Abstract
R-spondin proteins are adult stem cell growth factors capable of stimulating gut development by activating LGR4, 5, and 6 receptors to promote Wnt signaling. Although multiple Wnt ligands and cognate Frizzled receptors are expressed in the ovary, their physiological roles are unclear. Based on bioinformatic and in situ hybridization analyses, we demonstrated the exclusive expression of R-spondin2 in oocytes of ovarian follicles. In cultured somatic cells from preantral follicles, R-spondin2 treatment (ED50: 3 ng/ml) synergized with Wnt3a to stimulate Wnt signaling. In cultured ovarian explants from prepubertal mice containing preantral follicles, treatment with R-spondin2, similar to follicle stimulating hormone, promoted the development of primary follicles to the secondary stage. In vivo administration of an R-spondin agonist stimulated the development of primary follicles to the antral stage in both immature mice and gonadotropin releasing hormone antagonist-treated adult mice. Subsequent treatment with gonadotropins allowed the generation of mature oocytes capable of undergoing early embryonic development and successful pregnancy. Furthermore, R-spondin agonist treatment of immune-deficient mice grafted with human cortical fragments stimulated the development of primary follicles to the secondary stage. Thus, oocyte-derived R-spondin2 is a paracrine factor essential for primary follicle development, and R-spondin agonists could provide a new treatment regimen for infertile women with low responses to the traditional gonadotropin therapy.—Cheng, Y., Kawamura, K., Takae, S., Deguchi, M., Yang, Q., Kuo, C., Hsueh, A. J. W. Oocyte-derived R-spondin2 promotes ovarian follicle development.
Keywords: Wnt pathway, follicle development, FSH, ovulation, embryos
Wingless (Wnt) proteins are secreted signaling molecules conserved in vertebrates and invertebrates and act locally to regulate diverse developmental processes (1). A large family of Wnt ligands activates several G-protein-coupled Frizzled receptors, and this ligand-receptor interaction requires the participation of low-density lipoprotein-related receptors 5 and 6 (LRP5 and 6). The activation of Frizzled receptors and LRP5 and 6 can be inhibited by other secreted proteins, including Dickkopfs and soluble Frizzled-related proteins (2). Following binding to Frizzled receptors, Wnt ligands activate the canonical Wnt pathway mediated by dishevelled, glycogen synthase kinase 3 (GSK3), and β-catenin, leading to transcriptional activation of T-cell factor/lymphoid enhancer factor-regulated genes (3).
Multiple Wnt ligands, Frizzled receptors, and intracellular signaling molecules in the Wnt signaling pathway are expressed in the ovary at specific stages of follicular development and during luteinization (4–6). In human cumulus cells, Wnt2 acts through its receptor Frizzled9 to regulate the β-catenin pathway (7). In cultured rodent granulosa cells, knockdown of Wnt2 expression using siRNA decreased DNA synthesis, whereas Wnt2 overexpression enhanced it (8). In a mouse model overexpressing a dominant stable β-catenin mutant in granulosa cells of small antral and preovulatory follicles, facilitation of follicle-stimulating hormone (FSH)-induced follicular growth and suppression of follicle atresia were evident (9). Although these findings suggested the existence of the Wnt-Frizzled-β-catenin pathway in ovarian follicles, only minor ovarian effects of Wnt ligands were evident, and the exact physiological roles of Wnt and related molecules in the ovary remain to be elucidated.
R-spondin1, a gene with the thrombospondin type 1 domain expressed in the embryonic roof plate and dorsal neural tube (10), was found to induce intestinal cell proliferation by activating the Wnt signaling pathway (11). The 4 paralogous R-spondin proteins stimulate Wnt signaling by activating leucine-rich repeat-containing G-protein-coupled receptors 4–6 (LGR4–6; refs. 12, 13) and by preventing DKK1-mediated LRP6 and Kremen1 association and internalization (14). Studies using the T-cell factor (TCF)-reporter assay demonstrated strong synergistic effects of R-spondin and Wnt proteins in the stimulation of the canonical Wnt signaling pathway. Of interest, a transgene insertional mutant, footless, with a hypomorph of R-spondin2 function, was associated with asymmetric limb malformations and premature ovarian failure (15). Although R-spondin2-null mice died immediately after birth, R-spondin2+/− female mice lost their fertility beginning at about 4 mo of age (16). These findings suggest that R-spondin2 could be important in regulating ovarian follicle development.
METHODS AND MATERIALS
Animals
Female ICR and B6D2F1 mice at different ages were obtained from Harlan Sprague-Dawley (Indianapolis, IN, USA), whereas severe combined immune deficient (SCID) mice were purchased from CLEA Japan (Tokyo, Japan). Animals were housed with a 12-h dark-light cycle and free access to food and water. The mice were treated in accordance with the guidelines of the local Animal Research Committees at Stanford University and Akita University.
To identify ovarian cell types expressing R-spondin2, in situ hybridization analysis was performed as described previously (17). Briefly, [35S]-uridine triphosphate (35S-UTP, PerkinElmer, Wellesley, MA, USA)-labeled antisense and sense probes of mouse R-spondin2 cDNA (GenBank accession no. BC156617.1, nt 542–726) were generated using the Riboprobe In-Vitro Transcription Systems kit (Promega, Madison, WI, USA). Frozen sections of ovaries were cut at 8 μm and incubated with labeled probes (∼5×106 cpm/ml) overnight at 56°C. After hybridization, slides were treated with RNase A at 37°C for 30 min to inactivate nonhybridized probes, followed by washes in descending series of SSC buffer (2×, 1×, 0.5×, and 0.1× SSC) at 65°C for 30 min before dehydration. To visualize the radiolabel, slides were dipped in photographic NTB-2 autoradiographic emulsion (Kodak, Rochester, NY, USA) and stored at 4°C for 10 d. D-19 developer and fixer were used to develop signals, followed by hematoxylin counterstaining.
Real-time RT-PCR analyses
Transcript levels for R-spondin2, diverse Wnt, and Frizzled genes, as well as LGR4–6, were analyzed in oocytes and somatic cells together with those for markers of different cell types. Ovaries from d 10 mice were treated with 0.25% trypsin, 0.1% collagenase I, and 0.02% DNase I for 15 min at 37°C. After adding 1 mM EDTA, ovaries were incubated at 37°C for 30 min before collecting oocytes and remaining somatic cells. Total RNA was extracted using an RNeasy Micro Kit (Qiagen, Valencia, CA, USA), and cDNA was synthesized using a Sensicript RT Kit (Qiagen) according to the manufacturer's protocol. Real-time PCR was performed using iTaq SYBR Green SuperMix (Bio-Rad, Richmond, CA, USA) on a Smart Cycler TD System (Cepheid, Sunnyvale, CA, USA) as follows: 15 min at 95°C and then 45 cycles of 15 s at 95°C and 60 s at 60°C. The primers used are shown in Table 1. Relative abundance of specific genes was normalized to those of β-actin levels.
Table 1.
Primers used
| Gene | Primer set sequences |
|
|---|---|---|
| Primer | Sequence | |
| R-spodin2 | Forward | 5′-GCAGCCGATGTCAACAGAA-3′ |
| Reverse | 5′-ATACCCTGATGGGCAGGAAT-3′ | |
| GDF9 | Forward | 5′-TCTGAACAACTCTGCCTCTTCC-3′ |
| Reverse | 5′-GCTAAACACTCCGTCCTCTGG-3′ | |
| FSHR | Forward | 5′-TCTAACAGGGTCTTCCTCTGC-3′ |
| Reverse | 5′-CTCAGTTCAATGGCGTTCC-3′ | |
| Wnt2 | Forward | 5′-AGAGTGCCAACACCAGTTCC-3′ |
| Reverse | 5′-CCGATTCCCGACTACTTCG-3′ | |
| Wnt2b | Forward | 5′-CGTCCTGGTGGTACATAGGG-3′ |
| Reverse | 5′-TGATGTCTGGGTAGCGTTGAC-3′ | |
| Wnt3a | Forward | 5′-TTGGAGGAATGGTCTCTCG-3′ |
| Reverse | 5′-CCTCATTGTTGTGACGGTTC-3′ | |
| Wnt4 | Forward | 5′-GGAGAACTGGAGAAGTGTGG-3′ |
| Reverse | 5′-AGGACTGTGAGAAGGCTACG-3′ | |
| Wnt5a | Forward | 5′-AAGACAGTGCAGACCGAACG-3′ |
| Reverse | 5′-CACGAACTGATCCACAATCTCC-3′ | |
| Wnt5b | Forward | 5′-GAGTACGGCTACCGCTTTGC-3′ |
| Reverse | 5′-CCAGCCTCGTTGTTCTGTAGG-3′ | |
| FZD1 | Forward | 5′-ACATCGCGTACAACCAGACC-3′ |
| Reverse | 5′-CACTGCACCTTCACCAGAGG-3′ | |
| FZD2 | Forward | 5′-CACACGAACCAGGAAGACG-3′ |
| Reverse | 5′-CATGGAGCACAGGAAGAAGC-3′ | |
| FZD4 | Forward | 5′-GATGCCGATGAACTGACTGG-3′ |
| Reverse | 5′-GAGAGGAGCCACCACAAAGC-3′ | |
| FZD6 | Forward | 5′-AAAGCAGTGTGGTTCCATGC-3′ |
| Reverse | 5′-CGCCGCTAATGTTGTCTCC-3′ | |
| FZD7 | Forward | 5′-AGAGCGACCCATCATCTTCC-3′ |
| Reverse | 5′-GTAGCCATCGTCCGAGAAGC-3′ | |
| FZD8 | Forward | 5′-CGCTGGTGGAGATACAGTGC-3′ |
| Reverse | 5′-TCACACACAGAGCGACAAGG-3′ | |
| FZD9 | Forward | 5′-CACAGAGCAACCATGTACTGC-3′ |
| Reverse | 5′-GAGCATGAAGACAGCCACAG-3′ | |
| LGR4 | Forward | 5′-CATTGATCACGGCAATCTCC-3′ |
| Reverse | 5′-AGGACTGGATTCAGGCAAGC-3′ | |
| LGR5 | Forward | 5′-TGTCACTGTGAGCTGGATGG-3′ |
| Reverse | 5′-GGAGGTGAAGACGCTGAGG-3′ | |
| LGR6 | Forward | 5′-AAGAGGAGGCACCAAAGAGG-3′ |
| Reverse | 5′-TGGCTTTGAGTCCTCTGTCC-3′ | |
| β-Actin | Forward | 5′-GTATCCATGAAATAAGTGGTTACAGG-3′ |
| Reverse | 5′-GCAGTACATAATTTACACAGAAGCAAT-3′ | |
TCF-luciferase assay
Ovaries obtained from d 10 mice were digested with collagenase I, DNase, and trypsin. After centrifugation at 270 g for 10 min, the cell pellet was washed and plated in a 96-well plate (BD Falcon; BD Biosciences, San Jose, CA, USA) in McCoy's 5a medium (Gibco BRL, Carlsbad, CA, USA) containing 4 mg/ml BSA (Sigma-Aldrich, St. Louis, MO, USA), penicillin-streptomycin, and sodium pyruvate for 3 h at 37°C. After washing twice with PBS to remove floating oocytes, somatic cells were cultured overnight at 37°C in McCoy's 5a medium containing 10% FBS (Life Technologies, Gaithersburg, MD, USA) before evaluation of Wnt signaling based on the TCF-luciferase assay (18). Cells were transfected with 1 μg of pTOP Flash or pFOP Flash plasmid together with 0.1 μg of pRL-TK plasmid using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) for 6 h. After culturing in McCoy's 5a medium containing 10% FBS overnight, cells were cultured in serum-free medium for 8 h and treated with different doses of R-spondin2 or Wnt5b, Wnt3a (30 ng/ml), and/or FSH (100 ng/ml) for 18 h. Luciferase activity expressed as the relative light unit was determined using a Dual Luciferase Reporter Assay Kit (Promega) with a luminometer (Bio-Rad). Some cells were also treated with DKK1 (300 ng/ml) to inhibit Wnt signaling.
Ovarian explant cultures
Ovaries from d 10 mice were placed on culture plate insert (Millipore, Bedford, MA, USA) and cultured in 400 μl of DMEM/F12 (Life Technologies) containing 0.1% BSA, 0.1% Albumax II, insulin, transferrin, selenium, l-ascorbic acid, and penicillin-streptomycin under the membrane insert to cover ovaries with a thin layer of medium as described previously (19). Ovaries were treated with Wnt 3a, R-spondin2, and/or FSH with medium changes every 2 d for 4 d. For some groups, explants were also treated with DKK1 or R-spondin2 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) to block R-spondin actions. Explants treated with nonimmune IgG or boiled (100°C, 15 min) R-spondin2 antibodies served as negative controls. At the end of culture, ovaries were fixed in Bouin's solution, paraffin embedded, and sectioned before staining with hematoxylin and eosin. Follicle dynamics was determined by counting follicles at different stages using serial sections as described previously (20).
In vivo treatment with R-spondin1-Fc, in vitro fertilization, and early embryo cultures
Mouse R-spondin1-Fc fusion protein containing a C-terminal mouse IgG2-Fc fragment was purified from the conditioned medium of stably transfected 293T cells using protein A affinity chromatography (21). Recombinant protein levels were determined by Coomassie blue staining and immunoblotting, using the R-spondin1 antibody (R&D Systems, Minneapolis, MN, USA). Wnt signaling activity was estimated based on the TCF luciferase assay in 293T cells. For in vivo studies, R-spondin1-Fc was injected intraperitoneally into d 10 mice (10 μg/d) daily for 5 d, followed by a single injection of eCG (7 IU) and, at 48 h later, with an ovulatory dose (7 IU) of hCG. At 16 h after treatlater, numbers of ovulated oocytes in oviducts were determined. For studies in adult animals, BDF1 mice at 9 wk of age were pretreated s.c. with a gonadotropin-releasing hormone (GnRH) antagonist (1 μg/g body weight; Cetrotide, Shionogi & Co., Tokyo, Japan) daily for 4 d, followed by daily i.p. treatment with saline or R-spondin1-Fc (20 μg/animal/d), together with the GnRH antagonist for another 4 d. Animals were then treated i.p. with a single injection of eCG (5 IU) to stimulate preovulatory follicles and, at 48 h later, with hCG (5 IU) to induce ovulation. At 16 h after treatment, the number of ovulated oocytes was determined. For in vitro fertilization, sperm from BDF1 male mice were collected into human tubal fluid medium (Millipore) and preincubated for 1 h at 37C. Oocytes were fertilized with sperm (2–3×105/ml) for 6 h, and inseminated oocytes were transferred into KSOM-AA medium (Millipore) for development into blastocysts.
Xenografting of human follicles
For checking R-spondin1-Fc effects on human follicles, ovarian cortical fragments were obtained from a 28-yr-old patient who underwent a Caesarean section and was not planning for a future pregnancy. We obtained approval from the Akita University institutional ethics committee and informed consent from the patient to remove 3 small ovarian cortical fragments (5 mm3) from one ovary. Ovarian fragments were dissected into 1-mm3 cubes and xenotransplanted into kidney capsules of SCID mice as described earlier (20). At the day after transplantation, animals were treated with saline or R-spondin1-Fc (20 μg/animal/d) for 7 d before dissection and fixing of ovarian grafts with Bouin's solution for histological analyses.
Statistical analyses
Results are presented as means ± se of ≥3 independent determinations. Statistical significance was determined by using the ANOVA test followed by Fisher's protected least significant difference, with P < 0.05 being statistically significant.
RESULTS
Expression of R-spondin2 in oocytes of primary and larger follicles
As shown in Fig. 1A, ovaries from neonatal mice at d 3 of age contained multiple primordial follicles in the ovarian cortex and some primary follicles migrated into the medulla region (Fig. 1Aa). In situ hybridization analyses showed R-spondin2 mRNA signals in oocytes of primary follicles but not in cortical primordial follicles (Fig. 1Ab). In ovaries from juvenile mice at d 15 of age, follicles at primary, secondary, and early antral stages were present (Fig. 1Ac), and R-spondin2 signals were found in oocytes of primary and larger follicles (Fig. 1Ad). In ovaries of prepubertal mice (d 23 of age), the largest follicles reached the antral stage (Fig. 1Ae). Again, R-spondin2 expression was found in oocytes of primary and larger follicles (Fig. 1Af). No signals were found for sections with the sense probes (data not shown).
Figure 1.
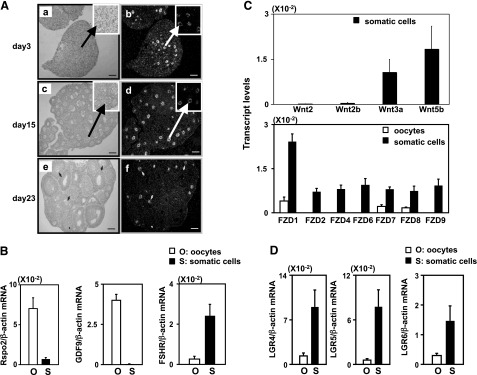
Expression of R-spondin2 together with different Wnt ligands, as well as Frizzled and LGR receptors, in different ovarian cell types. A) In situ hybridization analyses of R-spondin2 transcripts in ovaries of neonatal (d 3, a, b), juvenile (d 15, c, d), and prepubertal (d 23, e, f) mice were analyzed. a, c, e) Bright-field images. b, d, f) Dark-field images. Scale bars = 100 μm. Arrowheads indicate oocytes. B) Real-time RT-PCR analyses of R-spondin2 transcripts in isolated oocytes and somatic cells of preantral follicles. Ovaries from immature mice at 10 d of age containing secondary and smaller follicles were dissociated to obtain oocytes and somatic cells. The purity of these cells was confirmed using specific markers (GDF9 for oocyte, FSH receptor for somatic cells). For all Wnt genes, negligible expression was found in oocytes. For FZD genes, negligible expression was found for FZD2, 4, 6, and 9. n = 6. C) Real-time RT-PCR analyses of different Wingless (Wnt) and Frizzled (FZD) gene transcripts in oocytes and somatic cells; n = 6. D) Real-time RT-PCR analyses of LGR4–6 in somatic cells and oocytes of ovaries from d 10 mice; n = 6.
In ovaries of mice at d 10 of age, real-time RT-PCR analyses indicated the expression of R-spondin2 transcripts in oocytes with minimal levels in somatic cells (Fig. 1B). Transcripts for GDF9, an oocyte marker, were detected only in oocytes, whereas those for FSH receptor were found in somatic cells. As shown in Fig. 1C (top panel), transcript levels for Wnt2, 2b, 3a, and 5b were found in somatic cells but undetectable in oocytes. For Fizzled receptors (Fig. 1C, bottom panel), FZD2, 4, 6, and 9 transcripts were found only in somatic cells, whereas FZD1, 7, and 8 were found in both oocytes and somatic cells with higher levels in somatic cells. Furthermore, transcripts for LGR4–6, receptors for R-spondin proteins (12, 22), were found in somatic cells with lower levels in oocytes (Fig. 1D).
Treatment with R-spondin2 stimulated the canonical Wnt signaling pathway and promoted early follicle growth in ovarian explant cultures
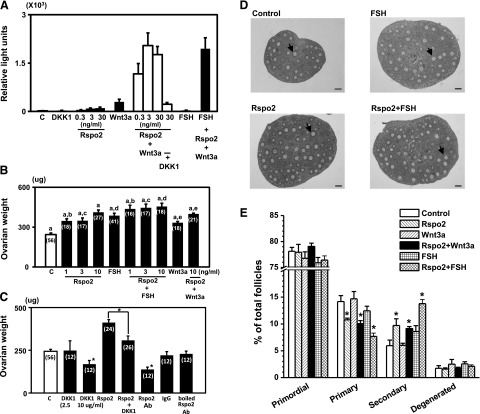
Somatic cells obtained from ovaries of d 10 mice were transfected with TCF-luciferase reporter plasmids before treatment with R-spondin2, Wnt3a, and/or FSH for 18 h. Luciferase activity was measured as an index of Wnt signaling. As shown in Fig. 2A, treatment with R-spondin2 or Wnt3a alone led to minimal stimulation of the TCF-luciferase activity, whereas treatment with FSH was ineffective. In the presence of both Wnt3a and R-spondin2, synergistic increases in TCF-luciferase activity were evident. In contrast to Wnt3a, treatment with Wnt5b showed less stimulation of luciferase activity even when R-spondin2 was included (Supplemental Fig. S1). Although cotreatment with R-spondin2 and FSH did not further increase luciferase activity, treatment with DKK1, an antagonist for Wnt signaling, blocked the stimulatory effects of R-spondin2 and Wnt3a. Also, cotreatment with FSH, together with R-spondin2 and Wnt3a, did not lead to further increases as compared with cells treated with R-spondin2 and Wnt3a. These findings suggest the functionality of the Wnt signaling pathway in ovarian cells, mediated by cognate receptors for R-spondin and Wnt3a together with downstream signaling molecules.
Figure 2.
Treatment with R-spondin2 and Wnt3a stimulated the canonical Wnt signaling pathway and preantral follicle growth in vitro. A) Synergistic stimulation of TCF-luciferase reporter activity by R-spondin2 and Wnt3a in cultured somatic cells obtained from ovaries of mice at 10 d of age. Cells were transfected with the TCF-luciferase reporter plasmid for 6 h before treatment with different reagents for 18 h: DKK1, 300 ng/ml; Wnt3a, 30 ng/ml; FSH, 100 ng/ml. C, control; Rspo2, R-spondin2. n = 9. B) Ovarian weight increases after treatment with increasing doses of R-spondin2 and/or FSH plus Wnt3a in vitro. Explant cultures of individual ovaries from mice at d 10 of age were incubated with different reagents for 4 d, with medium changes at d 2 of culture. Numbers in parentheses indicate the number of ovaries used. Bars with the same letter differ significantly (P<0.05). C) Antagonistic effects of DKK1 and R-spondin2 antibodies on basal and R-spondin2-stimulated ovarian weight. Ovarian explants were treated with different reagents for 4 d. Treatment with nonimmune IgG (500 ng/ml) and boiled R-spondin2 antibodies (500 ng/ml) served as controls. Ab, antibodies; R-spo2, R-spondin2. n = 9–18. *P < 0.05 vs. control or as indicated. D) Histology of ovaries treated with R-spondin2 and/or FSH. Arrowheads indicate secondary follicles. Scale bars = 100 μm. E) Follicle dynamics following treatment with R-spondin2, FSH, and Wnt3a. Serial sections of ovarian explants were used for detailed counting of follicles at different developmental stages. R-spondin2, 10 ng/ml; FSH, 25 ng/ml; Wnt3a, 3 ng/ml. n = 5–10. *P < 0.05 vs. control.
Ovaries from d 10 mice were cultured with different factors for 4 d with medium changes at d 2 of culture. As shown in Fig. 2B, treatment of ovarian explants with R-spondin 2 increased ovarian weight in a dose-dependent manner, with further increases when both R-spondin2 and FSH were added together. In contrast, cotreatment with Wnt3a and R-spondin2 did not further increase R-spondin2-stimulated weight gain, suggesting actions of endogenous Wnt factors. Furthermore, cotreatment with the antagonist DKK1 inhibited basal and R-spondin2-stimulated ovarian weight gain (Fig. 2C). To neutralize endogenous R-spondin2 actions, some ovarian explants were incubated with affinity-purified R-spondin2 antibodies (Fig. 2C). Treatment with R-spondin2 antibodies decreased basal ovarian weights, whereas treatment with nonimmune IgG or boiled R-spondin2 antibodies were ineffective, suggesting a role of endogenous R-spondin2 in follicle growth. Morphological analyses demonstrated the promotion of ovarian follicle development following treatment with either R-spondin2 and/or FSH (Fig. 2D). Histological analyses were performed using serial sections to evaluate follicles at different developmental stages. Although negligible changes in ratios of primordial and degenerated follicles were detected after treatment with R-spondin2, we found decreases in the percentage of primary follicles accompanied by increases in the percentage of secondary follicles (Fig. 2E). In contrast, FSH or Wnt3a treatment did not affect primordial, primary, or secondary follicle numbers but promoted early secondary follicles to the late secondary stage (data not shown). When explants were treated with either Wnt3a or FSH, together with R-spondin2 also stimulated the growth of primary follicles to the secondary stage.
In vivo treatment with R-spondin1-Fc promoted follicle development to allow gonadotropin stimulation of ovulation and early embryonic development
Because R-spondin1–3 are equipotent in stimulating the canonical Wnt signaling pathway (14), we used R-spondin1-Fc for in vivo studies. A chimeric R-spondin1-Fc protein was generated by fusing the C-terminal end of mouse R-spondin1 cDNA with the Fc fragment of IgG2 to facilitate secretion (21). After plasmid transfection and selection of 293T cell lines, secreted R-spondin1-Fc protein was purified and tested for its ability to stimulate Wnt signaling. As shown in Fig. 3A (left panel), a single band of purified R-spondin1-Fc could be detected, and treatment with increasing doses of R-spondin1-Fc, when added together with Wnt3a, stimulated TCF-luciferase activity in a dose-dependent manner (Fig. 3A, right panel). The potency of chimeric R-spondin1 is similar to that of recombinant human R-spondin2.
Figure 3.
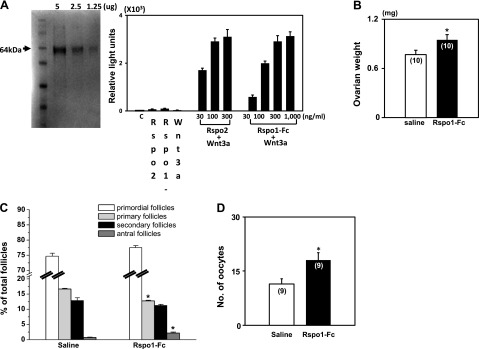
Treatment with R-Spondin1-Fc stimulated the development of primary follicles in immature mice to the antral stage. A) Purification of recombinant R-spondin1-Fc and its stimulation of the canonical Wnt signaling pathway. Left panel: Coomassie blue staining of R-spondin1-Fc. Right panel: TCF-luciferase assay. 293T cells were transfected with TCF-luciferase reporter plasmids before treatment with different doses of R-spondin2 or R-spondin1-Fc. n = 5. For testing the in vivo actions of R-spondin 1-Fc, prepubertal mice at d 10 of age were injected ip with R-spondin1-Fc (10 μg/d) or saline daily for 5 d for ovarian weight determination and follicle counting. Some animals were further treated with eCG (7 IU) for 48 h, followed by a single dose (7 IU) of hCG to induce ovulation. B) Increases in ovarian weight after treatment with R-spondin1-Fc. C) Follicle dynamics after R-spondin1-Fc treatment in vivo; n = 5–6. D) Ovulation efficiency. *P < 0.05 vs. control.
To test whether R-spondin could promote follicle development in vivo, prepubertal mice at d 10 of age were injected i.p. with R-spondin1-Fc (10 μg/d) daily for 5 d. As shown in Fig. 3B, increases in ovarian weight were found after R-spondin1-Fc treatment. Analyses of follicle dynamics indicated decreases in primary follicles, accompanied by increases in antral follicles (Fig. 3C). Some of these animals were further treated with eCG for 48 h, followed by an ovulating dose (7 IU) of hCG, before evaluating the number of ovulated eggs in oviducts 16 h later. As shown in Fig. 3D, the number of ovulated mature eggs retrieved was 58% higher in R-spondin1-Fc-pretreated group as compared with the controls. These data demonstrated the ability of R-spondin1-Fc to promote the development of primary follicles, leading to the formation of antral follicles capable of responding to gonadotropins to generate mature oocytes.
To further demonstrate the stimulatory effects of the R-spondin agonist in adult animals, mice at 9–10 wk of age were treated with a GnRH antagonist (1 μg/g body weight) daily for 4 d. After pretreatment with the GnRH antagonist, animals were treated with R-spondin1-Fc or saline for another 4 d. As shown in Fig. 4A, ovarian weight was increased after R-spondin1-Fc treatment. Follicle counting showed growth of primary follicles to the antral stage (Fig. 4B). Some animals were further treated with eCG to induce the final stage of follicle maturation. After 2 d, animals were injected with a single dose of hCG to induce ovulation. As shown in Fig. 4C, we retrieved 74% more mature oocytes from oviducts of R-spondin1-Fc-pretreated mice. Subsequent in vitro fertilization of ovulated oocytes and culturing of early embryos indicated that similar percentages of oocytes obtained from control and R-spondin1-Fc-pretreated animals progressed to the blastocyst stage (Fig. 4D). After mating with adult males, viable pups were delivered from mice pretreated with R-spondin1-Fc.
Figure 4.
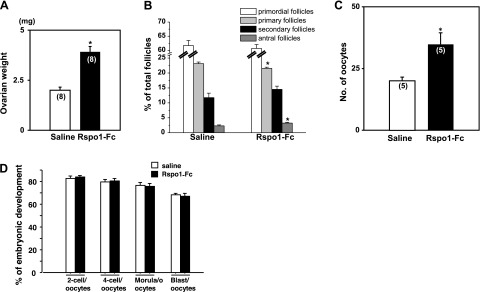
Treatment with R-Spondin1-Fc stimulated follicle development in adult mice to generate mature oocytes for fertilization and early embryonic development. Adult mice were treated with a GnRH antagonist (1 μg/g body weight) for 4 d, followed by treatment with the GnRH antagonist together with R-spondin1-Fc (20 μg/d) or saline for another 4 d. Ovaries were obtained for weighing, followed by serial sectioning and follicle counting. Some animals pretreated with R-spondin1-Fc or saline were further treated with eCG for 48 h, followed by hCG to induce ovulation and oocyte maturation. At 16 h after hCG injection, numbers of ovulated mature oocyte in oviducts were determined. Ovulated oocytes were inseminated with sperm in vitro, and early embryonic development was monitored daily for 4 d. Percentages of mature oocytes developed into different stages of early embryos were determined. A) Increases in ovarian weight after treatment with R-spondin1-Fc. B) Follicle dynamics after R-spondin1-Fc treatment in vivo; n = 5–6. C) Treatment with R-spondin1-Fc increased ovulation efficiency. D) Development of early embryos after in vitro fertilization of mature oocytes; n = 70–150. *P < 0.05 vs. control.
Treatment with an R-spondin agonist promoted human early follicle development
To test the ability of R-spondin to stimulate human follicle development, we obtained ovarian cortical fragments from a patient with informed consent. Ovarian fragments were dissected into 1-mm3 cubes and xenotransplanted into kidney capsules of SCID mice. At the day after transplantation, animals were treated with saline or R-spondin1-Fc (20 μg/animal/d) for 7 d. As shown in Fig. 5A, secondary follicles were found in grafts from R-spondin1-Fc-treated hosts, whereas many primary follicles were found in the saline-treated group. Detailed counting of follicles at different stages indicated minimal loss of total follicles after grafting in SCID mice when comparing groups before and after xenografting. Although comparable decreases in primordial follicles were found in both saline and R-spondin1-Fc-treated groups, treatment with R-spondin1-Fc promoted the development of primary follicles to the secondary stage (Fig. 5B).
Figure 5.
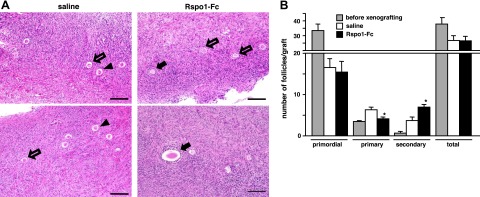
R-spondin treatment promoted human early follicle development. Human cortical cubes were xenotransplanted into kidney capsules of SCID mice. Animals were treated with vehicle (saline) or R-spondin1-Fc (Rspo1-Fc: 20 μg/animal/d) for 7 d. Follicle dynamics were determined in serial sections of ovarian grafts before and after treatments. A) Morphology of grafts after 7 d treatment with saline or R-spondin1-Fc. Solid arrows indicate secondary follicles; open arrows indicate primary follicles; arrowheads indicate primordial follicles. Scale bars = 50 μm. B) Follicle dynamics before and after treatment with saline or R-spondin1-Fc; n = 4–5. *P < 0.05 vs. control.
DISCUSSION
Our data indicated that R-spondin2 is an oocyte-expressed gene capable of promoting granulosa cell proliferation through the canonical Wnt signaling pathway. A strong synergism was found for oocyte-derived R-sponind2 and granulosa cell-derived Wnt ligands in the stimulation of the canonical β-catenin pathway, leading to the promotion of primary to secondary and antral follicles. These data underscore the important role of oocytes in controlling the fate of a given follicle. It is clear that the development of ovarian follicles is dependent on both gonadotropins produced by the anterior pituitary and local factors secreted by the oocyte. Observed promotion of human follicle development by an R-spondin agonist underscores potential clinical importance of these findings.
The exclusive localization of R-spondin2 in the oocyte is supported by in situ hybridization and RT-PCR analyses. R-spondin2 transcripts were not found in primordial follicles but expressed in oocytes of primary and large follicles until the preovulatory stage. RT-PCR analyses further indicated negligible R-spondin2 expression in somatic cells. Once the dormant follicles initiate growth, oocytes of growing follicles produce R-spondin2 to promote the proliferation of surrounding somatic cells. Consistent with our findings of multiple Wnt ligands and Frizzled receptors in somatic cells, earlier studies demonstrated the expression of Wnt2 (5) and Frizzled4 (23) in granulosa cells throughout follicle development. We also demonstrated the expression of LGR4–6 transcripts in ovarian somatic cells, consistent with recent findings showing R-spondin proteins as ligands for LGR4–6 (12, 13) and earlier studies showing ovarian expression of LGR4 (24, 25).
Optimal activation of the canonical Wnt signaling in somatic cells requires synergistic stimulation by oocyte-derived R-spondin2 and somatic cell-derived Wnt proteins. The Wnt antagonist DKK1 prevents Wnt signaling by binding to the coreceptors LRP6 and Kremen1, leading to their internalization (26). Suppressive effects of DKK1 observed in ovarian explant cultures indicated that promotion of follicle growth by R-spondin2 is mediated through the canonical Wnt pathway. Although FSH treatment in vivo also stimulates the development of preantral follicles (27), R-spondin and FSH likely act through independent pathways, because FSH does not stimulate the TCF-luciferase reporter. The important role of Wnt signaling in ovarian somatic cell proliferation and follicle growth observed here is consistent with reported suppression of follicle atresia found in transgenic mice overexpressing a dominant stable β-catenin mutant in granulosa cells (9). Likewise, knockdown of WNT2 expression in rodent granulosa cells using transfected siRNA decreased DNA synthesis, whereas WNT2 overexpression using a viral vector enhanced it (8). Observed decreases in ovarian weight gain following neutralization of endogenous R-spondin2 using specific antibodies further underscored the important role of endogenous oocyte-derived R-spondin2 in follicle development. Although multiple primordial follicles are initiated to start growth, only a few follicles developed to the preovulatory stage to release a mature oocyte. Because all follicles are under the influence of circulating FSH, follicles with optimal R-spondin2 expression in their oocyte likely become dominant and successfully release a competent egg.
The role of R-spondin2 in follicle development is supported by transgenic mouse studies. Footless mutant mice with a transgene insertion in the R-spondin2 gene (15) showed malformations of the limbs, kidney, and soft palate. Although Footless heterozygous animals were indistinguishable from wild-type animals through weaning, female animals were only fertile until 4 mo of age. Likewise, heterozygous R-spondin2 mutant female mice were 25% sterile at 4 mo and 85% sterile at >5 mo (16). The infertile phenotypes of these mutant mice resembled those found in patients with premature ovarian failure. It is likely that the initial waves of follicle growth were maintained in R-spondin2 heterozygous mutants, but inappropriate levels of R-sponind2 in oocytes eventually led to the failure of follicle development during late reproductive life. Of interest, mutations of the oocyte-specific homeobox gene Nobox were also associated with premature ovarian failure in mutant mice (28) and patients (29). In Nobox-null mice, ovarian R-spondin2 expression was decreased (30). It is, thus, of interest to evaluate the role of R-spondin2 in patients with premature ovarian failure. As an adult stem cell factor, R-spondin2 was also found to be involved in coat variation in domestic dogs (31), and in colorectal tumorigenesis (32).
We demonstrated the ability of R-spondin to promote follicle development in juvenile mice as well as in adult animals treated with a GnRH antagonist to suppress endogenous gonadotropin levels. At d 10 of age in mice, most advanced follicles reached the secondary stage and administration with an R-spondin agonist promoted the progression of primary follicles to the antral stage. Following gonadotropin stimulation, more mature oocytes were generated in animals pretreated with R-spondin1-Fc. In adult mice treated with the GnRH antagonist, administration of R-spondin1-Fc also increased the number of mature oocytes capable of undergoing fertilization and embryonic development, leading to successful pregnancy and delivery of viable pups. For these experiments, R-spondin1-Fc treatments were discontinued at the penultimate stage of follicle maturation during eCG and hCG injections because repressive effects of Wnt signaling on LH-stimulated ovulation were found in mutant mice overexpressing a dominant stable β-catenin gene in granulosa cells (9).
Although FSH treatment is widely used for infertility treatment, a subgroup of patients (FSH low responders) showed minimal responses to the gonadotropin (33). These patients exhibit low antral follicle count and elevated serum FSH and anti-Mullerian hormone levels at d 3 of their menstrual cycle. Although high doses of gonadotropins or adjuvant therapy with growth hormone or growth hormone-releasing hormone have been used, minimal benefit is evident (34). Despite the unknown etiologies for FSH low responders (33), our findings demonstrated that pretreatment with R-spondin could promote the development of human early follicles in a xenotransplantation model without FSH treatment. Recent studies indicated that R-spondin1 treatment ameliorates experimental colitis in mice (35) and protects mice from chemotherapy or radiation-induced oral mucositis (36) by stimulating adult stem cell proliferation. Future studies are needed to investigate whether R-spondin agonists could facilitate preantral follicle growth in FSH low responders to provide a potential therapy. Because R-spondin treatment reduces the number of primary follicles and causes reversible hyperplasia in gastrointestinal and oral tissues, the dosage and duration of R-spondin treatment for infertile patients requires further assessment.
Supplementary Material
Acknowledgments
This research was supported by funds from the U.S. National Institutes of Health National Institute of Child Health and Human Development (A.H.; R21 HD071259 and U54 HD068158 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research), the Japan Ministry of Education, Culture, Sports, Science, and Technology (K.K.; Grant-in-Aid for Scientific Research on Priority Area, The Germline: Its Developmental Cycle and Epigenome Network; 23013004 and B: 24390376), the Naito Foundation (K.K.), the Uehara Memorial Foundation (K.K.), and the Mochida Memorial Foundation for Medical and Pharmaceutical Research (K.K.).
The authors thank Dr. Nihar Nayak (Stanford University) for help with the in situ hybridization procedure.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- FSH
- follicle-stimulating hormone
- GnRH
- gonadotropin-releasing hormone
- LGR
- leucine-rich repeat-containing G-protein-coupled receptor
- LRP
- lipoprotein-related receptor
- SCID
- severe combined immune deficient
- TCF
- T-cell factor
- Wnt
- wingless
REFERENCES
- 1. Wodarz A., Nusse R. (1998) Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59–88 [DOI] [PubMed] [Google Scholar]
- 2. Kawano Y., Kypta R. (2003) Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116, 2627–2634 [DOI] [PubMed] [Google Scholar]
- 3. Mao J., Wang J., Liu B., Pan W., Farr G. H., 3rd, Flynn C., Yuan H., Takada S., Kimelman D., Li L., Wu D. (2001) Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7, 801–809 [DOI] [PubMed] [Google Scholar]
- 4. Hsieh M., Johnson M. A., Greenberg N. M., Richards J. S. (2002) Regulated expression of Wnts and Frizzleds at specific stages of follicular development in the rodent ovary. Endocrinology 143, 898–908 [DOI] [PubMed] [Google Scholar]
- 5. Ricken A., Lochhead P., Kontogiannea M., Farookhi R. (2002) Wnt signaling in the ovary: identification and compartmentalized expression of wnt-2, wnt-2b, and frizzled-4 mRNAs. Endocrinology 143, 2741–2749 [DOI] [PubMed] [Google Scholar]
- 6. Harwood B. N., Cross S. K., Radford E. E., Haac B. E., De Vries W. N. (2008) Members of the WNT signaling pathways are widely expressed in mouse ovaries, oocytes, and cleavage stage embryos. Dev. Dyn. 237, 1099–1111 [DOI] [PubMed] [Google Scholar]
- 7. Wang H. X., Tekpetey F. R., Kidder G. M. (2009) Identification of WNT/beta-CATENIN signaling pathway components in human cumulus cells. Mol. Hum. Reprod. 15, 11–17 [DOI] [PubMed] [Google Scholar]
- 8. Wang H. X., Li T. Y., Kidder G. M. (2010) WNT2 regulates DNA synthesis in mouse granulosa cells through beta-catenin. Biol. Reprod. 82, 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fan H. Y., O'Connor A., Shitanaka M., Shimada M., Liu Z., Richards J. S. (2010) Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol. Endocrinol. 24, 1529–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamata T., Katsube K., Michikawa M., Yamada M., Takada S., Mizusawa H. (2004) R-spondin, a novel gene with thrombospondin type 1 domain, was expressed in the dorsal neural tube and affected in Wnts mutants. Biochim. Biophys. Acta 1676, 51–62 [DOI] [PubMed] [Google Scholar]
- 11. Kim K. A., Kakitani M., Zhao J., Oshima T., Tang T., Binnerts M., Liu Y., Boyle B., Park E., Emtage P., Funk W. D., Tomizuka K. (2005) Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309, 1256–1259 [DOI] [PubMed] [Google Scholar]
- 12. Carmon K. S., Gong X., Lin Q., Thomas A., Liu Q. (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. U. S. A. 108, 11452–11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Lau W., Barker N., Low T. Y., Koo B. K., Li V. S., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M., Stange D. E., van Es J. E., Guardavaccaro D., Schasfoort R. B., Mohri Y., Nishimori K., Mohammed S., Heck A. J., Clevers H. (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 [DOI] [PubMed] [Google Scholar]
- 14. Kim K. A., Wagle M., Tran K., Zhan X., Dixon M. A., Liu S., Gros D., Korver W., Yonkovich S., Tomasevic N., Binnerts M., Abo A. (2008) R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 19, 2588–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bell S. M., Schreiner C. M., Hess K. A., Anderson K. P., Scott W. J. (2003) Asymmetric limb malformations in a new transgene insertional mutant, footless. Mech. Dev. 120, 597–605 [DOI] [PubMed] [Google Scholar]
- 16. Nam J. S., Park E., Turcotte T. J., Palencia S., Zhan X., Lee J., Yun K., Funk W. D., Yoon J. K. (2007) Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev. Biol. 311, 124–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nayak N. R., Brenner R. M. (2002) Vascular proliferation and vascular endothelial growth factor expression in the rhesus macaque endometrium. J. Clin. Endocrinol. Metab. 87, 1845–1855 [DOI] [PubMed] [Google Scholar]
- 18. Caricasole A., Ferraro T., Iacovelli L., Barletta E., Caruso A., Melchiorri D., Terstappen G. C., Nicoletti F. (2003) Functional characterization of WNT7A signaling in PC12 cells: interaction with A FZD5 x LRP6 receptor complex and modulation by Dickkopf proteins. J. Biol. Chem. 278, 37024–37031 [DOI] [PubMed] [Google Scholar]
- 19. Sato Y., Cheng Y., Kawamura K., Takae S., Hsueh A. J. (2012) C-type natriuretic peptide stimulates ovarian follicle development. Mol. Endocrinol. 26, 1158–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J., Kawamura K., Cheng Y., Liu S., Klein C., Duan E. K., Hsueh A. J. (2010) Activation of dormant ovarian follicles to generate mature eggs. Proc. Natl. Acad. Sci. U. S. A. 107, 10280–10284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ootani A., Li X., Sangiorgi E., Ho Q. T., Ueno H., Toda S., Sugihara H., Fujimoto K., Weissman I. L., Capecchi M. R., Kuo C. J. (2009) Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Lau W., Barker N., Low T. Y., Koo B. K., Li V. S., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M., Stange D. E., van Es J., Guardavaccaro D., Schasfoort R. B., Mohri Y., Nishimori K., Mohammed S., Heck A. J., Clevers H. (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 [DOI] [PubMed] [Google Scholar]
- 23. Hsieh M., Boerboom D., Shimada M., Lo Y., Parlow A. F., Luhmann U. F., Berger W., Richards J. S. (2005) Mice null for Frizzled4 (Fzd4−/−) are infertile and exhibit impaired corpora lutea formation and function. Biol. Reprod. 73, 1135–1146 [DOI] [PubMed] [Google Scholar]
- 24. Hsu S. Y., Liang S. G., Hsueh A. J. (1998) Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol. Endocrinol. 12, 1830–1845 [DOI] [PubMed] [Google Scholar]
- 25. Van Schoore G., Mendive F., Pochet R., Vassart G. (2005) Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochem. Cell Biol. 124, 35–50 [DOI] [PubMed] [Google Scholar]
- 26. MacDonald B. T., Tamai K., He X. (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGee E. A., Perlas E., LaPolt P. S., Tsafriri A., Hsueh A. J. (1997) Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol. Reprod. 57, 990–998 [DOI] [PubMed] [Google Scholar]
- 28. Rajkovic A., Pangas S. A., Ballow D., Suzumori N., Matzuk M. M. (2004) NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 305, 1157–1159 [DOI] [PubMed] [Google Scholar]
- 29. Qin Y., Choi Y., Zhao H., Simpson J. L., Chen Z. J., Rajkovic A. (2007) NOBOX homeobox mutation causes premature ovarian failure. Am. J. Hum. Genet. 81, 576–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi Y., Qin Y., Berger M. F., Ballow D. J., Bulyk M. L., Rajkovic A. (2007) Microarray analyses of newborn mouse ovaries lacking Nobox. Biol. Reprod. 77, 312–319 [DOI] [PubMed] [Google Scholar]
- 31. Cadieu E., Neff M. W., Quignon P., Walsh K., Chase K., Parker H. G., Vonholdt B. M., Rhue A., Boyko A., Byers A., Wong A., Mosher D. S., Elkahloun A. G., Spady T. C., Andre C., Lark K. G., Cargill M., Bustamante C. D., Wayne R. K., Ostrander E. A. (2009) Coat variation in the domestic dog is governed by variants in three genes. Science 326, 150–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Starr T. K., Allaei R., Silverstein K. A., Staggs R. A., Sarver A. L., Bergemann T. L., Gupta M., O'Sullivan M. G., Matise I., Dupuy A. J., Collier L. S., Powers S., Oberg A. L., Asmann Y. W., Thibodeau S. N., Tessarollo L., Copeland N. G., Jenkins N. A., Cormier R. T., Largaespada D. A. (2009) A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science 323, 1747–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fauser B. C., Diedrich K., Devroey P. (2008) Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum. Reprod. Update 14, 1–14 [DOI] [PubMed] [Google Scholar]
- 34. Tarlatzis B. C., Zepiridis L., Grimbizis G., Bontis J. (2003) Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum. Reprod. Update 9, 61–76 [DOI] [PubMed] [Google Scholar]
- 35. Zhao J., de Vera J., Narushima S., Beck E. X., Palencia S., Shinkawa P., Kim K. A., Liu Y., Levy M. D., Berg D. J., Abo A., Funk W. D. (2007) R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology 132, 1331–1343 [DOI] [PubMed] [Google Scholar]
- 36. Zhao J., Kim K. A., De Vera J., Palencia S., Wagle M., Abo A. (2009) R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/beta-catenin pathway. Proc. Natl. Acad. Sci. U. S. A. 106, 2331–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.