Abstract
To identify human bone marrow stromal cell (BMSC) subsets with enhanced ability to engraft/contribute to the resident intestinal cellular pool, we transplanted clonally derived BMSCs into fetal sheep. Analysis at 75 d post-transplantation showed 2 of the 6 clones engrafting the intestine at 4- to 5-fold higher levels (5.03±0.089 and 5.04±0.15%, respectively) than the other clones (P<0.01), correlating with the percentage of donor-derived Musashi-1+ (12.01–14.17 vs. 1.2–3.8%; P<0.01) or leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5)+ cells within the intestinal stem cell (ISC) region. Phenotypic and transcriptome analysis determined that the clones with enhanced intestinal contribution expressed high levels of Ephrin type B receptor 2 (EphB2). Intestinal explants demonstrated proliferation of the engrafted cells and ability to generate crypt-like structures in vitro still expressing EphB2. Additional transplants based on BMSC EphB2 expression demonstrated that, at 7 d post-transplant, the EphB2high BMSCs engrafted in the ISC region at levels of 2.1 ± 0.2%, while control EphB2low BMSCs engrafted at 0.3 ± 0.1% (P<0.01). Therefore we identified a marker for isolating and culturing an expandable subpopulation of BMSCs with enhanced intestinal homing and contribution to the ISC region.—Colletti, E., El Shabrawy, D., Soland, M., Yamagami, T., Mokhtari, S., Osborne, C., Schlauch, K., Zanjani, E. D., Porada, C. D., Almeida-Porada, G. EphB2 isolates a human marrow stromal cell subpopulation with enhanced ability to contribute to the resident intestinal cellular pool.
Keywords: crypts of Lieberkühn, transcriptome, Musashi-1, epithelial, Lgr5, transplant, niche
The epithelial lining of the intestine has one of the highest cellular turnover rates in the body, with all the necessary cell types being fully renewed within a week and arising continuously from a stem cell pool that is tightly regulated by its niche (1, 2). These cells, located at the bottom of the intestinal crypts, give rise to transient amplifying cells that proliferate, differentiate, and migrate out of the stem cell compartment onto the villus surface, where they sit as postmitotic mature cells (3). In addition, the stem cell niche also contains pericytes, myofibroblasts, endothelial cells, and mesenchymal cells, each contributing to homeostasis of the intestinal crypt (4). On injury, or in response to neoplastic transformation, these cells can recruit replacements either from resident populations or from outside sources such as bone marrow (5–7). However, it has yet to be determined whether there are specific subsets of bone marrow-derived stromal cells more likely to be responsible for this process. Preservation of intestinal stem cells (ISCs) and activation of cell migration are regulated through Notch, bone morphogenic proteins (BMPs), and the Hedgehog and Wnt/β-catenin signaling pathways (8). β-Catenin ensures the correct positioning of epithelial cells along the crypt/villus axis by regulating the expression of members of the Ephrin and Ephrin receptor (Eph) families (9–11). Ephrins and Eph, both membrane-bound proteins, are differentially expressed in intestinal mucosa, with Eph localized in the intestinal crypt region, while ephrin proteins colonize the villi. The signaling between Ephrin and Eph is restricted to sites of direct cell-to-cell contact and is bidirectional in nature, since it is transmitted in both the receptor-expressing as well as in the ligand-expressing cells (12). In addition to the role of promoting cell proliferation of the intestinal epithelium, Ephrin type B receptors (EphBs) have been recognized to function as tumor suppressors by controlling cell migration and inhibiting tumoral invasive growth (13). Studies have also demonstrated that Ephrin/Eph signaling has a decisive role in tissue repair, acceleration of wound closure, and maintenance of homeostasis of the intestinal barrier in adults (14, 15). Studies have now shown that, similar to the endogenous regenerative process, bone marrow-derived stromal and mesenchymal cells are able to contribute, after transplantation, to the replacement of the radiation-depleted ISC compartment after abdominal and pelvic radiotherapy (16, 17). Other encouraging results also demonstrated that transplantation of these cells promotes healing in inflammatory bowel disease (IBD), drug-induced colitis (18, 19), and ulcer formation (7, 20). Therefore, cell-based therapies are thought to be of great promise for the reconstitution of damaged areas and as a means of increasing and promoting the regenerative process (20). While hematopoietic stem cells are able to migrate and contribute to the epithelial layer of the intestine (21), the promise of stromal and mesenchymal cells as cell therapy agents relies on the ability of these cells to migrate, on infusion, to areas of inflammation and injury, and to release soluble factors that promote healing through the modulation of immune activity, decreased inflammation, inhibition of apoptosis, and stimulation and support of resident stem and progenitor cells (22–24). Nevertheless, in order to develop successful cell therapies, it is necessary to find populations of cells that can home preferentially to the intestine and/or have the intrinsic ability to restore the integrity of the intestinal mucosa. Transplantation of human cells into xenogeneic fetal recipients, such as mice, rats, goats, pigs, sheep, monkeys, and baboons, have allowed the study of human stem cell biology, such as definition of phenotypes, homing molecules, and differentiative potential (25–32). During fetal life, the preimmune status of the fetus allows the engraftment of exogenous cells and the establishment of donor-specific tolerance (33–35). Furthermore, developmental cues arising from proliferating cells and microenvironmental niches induce the physiological homing and differentiation of donor cells, allowing the evaluation of their migratory patterns (36–39). Here we use a fetal sheep model to test the ability of clonally derived human bone marrow stromal cells (BMSCs) isolated based on Stro-1 positivity (40), to migrate and contribute to the intestinal mucosa. Using this model, we characterized several clonally derived BMSC populations and reported on the ability of these clones to engraft and to contribute to hematopoietic and hepatic sites (39). Here we further investigated the homing and differentiative potential of these clonally derived cells, addressing the question of whether any of these BMSC clones might be able to preferentially contribute to the cellular content of the intestinal mucosa.
MATERIALS AND METHODS
BMSC isolation and clonal expansion
Heparinized human bone marrow was obtained from healthy donors after informed consent according to guidelines from the Office of Human Research Protection at the University of Nevada. Clonal populations were obtained by single-cell deposition of Stro-1+ CD45−Gly-A− mononuclear cells into fibronectin-coated 96 well plates using a FACSVantage (BD Biosciences, San Jose, CA, USA) as described previously (39).
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD) labeling and transduction of human BMSCs
BMSCs were either labeled with DiD or transduced with a pEGFP-Lv105 vector (Capital Biosciences, Rockville, MD, USA) as described previously (41). In short, BMSCs were cultured in flasks to a density of 5 × 105 cells/cm2 in mesenchymal stem cell growth medium (MSCGM; Lonza Biologicals Inc. Hopkinton, MA, USA). To label cells, 25 μl of a stock solution of 1 mM DiD (Life Technologies, Grand Island, NY, USA) in DMSO (Sigma, St. Louis, MO, USA) was added to 2 ml of QBSF60 serum-free medium (Quality Biological, Gaithersburg, MD, USA), to give a final concentration of 12.5 μM. BMSC cultures were incubated in the DiD labeling solution for 20 min at 37°C; the labeling solution was removed, and cells were rinsed with medium and incubated for 10 min with warmed growth medium. Transduction with the pEGFP-Lv105 vector (1010 pfu/ml) was performed using subconfluent cultures of clonally derived BMSCs for 6 h in QBSF60 (Quality Biological) and 8 μg/ml protamine sulfate (Calbiochem, San Diego, CA, USA) at a MOI of 100. After transduction, cells were washed, and medium was changed to MSCGM. Efficiency of labeling or transduction (>95%) and viability of cells (>95%) were assessed prior to cell transplantation.
Flow cytometric analysis
BMSC cultures were analyzed as described previously (39) by labeling with various antibodies directly conjugated with fluorescein isothiocyanate or phycoerythrin according to the manufacturer's recommendations. In addition, unconjugated EphB2 antibody (Abcam, Cambridge, MA, USA) was added to single-cell suspensions of BMSCs and incubated at 4°C for 30 min. Cells were washed in phosphate-buffered saline (PBS) and stained for an additional 30 min with Alexa Fluor 488-conjugated secondary antibody (Life Technologies). After washing, flow cytometric analysis of the cells was performed on a FACScan (BD Biosciences) after setting the background fluorescence with an isotype control. Cells were sorted based on EphB2 fluorescence intensity using a FACSVantage (BD Biosciences).
In utero transplantation of sheep fetuses
All procedures were in accordance with the University of Nevada institutional animal care and use committee guidelines. Because the use of large animal models requires minimization of animal numbers without compromising reliability of results and statistical significance, in these studies we used a total of 13 animals; 7 animals were transplanted with EphB2−/low populations and 6 animals with EphB2high. Fetal sheep were injected intraperitoneally, by ultrasound-guided transabdominal percutaneous injection (39, 42), with different human BMSC populations at the concentrations indicated in Results, at 55–62 d of gestation. In short, after fetal visualization, a 22-gauge 15-cm echo-tip needle (Cook Medical, Bloomington, IN, USA) was inserted through the skin and the uterine wall into the amniotic cavity and then into the fetal peritoneal cavity under continuous ultrasound guidance. After confirmation of the appropriate positioning of the needle, the graft was injected slowly. The fetus was then checked to ensure adequate heartbeat after transplantation just prior to anesthetic being withdrawn from the ewe.
Immunofluorescence
BMSCs were grown in chamber slides and fixed in 1% paraformaldehyde in PBS. Slides were washed with PBS, and blocked in PBS containing 2% bovine serum albumin (BSA; Sigma). Slides were then incubated in PBS containing 2% BSA and primary antibody overnight at 4°C. Slides were washed with PBS with 2% BSA and then incubated with secondary antibody in PBS with 2% BSA for 1 h at 4°C. Secondary antibodies were conjugated to Alexa 488, 594, or 647 (Life Technologies). Finally, cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; BioGenex, Fremont, CA, USA), and coverslips were mounted with Cytoseal 60 (Thermo Fisher Scientific, Waltham, MA, USA). Frozen tissue sections of small intestine from nontransplanted and transplanted sheep were prepared by preserving tissues in 4% paraformaldehyde in PBS, cryoprotecting in increasing concentrations of sucrose, and embedding in 20% sucrose in PBS with optimal cutting temperature (OCT) compound (Ted Pella, Redding, CA, USA). Paraffin-embedded tissue sections were prepared by dewaxing in xylene, rehydrating in decreasing concentrations of ethanol, and immersing in PBS. The primary antibodies used were against the following proteins: pan-cytokeratin clone lu-5 (BioGenex), Musashi-1 (R&D, Minneapolis, MN, USA), EphB2, defensin, Notch-2, leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5), chromogranin A, group II phospholipase A2 (Abcam), and vimentin (Sigma). Specificity of the antibodies used in these experiments was confirmed by staining, in parallel, slides in which the primary antibody was either absent or was replaced by a nonspecific isotype-matched primary antibody. An Olympus Fluoview 1000 confocal system (Olympus, Tokyo, Japan) was used to visualize and capture the fluorescent images.
Fluorescence in situ hybridization (FISH)
To examine the contribution of transplanted clones engrafted in the fetal sheep small intestine, human species-specific DNA probes were constructed and used on paraffin-embedded sections as described previously (43). In short, probes were prepared by PCR containing 1× PCR buffer; 0.1 mM dATP, dCTP, and dGTP; 0.065 mM dTTP, 0.25 mM Texas Red dUTP (Life Technologies); 10 pmol of specific primers; and 100 ng of sheep or human DNA. The primers to amplify the human probe, specific for the human alu sequence, were sense, 5′-GTGGCTCACGCCTGTAATCC-3′, and antisense, 5′-TTTTTTGAGACGGAGTCTCGC-3′.
Probes were denatured for 5 min at 95°C, then allowed to renature at 37°C for 3 h. Tissue sections (10 μm thick) were washed in 2× SSC for 30 min at 37°C, dehydrated in ethanol, and treated with 10 μg/ml proteinase K for 10 min at room temperature. Sections were washed for 5 min with water, followed by 2× SSC for 5 min, and dehydrated in ice-cold ethanol. Sections were denatured at 85°C for 3 min in preheated 70% formamide in 2× SSC (pH 7.0) and then dehydrated with ice-cold ethanol. Probe was applied to sections at 45°C, sealed with a coverslip, and incubated overnight at 42°C. Coverslips were removed by immersing slides in 2× SSC at 45°C, and washed twice with preheated 50% formamide in 2× SSC (pH 7.0) for 5 min, and then washed with 0.1× SSC twice, 5 min each, at 45°C. Sections were washed with PBS, subjected to immunofluorescence, treated with DAPI, and sealed with Cytoseal 60 for use with confocal microscopy.
Microarray analysis of BMSCs
BMSCs from 4 different human donors, isolated and grown as described above, were washed and lysed directly in Trizol (Life Technologies) and RNA purified according to the manufacturer's instructions. The concentration of the RNA samples was determined by Ribogreen (Life Technologies), and the integrity was confirmed on an Agilent 2100 BioAnalyzer (Agilent Technologies, Santa Clara, CA, USA) by the University of Nevada Reno Genomics Center. Total RNA samples (n=4) were labeled using an Affymetrix 3′ one-cycle cDNA Synthesis kit (Affymetrix, Santa Clara, CA, USA) in accordance with manufacturer's protocols. Briefly, cDNA was synthesized from total RNA, followed by second-strand DNA synthesis. Biotin-labeled cRNA was synthesized, fragmented, and then hybridized to Affymetrix GeneChip Human Genome U133 Plus 2.0 arrays. The arrays were then washed and stained with streptavidin-phycoerythrin (Life Technologies) using the Affymetrix GeneChip Fluidics Station 450 and scanned using the Affymetrix GeneChip 3000 7G scanner.
Probe-level analyses of the images from scanning of chips were performed using Affymetrix GeneChip Operating Software (GCOS). Threshold detection P values were set to assign “present” (P<0.05), “marginal” (0.05≤P≤0.49), or “absent” (P>0.49) decision calls for each gene assigned by MAS 5.0 criteria using this software.
Real-time PCR
Total RNA was extracted from 3 different EphB2−/low BMSC populations and 3 different EphB2high BMSC populations using Trizol Reagent with PureLink RNA microkit (Life Technologies) following the manufacturer's instructions. RNA was DNase-treated using RNase-free DNase-I (Qiagen, Valencia, CA, USA) and purified with an RNeasy Mini Kit (Qiagen). RNA was quantified using RiboGreen (Life Technologies), and its quality was evaluated using Agilent 2100 Bioanalyzer. Only RNA samples that showed good 18s and 28s ribosomal RNA peaks were reverse transcribed with the RT2 first-strand kit (SABioscience, Frederick, MD, USA). Total RNA (1 μg for each sample) was used to synthesize cDNA. cDNA was amplified on a MicroAmp Optical 96-Well Reaction Plate using Applied Biosystems Prism 7500 Fast Real-Time PCR System. The sequences of EphB2 primers were as follows: EphB2, sense 5′-GAAGGAGCTCAGTGAGTACAACG-3′, antisense 5′-GCACCTGGAAGACATAG ATGG-3′. GAPDH (SABioscience, Valencia, CA, USA) and β-2 microglobulin (B2M; RealTimePrimers.com, Elkins Park, PA, USA) were used as endogenous controls. After denaturing at 95°C for 10 min, cDNA products were amplified for 40 cycles, each cycle consisting of denaturing at 95°C for 15 s, annealing, and extension at 60°C for 1 min. Quantitative measurements of EphB2, GAPDH, and B2M cDNAs were performed by kinetic PCR using SYBR green (SABioscience) as fluorescent dye and ROX as passive reference dye. The accumulating DNA products were monitored by the ABI7500 sequence detection system (Life Technologies), and data were stored continuously during the reaction. The results were validated based on the quality of dissociation curves and good efficiency of the primers. Each sample was analyzed in triplicate along with nontemplate controls to monitor contaminating DNA. For the target gene, the relative gene expression in each sample was normalized to that of GAPDH and B2M housekeeping genes and analyzed by the 2−ΔΔCT method (44).
Intestinal explant culture
Explant culture of sheep small intestine was established as described previously (45), with a few modifications. Briefly, small intestine isolated from 140 d sheep fetuses was immersed in sterile PBS and washed several times, with cavity contents removed by splitting the intestinal tube down its length along mesentery tissue connections. Small pieces (∼1 mm) were cut and separated into 12-well culture dishes and incubated in MSCGM at 37°C and 5% CO2. Medium was changed every 48 h. Cells were passed into Lab-Tek 2-well chamber slides (Thermo Fisher) for immunofluorescence examination by collecting supernatants and mechanically dislodging adherent cells, resuspending the supernatants and adherent cells together, and plating the combination at 5 × 104 cells/ml. Cultures were allowed to settle for 48 h, and cells were preserved in 1% paraformaldehyde in PBS for 1 h and analyzed by immunofluorescence protocols as described previously (45).
Data analysis and statistics
Small intestine samples from nontransplanted and transplanted sheep were collected at random, and tissue sections were prepared. At least 5 different sections were analyzed per tissue sample. An average of ≥30,000 total cells, quantified by DAPI-labeled nuclear fluorescence, were counted per tissue section. The percentage of human cells was determined by quantifying FISH+, EGFP+, or DiD+ cells in each section and dividing this by the total number of cells present in the same section. The percentage of human cells contributing to the ISC bed was quantified by calculating the number of cells positive for both Musashi and human-specific FISH per 100 Musashi-labeled cells. Experimental results are presented as means ± sem. Comparisons between experimental results were determined by 2-sided nonpaired Student's t test analysis. A value of P < 0.05 was considered statistically significant. Wilcoxon rank sum tests were also used for the same comparisons, yielding identical results to the Student's t test.
RESULTS
Clonally derived human BMSCs contribute to fetal sheep small intestine after transplantation
To investigate the ability of clonally derived BMSC populations to home, lodge, and contribute to the epithelium of the small intestine, we transplanted 6 animals, each with a unique clonally derived Stro-1+ BMSC population (106 cells/animal), and evaluated these animals for human cell engraftment at 75 d post-transplantation. Both bone marrow-derived Stro-1+ cells (40, 46, 47) and these clonally derived BMSC populations have been previously characterized (39). Identification and quantification of human cells present within the fetal sheep intestine was performed using a human-specific probe and FISH, as described in Materials and Methods. Human cells were present throughout the smooth muscle layers and the submucosa, but most of the donor cells were found within mucosal layers (Fig. 1A, B). Furthermore, the overall contribution of each of the BMSC clones to the cellular content of the fetal sheep intestine varied considerably among the different populations. While 4 of the 6 clones contributed with <2% of cell engraftment, specifically 1.13 ± 0.071, 1.20 ± 0.07, 1.46 ± 0.086, and 1.92 ± 0.04%, 2 of the transplanted clones engrafted at levels of 5.03 ± 0.089% (P<0.01) and 5.04 ± 0.15% (P<0.01), respectively; this represents a 4- to 5-fold increase over the other clones. Images of representative human-specific FISH labeling pan-cytokeratin+ cells can be seen in Fig. 1A, B. Specificity of the human-specific probe is demonstrated in Supplemental Fig. S1.
Figure 1.
Clonally derived human BMSCs contribute to the fetal sheep intestine, as identified by FISH pan-cytokeratin, Mushashi, and Lgr5 immunofluorescence. A) Human-specific FISH probe (red) identifies human cells engrafted in fetal sheep intestine (arrow). B) Immunofluorescence analysis using a pan-cytokeratin antibody demonstrates that transplanted human cells expressed cytokeratin after engrafting in the intestine (arrow). C) Total percentages of human cells expressing Musashi-1 present in the ISC region per transplanted animal. D) Human-specific FISH (red) and Musashi-1 immunofluorescence (green) of chimeric fetal sheep intestine demonstrated BMSC contribution to the tissue, including cells expressing Musashi-1. E) Human-specific FISH (red) and Lgr5 immunofluorescence (green) of chimeric fetal sheep intestine. F) Human cells (as identified by human-specific FISH) expressing Musashi-1 also expressed Lgr5.
Transplanted human BMSC clones that engraft within the villi and crypt areas express appropriate region-specific intestinal lineage markers
While determining the overall contribution of transplanted BMSCs to both the mucosal and submucosal layers of the intestine, we observed that human cells within the mucosa were positioned not only in the villi region, but also within the crypt area. Prior to transplant, clonally derived BMSCs were examined in vitro and found to be negative for both the expression of Mushashi-1 and a broad range of cytokeratins using a pan-cytokeratin antibody. Therefore, we used these same markers and immunofluorescence analysis, in combination with human-specific FISH, to investigate whether the BMSCs that engrafted within the intestinal mucosal layer had started to express markers of specific intestinal lineages. As can be seen in Fig. 1B, some of the engrafted human cells (as determined by FISH) show cytokeratin expression, a marker of intestinal epithelium. No human-derived neuroendocrine or paneth cells were found in the intestine of the transplanted animals as determined, respectively, by chromogranin A and either group II phospholipase A2 or defensin staining (data not shown).
To examine the fetal sheep ISC compartment, we used Musashi-1 positivity as an ISC marker (48, 49), and visualized and quantified human cell contribution to this area by double positivity for Musashi-1 and human-specific FISH labeling, as shown in Fig. 1D.
For each of the BMSC clonal populations, the percentage of human Musashi-1+ cells present in the ISC region correlated directly with the overall percentage of engraftment, with 4 of the clones contributing with 1.18 ± 0.095, 1.42 ± 0.16, 2.90 ± 0.254, and 3.85 ± 0.18% of cells, while the other 2 clones generated high levels of Musashi-1+ cells: 12.01 ± 0.45% (P<0.01) and 14.17 ± 0.09% (P<0.01), respectively (Fig. 1C). We further investigated whether Lgr5 (50), another putative ISC marker, was expressed by the engrafted human cells; as can be seen in Fig. 1, most of the human cells (as identified by human-specific FISH) that expressed Musashi-1 (Fig. 1D) also expressed Lgr5 (Fig. 1E, F).
Identification of putative markers for isolating BMSC subsets with enhanced intestinal potential
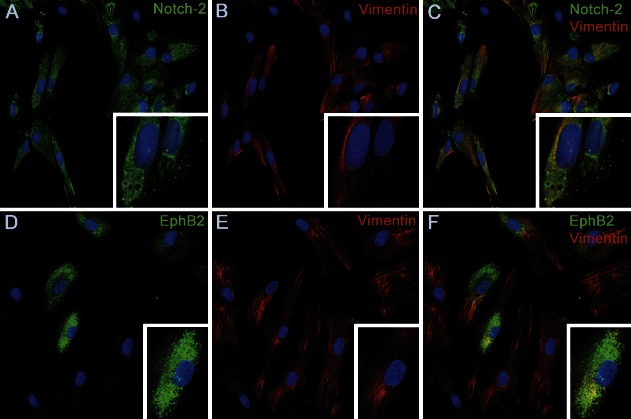
No correlation was found between the levels of expression of integrin-β1, CD44, desmin, vimentin, fibronectin, or laminin on the different BMSC clones prior to transplant (39) and the enhanced levels of intestinal engraftment obtained with the 2 clonally derived BMSC populations. Therefore, we further tested the different BMSC clones by immunofluorescence for the expression of previously proposed ISC markers, such as GPR49, PROM-1, and DCAMKL1 (2, 51), and found them to be negative (data not shown). Next, we used microarray analysis to profile nonclonally derived BMSC populations (n=4) and thereby identify putative markers that would justify the advantage seen with the clonally derived BMSCs to migrate or repopulate the intestinal mucosa. From this analysis, we were able to confirm the overall absence of GPR49, PROM-1, and DCAMKL1 at the transcriptional level. We also searched for RNA transcripts present in BMSCs that have been reported in the literature (3, 51–55) to be preferentially transcribed by intestinal mesenchyme and/or epithelial cells in the crypt or in the villus area, and the data are presented in Table 1. We found that, in similarity to cells located in the stem cell region at the base of the intestinal crypt (3, 51–55), BMSCs expressed transcripts for the transmembrane proteins EphB2, and Notch 2. In addition, BMSCs also expressed the transcription factors BAMBI, HOXB5, Sox-9, TCF3, and TCF4, which are known to be present in crypt epithelial cells (Table 1). Confirmation of the expression of EphB2, and Notch 2 at the protein level was performed by immunofluorescence and confocal microscopy. A representative image of the results obtained can be seen in Fig. 2. While Notch-2 was found to be expressed uniformly on nonclonally derived cultured BMSCs (Fig. 2A, C), expression of EphB2 was only seen in some of the nonclonally derived cultured BMSCs (Fig. 2D, F), indicating that high expression of EphB2 can be used to select a subpopulation of BMSCs. Constitutive expression of vimentin by EphB2−/low cells confirmed that lack of expression of EphB2 was not due to poor viability (Fig. 2E, F). Since EphB2 positivity was able to mark a subpopulation within the BMSCs, we next stained BMSC clones that had engrafted at low and high levels in the intestine of the transplanted animals and found that, overall, the former clones had lower levels of EphB2 than the latter ones (data not shown). The levels of EphB2 expression within the different cell populations were also confirmed at the transcriptional level by real-time PCR and found to be 9 fold higher in EphB2high clonal cells when compared with EphB2low populations (P<0.01; Supplemental Fig. S2).
Table 1.
Evaluation of MSCs for the transcription of genes expressed in cells of the intestinal crypt and villus
| Expressing cells and gene ID | Overall present call rate in BMSCs, n = 4 (%) |
|---|---|
| Crypt epithelial cells and MSCs | |
| BAMBI | 100 |
| EphB2 | 25 |
| HOXB5 | 63 |
| ITGB1 | 100 |
| Notch 2 | 90 |
| Sox9 | 75 |
| TCF3 | 82 |
| TCF4 | 89 |
| Villus mesenchyme and MSCs | |
| PDGFRA | 50 |
| Ptch1 | 33 |
| SMAD1 | 50 |
| Villus epithelium and MSCs | |
| AGPAT2 | 63 |
| ALDH3B1 | 100 |
| EFNB2 | 100 |
| HADHB | 100 |
| PITPNA | 100 |
| PTPRF | 66 |
| SLC7A8 | 33 |
Probe set specificity was confirmed by reviewing genotype mapping for each probe set at the Center for Computational Biology and Integrative Biology Gene Enrichment Profiler web page. Evaluation is according to previously published reports for the intestinal cells. Overall present call rates were computed as the percentage of Affymetrix present calls across multiple probe sets and sample replicates for each gene.
Figure 2.
Immunofluorescence analysis of unsorted and clonal BMSC populations. A–C) Nonclonally derived cultured BMSCs uniformly expressed Notch-2 (A), as confirmed by determining expression of the cytoskeleton protein vimentin (B) and coexpression of both (C). D–F) Expression of EphB2 was only seen in some of the nonclonally derived cultured BMSCs (D, F), indicating that high expression of EphB2 can be used to select a subpopulation of BMSCs. Constitutive expression of Vimentin by EphB2−/low cells confirmed that lack of expression of EphB2 was not due to poor viability (E, F). DAPI (blue) labels all nuclei.
We also investigated whether there was direct contribution of transplanted clonally derived BMSCs to the EphB2+ population of the animals' intestinal crypts. As can be seen in Supplemental Fig. S3, using immunofluorescence analysis in combination with human-specific FISH, transplanted BMSC contributed to the endogenous (sheep) EphB2+ intestinal population, albeit at low numbers.
Sorting of BMSCs based on EphB2 expression demonstrates that EphB2high BMSCs have increased contribution to fetal sheep intestine starting shortly after transplantation
Since clonally derived EphB2high BMSCs contributed at higher levels to both the differentiated epithelial cytokeratin+ layer and Musashi-1+ and Lgr5+ populations in the ISC compartment, we next confirmed that sorting BMSCs based on EphB2 expression would allow us to isolate a population of cells with enhanced ability to replenish the ISC compartment. We sorted EphB2high and EphB2low/neg BMSC populations (Supplemental Fig. S4) labeled with DiD, and transplanted each one of the cell populations at a concentration of 3.6 × 105 cells/fetus into fetal sheep recipients (n=4). One week after transplantation, we evaluated the transplanted animals for the presence of DiD-labeled cells in the intestinal mucosa and found that, although both populations had migrated and lodged in the fetal sheep intestine, EphB2high BMSCs contributed to 2.1 ± 0.2% of total cells in this area, while contribution to this layer by EphB2low BMSCs was 7-fold less, displaying only 0.3 ± 0.1% engraftment.
Contribution of clonally derived EphB2high BMSCs to the ISC was evaluated within the intestine of transplanted animals and by in vitro intestinal explant cultures
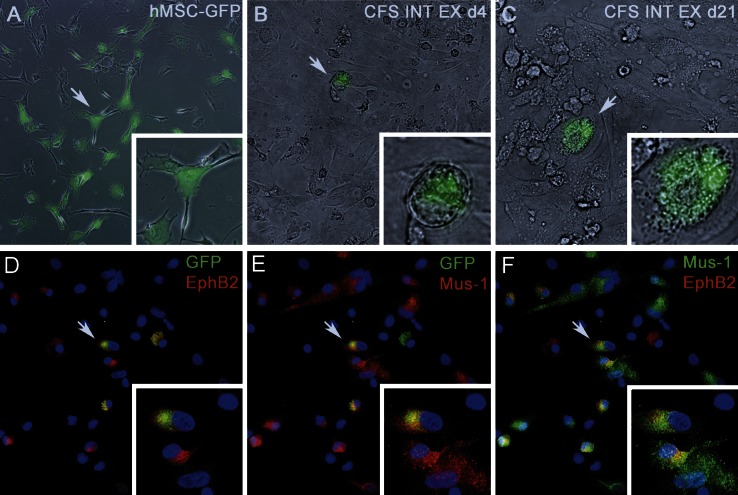
To further evaluate the contribution of EphB2high clonal cells to the ISC bed, clonally derived EphB2high BMSCs were transduced with a lentiviral vector encoding EGFP (Fig. 3A) and transplanted into another set of fetal sheep recipients (n=3).
Figure 3.
Serial culture of intestinal explant demonstrates that clonally derived BMSCs gave rise to cells able to form crypt-like structures by 20 d postculture. A) EphB2high BMSCs, prior to transplant, after transduction with a lentiviral vector encoding EGFP. B) At d 4 of culture, clusters of EGFP+ cells were observed within the explants. C) These cells proliferated to give rise to a crypt-like structure by 20 d postexplant. D, E) Immnostaining of d 20 cells showed that EGFP+ cells continued to express EphB2 (D) and had acquired Musashi-1 (E). F) EphB2+ cells express Musashi-1. Arrows indicate features enlarged in insets.
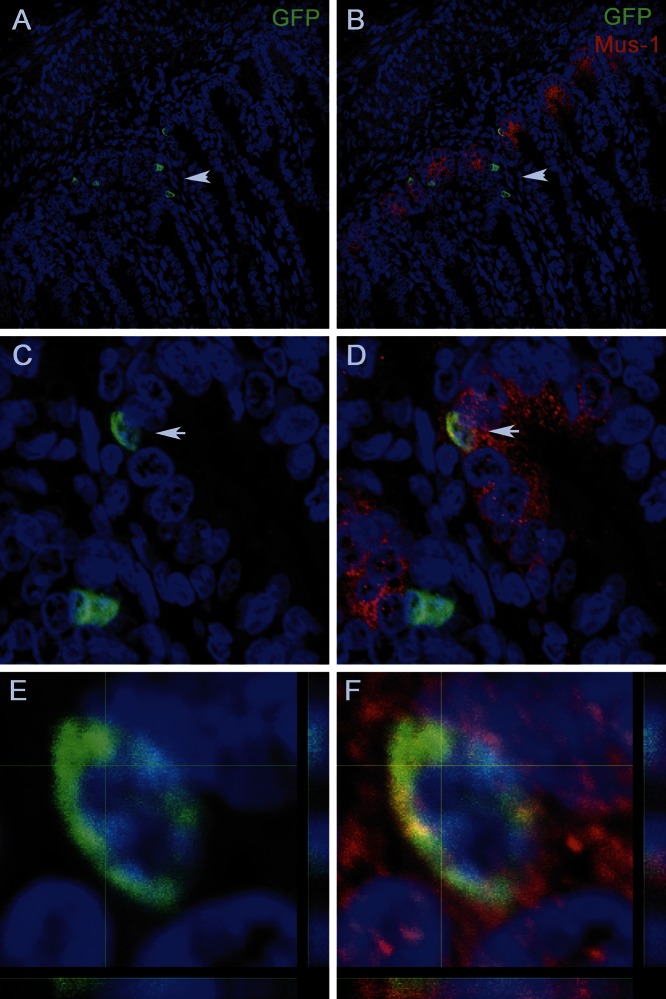
At 60 d post-transplant, immunofluorescence analysis of tissue sections of the intestinal crypts of the transplanted animals demonstrated that EGFP+ BMSCs, after engraftment, expressed Lgr5 (Fig. 4A) and Mushashi-1 (Fig. 4B) and continued to express EphB2 (Fig. 4C). Analysis of sagittal sections of the intestinal crypts of the transplanted animals enabled us to confirm the localization of the EGFP+ cells within the crypt (Fig. 5A, C) and the coexpression of Musahi-1 (Fig. 5B, D) and confirm the double positivity of these cells (Fig. 5E, F).
Figure 4.
EphB2high BMSC and EGFP BMSCs express markers of ISCs after transplant and proliferate on engraftment. A–C) EGFP+ BMSCs after engraftment express Lgr5 (A) and Mushashi-1 (B) and continue to express EphB2 (C). D–F) EphB2high BMSCs labeled with a lentiviral vector expressing EGFP+ cells engrafted in the crypt and villous area on transplantation (D); Ki67 labeling marks proliferating transplanted and endogenous cells (E); EGFP+ transplanted cells that are proliferating are marked with Ki67 labeling (F). Arrows indicate features enlarged in insets.
Figure 5.
Sagittal section of intestinal crypts of animals transplanted with clonally derived BMSC EGFP+ cells. A, C) Localization of the EGFP+ cells within the crypt (arrows). B, D) Coexpression of Musahi-1 (arrows). E, F) Z-stack analysis confirms that EGFP+ cell (E) is expressing Musashi-1 (F).
At the same time point of 60 d post-transplant, small intestine was also collected from these animals, and explant cultures were performed. Explant cultures remained viable past 50 d and were able to be serially seeded into chamber slides for examination by immunofluorescence microscopy. Human BMSC-derived EGFP+ cells were found in the intestinal explants of all transplanted animals, and images representative of the results obtained with these cultures can be seen in Fig. 3B–F. At d 4 of culture, clusters of EGFP+ cells were observed within the explants (Fig. 3B). These cells proliferated to give rise to a crypt-like structure (56) by 20 d postexplant (Fig. 3C). Immunostaining of d 20 explant cells demonstrated that EGFP+ cells continued to express EphB2 and had acquired Musashi-1 positivity Fig. 3D–F.
We next investigated whether the transplanted EGFP+EphB2high BMSCs underwent proliferation in vivo on engraftment, and as can be seen in a representative image of the results obtained (Fig. 4D–F) ∼50% of EGFP+ cells are positive for Ki67, a marker of cellular proliferation.
To investigate whether transplanted cells were fusing with endogenous sheep cells, we visualized cells that were positive for EGFP (Supplemental Fig. S5A, C, D, F) and performed FISH using either a sheep-specific (Fig. S5B, C) or human-specific probe (Fig. S5E, F). By examining the species origin of the genomic DNA present within the nuclei of the EGFP+ cells, we were able to determine whether the transplanted BMSCs had undergone fusion. Supplemental Fig. S5 shows that EGFP+ cells are the only ones hybridizing with a human-specific probe, while Fig. 5C demonstrates that using a sheep-specific probe, all the nuclei, except the EGFP+ cells, are labeled by FISH.
DISCUSSION
Previous studies in animal models have shown that transplantation of bone marrow-derived cells can contribute to the regeneration of the gastrointestinal tract in IBD and radiation-induced injury (17–19, 57, 58). However, the reduced levels of engraftment, and low or absent differentiation of bone marrow-derived cells into intestinal cells, reported in most of these studies, discouraged the practical application of these cells in a clinical setting. In contrast, another study showed that transplantation with BMSCs genetically modified to express CXCR-4 resulted in levels of engraftment able to ameliorate radiation enteritis (59). Even though BMSCs are known to have the innate ability to migrate and home to areas of injury, this study demonstrates that this natural aptitude might not be adequate to induce migration of therapeutic numbers of cells to the site of damage. Therefore, it is necessary to find populations of cells that home preferentially to the intestine and/or have the intrinsic ability to restore the integrity of the intestinal mucosa. Here we show that clonally derived human adult BMSCs (at a dosage of 107cells/kg) are able, on intraperitoneal injection, to migrate and home to the intestinal mucosa, with some of the BMSC clones engrafting at much higher levels than the others. We also demonstrated that the transplanted human cells within the mucosa were positioned mostly within the crypt area, although some could also be found in the villi region. Using FISH and antibodies against cytokeratin, Lgr5, and Musashi-1, expression of which was absent in BMSCs prior to transplant, we showed that the transplanted cells had started to express specific markers of intestinal lineages. While the mechanism responsible for the transplanted BMSCs acquiring markers of intestinal cells is not currently known, this process does not involve fusion between donor and host cells in the fetal sheep model. Given the highly proliferative/inductive nature of the fetal environment, it is likely that soluble factors, signaling molecules, and microvesicles (60) containing RNAs and proteins may all be present and contribute to this process.
For each of the BMSC clonal populations, the percentage of human Musashi-1+ and Lgr5 cells found in the ISC region correlated directly with the overall percentage of engraftment, demonstrating that contribution to the stem cell bed was crucial for the overall level of engraftment. To identify a plausible cause for why certain clones displayed enhanced intestinal engraftment, we used microarray analysis in nonclonally derived BMSCs, and looked for commonalities between the gene expression profile of BMSCs and of that which had been reported in the literature for ISCs. From the few that were common, we found that EphB2 was differentially expressed on unsorted BMSCs and that the clones with higher levels of intestinal engraftment also expressed higher levels of EphB2. We next demonstrated that sorting of BMSCs based on EphB2 expression allowed us to isolate a population of cells with enhanced ability to replenish the ISC compartment. EphB2high BMSCs engrafted at 7-fold higher levels than EphB2dim/− when transplanted at the same concentration of 3.6 × 106/kg. Of note is that the overall intestinal engraftment of the EphB2high BMSC clones was ∼5%, but when BMSCs were selected and sorted specifically for EphB2high the engraftment was only 2%. This is most likely due to the fact that the cell dose used with BMSC EphB2high was 2.7-fold less than that used with the BMSC clones and/or the time that elapsed until evaluation. Taking these factors into consideration, it thus seems that, irrespective of clonality, both EphB2high populations contributed in a similar way to the intestinal mucosa. In addition, when we examined the ability of BMSCs to reconstitute the ISC compartment, we found that clonal EphB2high BMSCs replenished the stem cell bed at levels of 14%, as attested by their Musashi-1 and Lgr5 positivity. Because the model used to test these cells is a noninjury model, inflammatory signals inducing apoptosis of incoming cells are absent; therefore, these studies are able to reflect the intrinsic aptitude of EphB2high BMSCs to migrate to the intestine and, in particular, to the ISC region. The results presented here are also in agreement with previous reports regarding Eph-Ephrin expression patterns in the intestine, in which it was demonstrated that the proliferative basal crypt cells were EphB2+, while the postmitotic differentiated cells were EphrinB1+ (61), and that epithelial intestinal cells with the longest telomeres and elevated expression of ISC-specific genes also possess the highest EphB2 surface levels (62). Furthermore, it has also been shown that EphB2 expression, can be used to purify and expand populations of intestinal undifferentiated cells (62). Therefore, it is possible that bone marrow cells expressing high levels of EphB2 are recruited to sites of the intestine where this molecule is normally expressed. Nevertheless, because Ephrin/Eph signaling has a decisive role in tissue repair, acceleration of wound closure, and maintenance of homeostasis of the intestinal barrier in adults (14, 15), the use of EphB2high BMSCs in cell therapies to target the intestine could potentially result in enhanced levels of homing and repopulation of the ISC pool, decreasing the healing time in severe intestinal damage. However, studies using models of chronic injury or induced colitis will be needed to determine whether EphB2high BMSCs play an important role in the endogenous repair and regeneration process, and to test the ability of exogenously supplied EphB2high BMSCs to produce a therapeutic effect in the presence of intestinal injury.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Heart, Lung, and Blood Institute grants HL097623 and HL073737 and grant P20 RR-016464 from the Idea Network of Biological Research Excellence (INBRE) Program of the National Center for Research Resources.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BMSC
- bone marrow stromal cell
- BSA
- bovine serum albumin
- DAPI
- 4′,6-diamidino-2-phenylindole
- DiD
- 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate
- Eph
- Ephrin receptor
- EphB2
- Ephrin type B receptor 2
- FISH
- fluorescence in situ hybridization
- IBD
- inflammatory bowel disease
- ISC
- intestinal stem cell
- Lgr5
- leucine-rich repeat-containing G-protein-coupled receptor 5
- MSCGM
- mesenchymal stem cell growth medium
- PBS
- phosphate-buffered saline
REFERENCES
- 1. Gordon J. I., Schmidt G. H., Roth K. A. (1992) Studies of intestinal stem cells using normal, chimeric, and transgenic mice. FASEB J. 6, 3039–3050 [DOI] [PubMed] [Google Scholar]
- 2. Clevers H. (2009) Searching for adult stem cells in the intestine. EMBO Mol. Med. 1, 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crosnier C., Stamataki D., Lewis J. (2006) Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat. Rev. Genet. 7, 349–359 [DOI] [PubMed] [Google Scholar]
- 4. Powell D. W., Adegboyega P. A., Di Mari J. F., Mifflin R. C. (2005) Epithelial cells and their neighbors. I. Role of intestinal myofibroblasts in development, repair, and cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G2–G7 [DOI] [PubMed] [Google Scholar]
- 5. Uccelli A., Moretta L., Pistoia V. (2008) Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 [DOI] [PubMed] [Google Scholar]
- 6. Okamoto R., Watanabe M. (2004) Molecular and clinical basis for the regeneration of human gastrointestinal epithelia. J. Gastroenterol. 39, 1–6 [DOI] [PubMed] [Google Scholar]
- 7. Okamoto R., Yajima T., Yamazaki M., Kanai T., Mukai M., Okamoto S., Ikeda Y., Hibi T., Inazawa J., Watanabe M. (2002) Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat. Med. 8, 1011–1017 [DOI] [PubMed] [Google Scholar]
- 8. Medema J. P., Vermeulen L. (2011) Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 474, 318–326 [DOI] [PubMed] [Google Scholar]
- 9. Batlle E., Henderson J. T., Beghtel H., van den Born M. M., Sancho E., Huls G., Meeldijk J., Robertson J., van de Wetering M., Pawson T., Clevers H. (2002) Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111, 251–263 [DOI] [PubMed] [Google Scholar]
- 10. Hafner C., Schmitz G., Meyer S., Bataille F., Hau P., Langmann T., Dietmaier W., Landthaler M., Vogt T. (2004) Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin. Chem. 50, 490–499 [DOI] [PubMed] [Google Scholar]
- 11. Van de Wetering M., Sancho E., Verweij C., de Lau W., Oving I., Hurlstone A., van der Horn K., Batlle E., Coudreuse D., Haramis A. P., Tjon-Pon-Fong M., Moerer P., van den Born M., Soete G., Pals S., Eilers M., Medema R., Clevers H. (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 [DOI] [PubMed] [Google Scholar]
- 12. Holmberg J., Genander M., Halford M. M., Anneren C., Sondell M., Chumley M. J., Silvany R. E., Henkemeyer M., Frisen J. (2006) EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell 125, 1151–1163 [DOI] [PubMed] [Google Scholar]
- 13. Genander M., Halford M. M., Xu N. J., Eriksson M., Yu Z., Qiu Z., Martling A., Greicius G., Thakar S., Catchpole T., Chumley M. J., Zdunek S., Wang C., Holm T., Goff S. P., Pettersson S., Pestell R. G., Henkemeyer M., Frisen J. (2009) Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell 139, 679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hafner C., Meyer S., Hagen I., Becker B., Roesch A., Landthaler M., Vogt T. (2005) Ephrin-B reverse signaling induces expression of wound healing associated genes in IEC-6 intestinal epithelial cells. World J. Gastroenterol. 11, 4511–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hafner C., Meyer S., Langmann T., Schmitz G., Bataille F., Hagen I., Becker B., Roesch A., Rogler G., Landthaler M., Vogt T. (2005) Ephrin-B2 is differentially expressed in the intestinal epithelium in Crohn's disease and contributes to accelerated epithelial wound healing in vitro. World J. Gastroenterol. 11, 4024–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Semont A., Francois S., Mouiseddine M., Francois A., Sache A., Frick J., Thierry D., Chapel A. (2006) Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv. Exp. Med. Biol. 585, 19–30 [DOI] [PubMed] [Google Scholar]
- 17. Kudo K., Liu Y., Takahashi K., Tarusawa K., Osanai M., Hu D. L., Kashiwakura I., Kijima H., Nakane A. (2010) Transplantation of mesenchymal stem cells to prevent radiation-induced intestinal injury in mice. J. Radiat. Res. (Tokyo) 51, 73–79 [DOI] [PubMed] [Google Scholar]
- 18. Yabana T., Arimura Y., Tanaka H., Goto A., Hosokawa M., Nagaishi K., Yamashita K., Yamamoto H., Adachi Y., Sasaki Y., Isobe M., Fujimiya M., Imai K., Shinomura Y. (2009) Enhancing epithelial engraftment of rat mesenchymal stem cells restores epithelial barrier integrity. J. Pathol. 218, 350–359 [DOI] [PubMed] [Google Scholar]
- 19. Hayashi Y., Tsuji S., Tsujii M., Nishida T., Ishii S., Nakamura T., Eguchi H., Kawano S. (2007) The transdifferentiation of bone-marrow-derived cells in colonic mucosal regeneration after dextran-sulfate-sodium-induced colitis in mice. Pharmacology 80, 193–199 [DOI] [PubMed] [Google Scholar]
- 20. Lanzoni G., Roda G., Belluzzi A., Roda E., Bagnara G. P. (2008) Inflammatory bowel disease: Moving toward a stem cell-based therapy. World J. Gastroenterol. 14, 4616–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krause D. S., Theise N. D., Collector M. I., Henegariu O., Hwang S., Gardner R., Neutzel S., Sharkis S. J. (2001) Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105, 369–377 [DOI] [PubMed] [Google Scholar]
- 22. Caplan A. I. (2009) Why are MSCs therapeutic? New data: new insight. J. Pathol. 217, 318–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Blanc K. (2006) Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy 8, 559–561 [DOI] [PubMed] [Google Scholar]
- 24. Swenson E. S. T. N. (2010) Stem cell therapeutics: potential in the treatment of inflammatory bowel disease. Clin. Exp. Gastroenterol. 2010, 1–10 [PMC free article] [PubMed] [Google Scholar]
- 25. Sun Y., Xiao D., Zhang R. S., Cui G. H., Wang X. H., Chen X. G. (2007) Formation of human hepatocyte-like cells with different cellular phenotypes by human umbilical cord blood-derived cells in the human-rat chimeras. Biochem. Biophys. Res. Commun. 357, 1160–1165 [DOI] [PubMed] [Google Scholar]
- 26. Schoeberlein A., Schatt S., Troeger C., Surbek D., Holzgreve W., Hahn S. (2004) Engraftment kinetics of human cord blood and murine fetal liver stem cells following in utero transplantation into immunodeficient mice. Stem Cells Dev. 13, 677–684 [DOI] [PubMed] [Google Scholar]
- 27. Xiao Y. P., Chen M. J., Sheng M., Gong Z. J., Wang S., Huang S. Z. (2003) [Analysis of human cells in transplanted goats using fluorescence in situ hybridization]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 25, 129–133 [PubMed] [Google Scholar]
- 28. Tarantal A. F., Goldstein O., Barley F., Cowan M. J. (2000) Transplantation of human peripheral blood stem cells into fetal rhesus monkeys (Macaca mulatta). Transplantation 69, 1818–1823 [DOI] [PubMed] [Google Scholar]
- 29. Shields L. E., Bryant E. M., Easterling T. R., Andrews R. G. (1995) Fetal liver cell transplantation for the creation of lymphohematopoietic chimerism in fetal baboons. Am. J. Obstet. Gynecol. 173, 1157–1160 [DOI] [PubMed] [Google Scholar]
- 30. Pixley J. S., Tavassoli M., Zanjani E. D., Shaft D. M., Futamachi K. J., Sauter T., Tavassoli A., MacKintosh F. R. (1994) Transplantation in utero of fetal human hematopoietic stem cells into mice results in hematopoietic chimerism. Pathobiology 62, 238–244 [DOI] [PubMed] [Google Scholar]
- 31. Fujiki Y., Fukawa K., Kameyama K., Kudo O., Onodera M., Nakamura Y., Yagami K., Shiina Y., Hamada H., Shibuya A., Nakauchi H. (2003) Successful multilineage engraftment of human cord blood cells in pigs after in utero transplantation. Transplantation 75, 916–922 [DOI] [PubMed] [Google Scholar]
- 32. Flake A. W., Harrison M. R., Adzick N. S., Zanjani E. D. (1986) Transplantation of fetal hematopoietic stem cells in utero: the creation of hematopoietic chimeras. Science 233, 776–778 [DOI] [PubMed] [Google Scholar]
- 33. Peranteau W. H., Heaton T. E., Gu Y. C., Volk S. W., Bauer T. R., Alcorn K., Tuschong L. M., Johnson M. P., Hickstein D. D., Flake A. W. (2009) Haploidentical in utero hematopoietic cell transplantation improves phenotype and can induce tolerance for postnatal same-donor transplants in the canine leukocyte adhesion deficiency model. Biol. Blood Marrow Transplant 15, 293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rubin J. P., Cober S. R., Butler P. E., Randolph M. A., Gazelle G. S., Ierino F. L., Sachs D. H., Lee W. P. (2001) Injection of allogeneic bone marrow cells into the portal vein of swine in utero. J. Surg. Res. 95, 188–194 [DOI] [PubMed] [Google Scholar]
- 35. Sabatino D. E., Mackenzie T. C., Peranteau W., Edmonson S., Campagnoli C., Liu Y. L., Flake A. W., High K. A. (2007) Persistent expression of hF.IX After tolerance induction by in utero or neonatal administration of AAV-1-F.IX in hemophilia B mice. Mol. Ther. 15, 1677–1685 [DOI] [PubMed] [Google Scholar]
- 36. Almeida-Porada G., Crapnell K., Porada C., Benoit B., Nakauchi H., Quesenberry P., Zanjani E. D. (2005) In vivo haematopoietic potential of human neural stem cells. Br. J. Haematol. 130, 276–283 [DOI] [PubMed] [Google Scholar]
- 37. Almeida-Porada G., El Shabrawy D., Porada C., Zanjani E. D. (2002) Differentiative potential of human metanephric mesenchymal cells. Exp. Hematol. 30, 1454–1462 [DOI] [PubMed] [Google Scholar]
- 38. Almeida-Porada G., Porada C. D., Chamberlain J., Torabi A., Zanjani E. D. (2004) Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood 104, 2582–2590 [DOI] [PubMed] [Google Scholar]
- 39. Chamberlain J., Yamagami T., Colletti E., Theise N. D., Desai J., Frias A., Pixley J., Zanjani E. D., Porada C. D., Almeida-Porada G. (2007) Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology 46, 1935–1945 [DOI] [PubMed] [Google Scholar]
- 40. Simmons P. J., Gronthos S., Zannettino A., Ohta S., Graves S. (1994) Isolation, characterization and functional activity of human marrow stromal progenitors in hemopoiesis. Prog. Clin. Biol. Res. 389, 271–280 [PubMed] [Google Scholar]
- 41. Colletti E. J., Airey J. A., Liu W., Simmons P. J., Zanjani E. D., Porada C. D., Almeida-Porada G. (2009) Generation of tissue-specific cells from MSC does not require fusion or donor-to-host mitochondrial/membrane transfer. Stem Cell Res. 2, 125–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shaw S. W., Bollini S., Nader K. A., Gastadello A., Mehta V., Filppi E., Cananzi M., Gaspar H. B., Qasim W., De Coppi P., David A. L. (2011) Autologous transplantation of amniotic fluid-derived mesenchymal stem cells into sheep fetuses. Cell Transplant. 20, 1015–1031 [DOI] [PubMed] [Google Scholar]
- 43. Ishikawa F., Drake C. J., Yang S., Fleming P., Minamiguchi H., Visconti R. P., Crosby C. V., Argraves W. S., Harada M., Key L. L., Jr., Livingston A. G., Wingard J. R., Ogawa M. (2003) Transplanted human cord blood cells give rise to hepatocytes in engrafted mice. Ann. N. Y. Acad. Sci. 996, 174–185 [DOI] [PubMed] [Google Scholar]
- 44. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 45. Batman P. A., Fleming S. C., Sedgwick P. M., MacDonald T. T., Griffin G. E. (1994) HIV infection of human fetal intestinal explant cultures induces epithelial cell proliferation. AIDS 8, 161–167 [PubMed] [Google Scholar]
- 46. Gronthos S., Zannettino A. C. (2008) A method to isolate and purify human bone marrow stromal stem cells. Methods Mol. Biol. 449, 45–57 [DOI] [PubMed] [Google Scholar]
- 47. Dennis J. E., Carbillet J. P., Caplan A. I., Charbord P. (2002) The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs 170, 73–82 [DOI] [PubMed] [Google Scholar]
- 48. Potten C. S., Booth C., Tudor G. L., Booth D., Brady G., Hurley P., Ashton G., Clarke R., Sakakibara S., Okano H. (2003) Identification of a putative intestinal stem cell and early lineage marker; Musashi-1. Differentiation 71, 28–41 [DOI] [PubMed] [Google Scholar]
- 49. Nishimura S., Wakabayashi N., Toyoda K., Kashima K., Mitsufuji S. (2003) Expression of Musashi-1 in human normal colon crypt cells: a possible stem cell marker of human colon epithelium. Dig. Dis. Sci. 48, 1523–1529 [DOI] [PubMed] [Google Scholar]
- 50. Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., Clevers H. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 51. Montgomery R. K., Breault D. T. (2008) Small intestinal stem cell markers. J. Anat. 213, 52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van der Flier L. G., Clevers H. (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241–260 [DOI] [PubMed] [Google Scholar]
- 53. Van der Flier L. G., Sabates-Bellver J., Oving I., Haegebarth A., De Palo M., Anti M., Van Gijn M. E., Suijkerbuijk S., Van de Wetering M., Marra G., Clevers H. (2007) The intestinal Wnt/TCF signature. Gastroenterology 132, 628–632 [DOI] [PubMed] [Google Scholar]
- 54. George M. D., Wehkamp J., Kays R. J., Leutenegger C. M., Sabir S., Grishina I., Dandekar S., Bevins C. L. (2008) In vivo gene expression profiling of human intestinal epithelial cells: analysis by laser microdissection of formalin fixed tissues. BMC Genomics 9, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kosinski C., Li V. S., Chan A. S., Zhang J., Ho C., Tsui W. Y., Chan T. L., Mifflin R. C., Powell D. W., Yuen S. T., Leung S. Y., Chen X. (2007) Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc. Natl. Acad. Sci. U. S. A. 104, 15418–15423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J., Clevers H. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 [DOI] [PubMed] [Google Scholar]
- 57. Gonzalez M. A., Gonzalez-Rey E., Rico L., Buscher D., Delgado M. (2009) Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 136, 978–989 [DOI] [PubMed] [Google Scholar]
- 58. Mouiseddine M., Francois S., Semont A., Sache A., Allenet B., Mathieu N., Frick J., Thierry D., Chapel A. (2007) Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br. J. Radiol. 80(Spec. 1), S49–S55 [DOI] [PubMed] [Google Scholar]
- 59. Zhang J., Gong J. F., Zhang W., Zhu W. M., Li J. S. (2008) Effects of transplanted bone marrow mesenchymal stem cells on the irradiated intestine of mice. J. Biomed. Sci. 15, 585–594 [DOI] [PubMed] [Google Scholar]
- 60. Aliotta J. M., Lee D., Puente N., Faradyan S., Sears E. H., Amaral A., Goldberg L., Dooner M. S., Pereira M., Quesenberry P. J. (2012) Progenitor/stem cell fate determination: interactive dynamics of cell cycle and microvesicles. Stem Cells Dev. 21, 1627–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Koo B. K., Lim H. S., Chang H. J., Yoon M. J., Choi Y., Kong M. P., Kim C. H., Kim J. M., Park J. G., Kong Y. Y. (2009) Notch signaling promotes the generation of EphrinB1-positive intestinal epithelial cells. Gastroenterology 137, 145–155, 155 e141–e143 [DOI] [PubMed] [Google Scholar]
- 62. Jung P., Sato T., Merlos-Suarez A., Barriga F. M., Iglesias M., Rossell D., Auer H., Gallardo M., Blasco M. A., Sancho E., Clevers H., Batlle E. (2011) Isolation and in vitro expansion of human colonic stem cells. Nat. Med. 17, 1225–1227 [DOI] [PubMed] [Google Scholar]
- 63. Cogle C. R., Theise N. D., Fu D., Ucar D., Lee S., Guthrie S. M., Lonergan J., Rybka W., Krause D. S., Scott E. W. (2007) Bone marrow contributes to epithelial cancers in mice and humans as developmental mimicry. Stem Cells 25, 1881–1887 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.