., Padfield K. E., Zhang Q., Gopalan S., Mindrinos M. N., Dai G., Yu Y. M., Martyn J. A., Tompkins R. G., Rahme L. G., Tzika A. A. (2005) Proton NMR spectroscopy shows lipids accumulate in skeletal muscle in response to burn trauma-induced apoptosis. FASEB J. 19, 1431–1440 [DOI] [PubMed] [Google Scholar]
- 7. Padfield K. E., Zhang Q., Gopalan S., Tzika A. A., Mindrinos M. N., Tompkins R. G., Rahme L. G. (2006) Local and distant burn injury alter immuno-inflammatory gene expression in skeletal muscle. J. Trauma. 61, 280–292 [DOI] [PubMed] [Google Scholar]
- 8. Zhang Q., Cao H., Astrakas L. G., Mintzopoulos D., Mindrinos M. N., Schulz J. r., Tompkins R. G., Rahme L. G., Tzika A. A. (2006) Uncoupling protein 3 expression and intramyocellular lipid accumulation by NMR following local burn trauma. Int. J. Mol. Med. 18, 1223–1229 [PubMed] [Google Scholar]
- 9. Tzika A. A., Astrakas L. G., Cao H., Mintzopoulos D., Zhang Q., Padfield K., Yu H., Mindrinos M. N., Rahme L. G., Tompkins R. G. (2008) Murine intramyocellular lipids quantified by NMR act as metabolic biomarkers in burn trauma. Int. J. Mol. Med. 21, 825–832 [PubMed] [Google Scholar]
- 10. Tzika A. A., Mintzopoulos D., Padfield K., Wilhelmy J., Mindrinos M. N., Yu H., Cao H., Zhang Q., Astrakas L. G., Zhang J., Yu Y. M., Rahme L. G., Tompkins R. G. (2008) Reduced rate of adenosine triphosphate synthesis by in vivo 31P nuclear magnetic resonance spectroscopy and downregulation of PGC-1beta in distal skeletal muscle following burn. Int. J. Mol. Med. 21, 201–208 [PubMed] [Google Scholar]
- 11. Righi V., Andronesi O., Mintzopoulos D., Tzika A. A. (2009) Molecular characterization and quantification using state of the art solid-state adiabatic TOBSY NMR in burn trauma. Int. J. Mol. Med. 24, 749–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tzika A. A., Mintzopoulos D., Mindrinos M., Zhang J., Rahme L. G., Tompkins R. G. (2009) Microarray analysis suggests that burn injury results in mitochondrial dysfunction in human skeletal muscle. Int. J. Mol. Med. 24, 387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Min K., Smuder A. J., Kwon O. S., Kavazis A. N., Szeto H. H., Powers S. K. (2011) Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J. Appl. Physiol. 111, 1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Powers S. K., Hudson M. B., Nelson W. B., Talbert E. E., Min K., Szeto H. H., Kavazis A. N., Smuder A. J. (2011) Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit. Care. Med. 39, 1749–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao K., Zhao G. M., Wu D., Soong Y., Birk A. V., Schiller P. W., Szeto H. H. (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J. Biol. Chem. 279, 34682–34690 [DOI] [PubMed] [Google Scholar]
- 16. Zhao K., Luo G., Giannelli S., Szeto H. H. (2005) Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem. Pharmacol. 70, 1796–1806 [DOI] [PubMed] [Google Scholar]
- 17. Anderson E. J., Lustig M. E., Boyle K. E., Woodlief T. L., Kane D. A., Lin C. T., Price J. W., 3rd, Kang L., Rabinovitch P. S., Szeto H. H., Houmard J. A., Cortright R. N., Wasserman D. H., Neufer P. D. (2009) Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 119, 573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dai D. F., Chen T., Szeto H., Nieves-Cintron M., Kutyavin V., Santana L. F., Rabinovitch P. S. (2011) Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J. Am. Coll. Cardiol. 58, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee H. Y., Kaneki M., Andreas J., Tompkins R. G., Martyn J. A. (2011) Novel mitochondria-targeted antioxidant peptide ameliorates burn-induced apoptosis and endoplasmic reticulum stress in the skeletal muscle of mice. Shock 36, 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carter E. A., Bonab A. A., Goverman J., Paul K., Yerxa J., Tompkins R. G., Fischman A. J. (2011) Evaluation of the antioxidant peptide SS31 for treatment of burn-induced insulin resistance. Int. J. Mol. Med. 28, 589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tomera J. F., Martyn J. (1988) Systemic effects of single hindlimb burn injury on skeletal muscle function and cyclic nucleotide levels in the murine model. Burns Incl. Therm. Inj. 14, 210–219 [DOI] [PubMed] [Google Scholar]
- 22. Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. (1980) Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature 283, 167–170 [DOI] [PubMed] [Google Scholar]
- 23. Hitzig B. M., Prichard J. W., Kantor H. L., Ellington W. R., Ingwall J. S., Burt C. T., Helman S. I., Koutcher J. (1987) NMR spectroscopy as an investigative technique in physiology. FASEB J. 1, 22–31 [DOI] [PubMed] [Google Scholar]
- 24. Brindle K. M., Blackledge M. J., Challiss R. A., Radda G. K. (1989) 31P NMR magnetization-transfer measurements of ATP turnover during steady-state isometric muscle contraction in the rat hind limb in vivo. Biochemistry 28, 4887–4893 [DOI] [PubMed] [Google Scholar]
- 25. Jucker B. M., Dufour S., Ren J., Cao X., Previs S. F., Underhill B., Cadman K. S., Shulman G. I. (2000) Assessment of mitochondrial energy coupling in vivo by 13C/31P NMR. Proc. Natl. Acad. Sci. U. S. A. 97, 6880–6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kingsley-Hickman P. B., Sako E. Y., Ugurbil K., From A. H., Foker J. E. (1990) 31P NMR measurement of mitochondrial uncoupling in isolated rat hearts. J. Biol. Chem. 265, 1545–1550 [PubMed] [Google Scholar]
- 27. Sako E. Y., Kingsley-Hickman P. B., From A. H., Foker J. E., Ugurbil K. (1988) ATP synthesis kinetics and mitochondrial function in the postischemic myocardium as studied by 31P NMR. J. Biol. Chem. 263, 10600–10607 [PubMed] [Google Scholar]
- 28. Forsen S., Hoffman R. (1963) Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J. Chem. Phys. 39, 2892–2901 [Google Scholar]
- 29. Bailey I. A., Williams S. R., Radda G. K., Gadian D. G. (1981) Activity of phosphorylase in total global ischaemia in the rat heart. A phosphorus-31 nuclear-magnetic-resonance study. Biochem. J. 196, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leimer K. R., Rice R. H., Gehrke C. W. (1977) Complete mass spectra of N-trifluoroacetyl-n-butyl esters of amino acids. J. Chromatogr. 141, 121–144 [DOI] [PubMed] [Google Scholar]
- 31. Cline G. W., Vidal-Puig A. J., Dufour S., Cadman K. S., Lowell B. B., Shulman G. I. (2001) In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. J. Biol. Chem. 276, 20240–20244 [DOI] [PubMed] [Google Scholar]
- 32. Shestov A. A., Valette J., Deelchand D. K., Ugurbil K., Henry P. G. (2012) Metabolic modeling of dynamic brain 13C NMR multiplet data: concepts and simulations with a two-compartment neuronal-glial model. Neurochem. Res. 37, 2388–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yo K., Yu Y. M., Zhao G., Bonab A. A., Aikawa N., Tompkins R. G., Fischman A. J. (2013) Brown adipose tissue and its modulation by a mitochondria-targeted peptide in rat burn injury-induced hypermetabolism. Am. J. Physiol. Endocrinol. Metab. 304, E331–E341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Befroy D. E., Falk Petersen K., Rothman D. L., Shulman G. I. (2009) Assessment of in vivo mitochondrial metabolism by magnetic resonance spectroscopy. Methods Enzymol. 457, 373–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alger J. R., Shulman R. G. (1984) NMR studies of enzymatic rates in vitro and in vivo by magnetization transfer. Q. Rev. Biophys. 17, 83–124 [DOI] [PubMed] [Google Scholar]
- 36. Zhang S. J., Sandstrom M. E., Lanner J. T., Thorell A., Westerblad H., Katz A. (2007) Activation of aconitase in mouse fast-twitch skeletal muscle during contraction-mediated oxidative stress. Am. J. Physiol. Cell Physiol. 293, 1154–1159 [DOI] [PubMed] [Google Scholar]
- 37. Siegel M. P., Kruse S. E., Knowels G., Salmon A., Beyer R., Xie H., Van Remmen H., Smith S. R., Marcinek D. J. (2011) Reduced coupling of oxidative phosphorylation in vivo precedes electron transport chain defects due to mild oxidative stress in mice. PLoS One 6, e26963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bulteau A. L., Ikeda-Saito M., Szweda L. I. (2003) Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry 42, 14846–14855 [DOI] [PubMed] [Google Scholar]
- 39. Vasquez-Vivar J., Kalyanaraman B., Kennedy M. C. (2000) Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. J. Biol. Chem. 275, 14064–14069 [DOI] [PubMed] [Google Scholar]
- 40. Verniquet F., Gaillard J., Neuburger M., Douce R. (1991) Rapid inactivation of plant aconitase by hydrogen peroxide. Biochem. J. 276, 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gardner P. R. (2002) Aconitase: sensitive target and measure of superoxide. Methods Enzymol. 349, 9–23 [DOI] [PubMed] [Google Scholar]
- 42. Beinert H., Kennedy M. C., Stout C. D. (1996) Aconitase as Ironminus sign sulfur protein, enzyme, and iron-regulatory protein. Chem. Rev. 96, 2335–2374 [DOI] [PubMed] [Google Scholar]
- 43. Apidianakis Y., Que Y. A., Xu W., Tegos G. P., Zimniak P., Hamblin M. R., Tompkins R. G., Xiao W., Rahme L. G. (2012) Down-regulation of glutatione S-transferase alpha 4 (hGSTA4) in the muscle of thermally injured patients is indicative of susceptibility to bacterial infection. FASEB J. 26, 730–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu Y. M., Ryan C. M., Fei Z. W., Lu X. M., Castillo L., Schultz J. T., Tompkins R. G., Young V. R. (2002) Plasma L-5-oxoproline kinetics and whole blood glutathione synthesis rates in severely burned adult humans. Am. J. Physiol. Endocrinol. Metab. 282, E247–E258 [DOI] [PubMed] [Google Scholar]
- 45. Cho S., Szeto H. H., Kim E., Kim H., Tolhurst A. T., Pinto J. T. (2007) A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J. Biol. Chem. 282, 4634–4642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.