Abstract
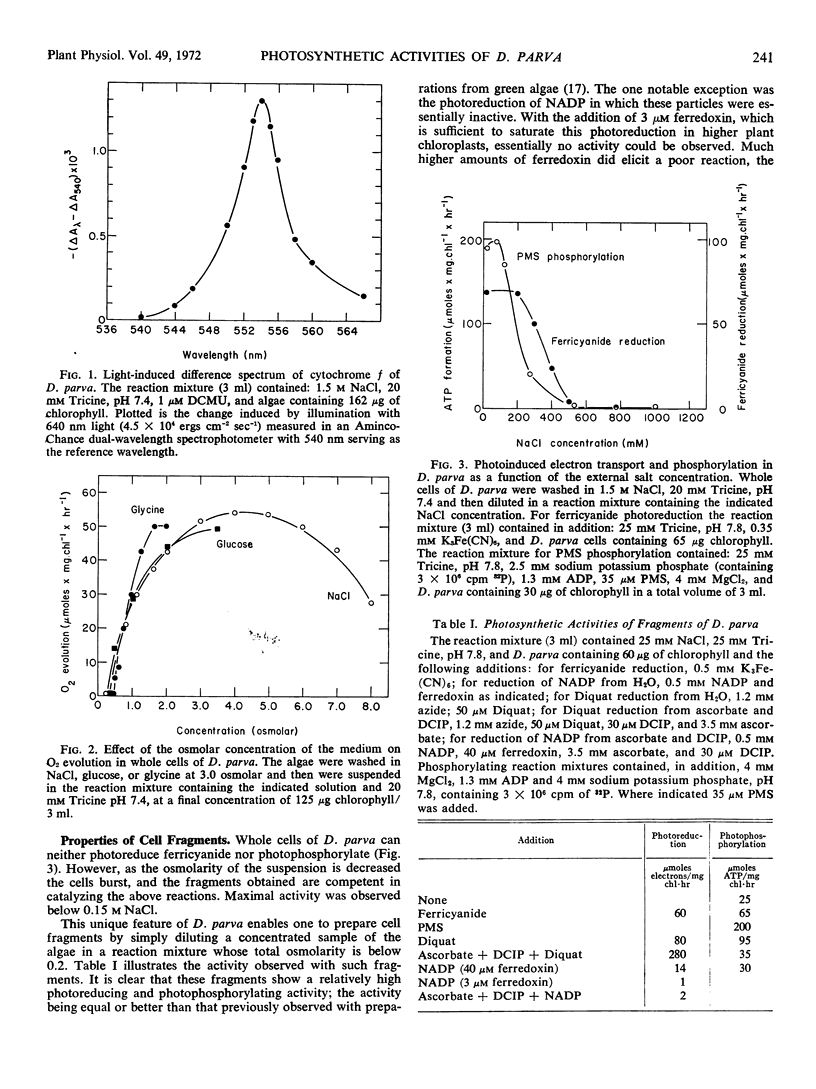
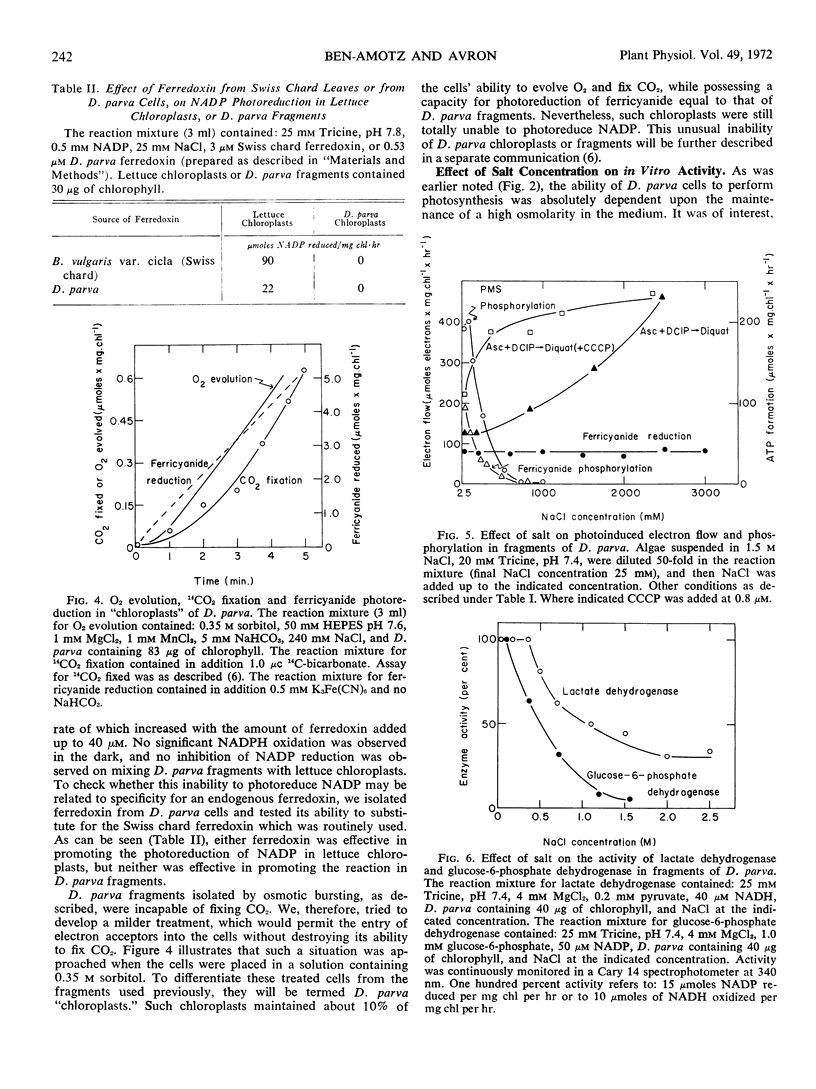
Dunaliella parva, a unicellular halophilic alga, was found to evolve oxygen photosynthetically only in the presence of a high osmolar concentration. Cell free preparations were obtained by placing the cells in a medium of low osmolarity. The fragments obtained showed a high photoreducing and photophosphorylating activity except for their inability to catalyze all ferredoxin dependent photoreactions. Placing the cells in a medium of intermediate osmolarity produced a “chloroplast” preparation which maintained some capacity for O2 evolution and CO2 fixation, while possessing the ability to catalyze the photoinduced reduction of ferricyanide. Enzymic and photosynthetic reactions of cell-free preparations from D. parva were inhibited, rather than stimulated, by the salt concentration optimal for growth. These results were interpreted as indicating the existence of a steep NaCl gradient in vivo between the medium and the cell compartments which are not permeable to salt.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation as a tool for the synthesis of specifically labeled nucleotides. Anal Biochem. 1961 Dec;2:535–543. doi: 10.1016/0003-2697(61)90021-5. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Chance B. Relation of phosphorylation to electron transport in isolated chloroplasts. Brookhaven Symp Biol. 1966;19:149–160. [PubMed] [Google Scholar]
- Ben-Amotz A., Avron M. Is nicotinamide adenine dinucleotide phosphate an obligatory intermediate in photosynthesis? Plant Physiol. 1972 Feb;49(2):244–248. doi: 10.1104/pp.49.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Ginzburg B. Z. Light-induced proton uptake in whole cells of Dunaliella parva. Biochim Biophys Acta. 1969 Jun 3;183(1):144–154. doi: 10.1016/0005-2736(69)90138-2. [DOI] [PubMed] [Google Scholar]
- Katoh S., San Pietro A. Activities of chloroplast fragments. I. Hill reaction and ascorbate-indophenol photoreductions. J Biol Chem. 1966 Aug 10;241(15):3575–3581. [PubMed] [Google Scholar]
- Shavit N., Avron M. The relation of electron transport and photophosphorylation to conformational changes in chloroplasts. Biochim Biophys Acta. 1967 May 9;131(3):516–525. doi: 10.1016/0005-2728(67)90011-4. [DOI] [PubMed] [Google Scholar]
- Shneyour A., Avron M. High biological activity in chloroplasts from Euglena gracilis prepared with a new gas pressure device. FEBS Lett. 1970 Jun 1;8(3):164–166. doi: 10.1016/0014-5793(70)80253-8. [DOI] [PubMed] [Google Scholar]
- Vernon L. P., Shaw E. Photochemical Activities of Spinach Chloroplasts Following Treatment with the Detergent Triton X-100. Plant Physiol. 1965 Nov;40(6):1269–1277. doi: 10.1104/pp.40.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Crofts A. R. Photosynthesis. Annu Rev Biochem. 1970;39:389–428. doi: 10.1146/annurev.bi.39.070170.002133. [DOI] [PubMed] [Google Scholar]
- Witt H. T., Rumberg B., Schmidt-Mende P., Siggel U., Skerra B., Vater J., Weikard J. On the analysis of photosynthesis by flashlight techniques. Angew Chem Int Ed Engl. 1965 Oct;4(10):799–819. doi: 10.1002/anie.196507991. [DOI] [PubMed] [Google Scholar]
- Yocum C. F., Pietro A. S. Ferredoxin-reducing substance (FRS) from spinach. II. Separation and assay. Arch Biochem Biophys. 1970 Sep;140(1):152–157. doi: 10.1016/0003-9861(70)90018-4. [DOI] [PubMed] [Google Scholar]


