Abstract
Interleukin-10 (IL-10) plays an important role in a host's defense against human papillomavirus (HPV) infection. IL-10 promoter variants may affect its expression level or functional efficiency and, subsequently, susceptibility to and survival of HPV16-associated squamous cell carcinoma of oropharynx (SCCOP). We determined tumor HPV16 DNA and genotyped three IL-10 promoter polymorphisms in 309 incident patients with SCCOP. Compared with the patients with corresponding common homozygous genotypes, patients carrying variant genotypes of IL-10 rs1800871 and rs1800872 were ∼2.5 times more likely to have HPV16+ tumors among patients with SCCOP. Among HPV16+ patients with SCCOP only, compared to those with the corresponding variant genotypes, the patients with IL-10 rs1800871 and rs1800872 CC genotypes had significantly better survival and ∼70–80% reduced risk of death/recurrence after multivariable adjustment. Additionally, functional relevance of these variants was characterized to explore the genotype-phenotype correlation. Our findings indicate that IL-10 genetic variants may be associated with tumor HPV16+ SCCOP and predict survival of HPV16+ patients with SCCOP. Larger studies are needed to validate our findings.—Jin, L., Sturgis, E. M., Cao, X., Song, X., Salahuddin, T., Wei, Q., Li, G. Interleukin-10 promoter variants predict HPV-positive tumors and survival of squamous cell carcinoma of the oropharynx.
Keywords: IL-10 variants, HPV, oropharyngeal cancer, smoking, biomarkers, survival
Squamous cell carcinoma of the head and neck (SCCHN) is estimated at 52,600 new cases and 11,500 deaths in the United States in 2012, and is characterized by moderately low survival rates, a high recurrence rate, and a high rate of second primary malignancy (1, 2). Despite the recent declines in U.S. smoking rates and accompanying decline in overall SCCHN rates, the incidence of squamous cell carcinoma of the oropharynx (SCCOP), a subset of SCCHN, continues to increase, particularly in young adults (3).
The growing incidence of SCCOP may be attributed to viral infection, as human papillomavirus (HPV) has been established as an etiologic risk factor for SCCOP, with 45–100% of these cases likely to be HPV+ (1, 4–6). Among the known types of HPV, HPV16 is the most common high-risk type of HPV in SCCOP, accounting for 90–95% of all HPV+ SCCOP (4, 6–8). Although SCCOP is strongly associated with HPV infection, only a small proportion of individuals with this risk factor develop SCCOP. It is suggested that genetic susceptibility may play a role in interindividual variations in development of HPV-associated SCCOP (8). Most studies found that HPV+ patients with SCCOP had improved survival and lower recurrence rates (5, 9, 10), while the significant heterogeneity in outcomes among HPV+ patients with SCCOP remains a clinical problem: most do exceptionally well, but smokers with HPV+ SCCOP have worse outcomes, in some studies similar to those of patients with HPV− SCCOP. So far, SCCOP have few prognostic markers targetable for improving prevention and treatment strategies. Since HPV status is highly relevant to SCCOP prognosis, and there is significant heterogeneity in outcomes of HPV+ patients with SCCOP, new biomarkers that determine HPV status and further stratify HPV+ patients with SCCOP will help avoid both overtreatment and undertreatment of SCCOP, resulting in better survival and quality of life for patients with this disease.
The host immune responses and chronic inflammation have been shown to be biologically important risk factors for HPV-related carcinogenesis, and increased duration of persistent HPV infection may be influential in determining disease development and outcome, indicating the importance of the host immune response to HPV clearance and HPV-related carcinogenesis. Cytokines are a group of host immune products involved in inflammation, immunity, defense against HPV infection, and modulation of HPV clearance. As interleukin-10 (IL-10) is an important and pleiotropic cytokine that plays a critical role in immune regulation through prominent anti-inflammatory and immunoregulatory activities, (11) its genetic variants may affect the host immune system and HPV infection and, subsequently, the HPV status and prognosis of patients with SCCOP.
Several common polymorphisms of IL-10 promoter have been identified, including rs1800896 (A→G), rs1800871 (C→T), and rs1800872 (C→A) (12). These polymorphisms may affect the expression level and function of IL-10, as well as HPV clearance (13–15). As yet, the association between IL-10 variants and HPV+ tumors and survival among patients with SCCOP has not been reported. Therefore, in this study, we aimed to evaluate whether IL-10 genetic variants in the promoter affect susceptibility to and survival of HPV16+ patients with SCCOP.
MATERIALS AND METHODS
Study subjects
A total of 309 incident patients with SCCOP with tumor tissue specimens were consecutively recruited, without restrictions on age, sex, ethnicity, or clinical stage, as a part of an ongoing molecular epidemiological study at The University of Texas M. D. Anderson Cancer Center. These patients were newly diagnosed and previously untreated and had histopathologically confirmed SCCOP. The patients completed an Institutional Review Board-approved informed consent form before they were enrolled. The details of the subject recruitment have been previously described (16). All patients donated 30 ml of blood for IL-10 genotyping. Medical record review for follow-up status was performed under direct supervision of the senior author and staff head and neck surgeon. Primary tumor subsite, clinical stage, treatment, recurrence-free status, specific disease, and overall survival were reviewed from medical records as assessed between the initial and final patient contact recorded and medical comorbidites. All patients included in survival analyses were treated with chemoradiation for curative intent at our institution.
Tumor HPV16 determination
Paraffin-embedded tissues were tested for HPV16 DNA using polymerase chain reaction (PCR)-based, type-specific assays with modification and quality control for the E6 and E7 regions (17). Assays of the samples were run in triplicate, with positive and negative controls (Siha and TPC-1 cell lines, respectively). β-Actin was used as a DNA quality control. Specificity for HPV16 E6 and E7 was confirmed by Southern blot analysis of paraffin-embedded tissue samples using a Roche Diagnostics labeling and hybridization system (Roche Applied Science, Indianapolis, IN, USA; ref. 5). HPV16 E6 and E7 specificity was confirmed by retesting 10% of the samples using restriction digestion of the PCR products with BanII and MspI to verify the presence of E6- and E7-specific fragments. The results of both methods were 100% concordant (18).
IL-10 genotyping
Leukocyte cell pellets of blood samples were used to genotype for IL-10 polymorphisms by PCR-restriction fragment length polymorphism analysis. For IL-10 rs1800871, the 110-bp PCR products were digested with SspI (New England BioLabs, Beverly, MA, USA) at 37°C overnight; the cytosine (C) allele was uncut, and the thymine (T) allele was cut into 86- and 24-bp bands. For IL-10 rs1800872, 300-bp PCR products were digested with RsaI (New England BioLabs) at 37°C overnight; the C allele was uncut, and the adenine (A) allele was cut into 211- and 89-bp bands. For IL-10 rs1800896, 80-bp PCR products were digested with Bst BI (New England BioLabs) at 37°C overnight; the guanine (G) allele was uncut, and the A allele was cut into 58- and 22-bp bands. At least 10% of the samples were randomly selected for retesting and provided 100% concordant results.
Serum IL-10 assessment
Plasma was stored at −80°C until use. Plasma levels of IL-10 were measured using eBioscience Human Th1/Th2 11plex FlowCytomix Kit (eBioscience, San Diego, CA, USA) following manufacturer's instruction for sample collection, storage, and assay procedure. Each sample was tested in duplicate, and the mean of tests was used for analysis. Furthermore, 10% of samples were randomly chosen and tested again for quality assurance.
Statistical analysis
χ2 tests were used to assess differences in the distributions of IL-10 genotype frequencies between HPV16+ and HPV16− patients with SCCOP, and the t test was used to compare the expression levels of IL-10 between the groups with different tumor HPV16 status and genotypes of IL-10 polymorphisms. The associations between IL-10 genotypes and tumor HPV16 positivity among patients with SCCOP were estimated by calculating odds ratios (ORs) and 95% confidence intervals (95% CI) using multivariate logistic regression analyses. Fifteen HPV16+ patients with SCCOP were omitted from survival analyses because of palliative treatment and/or treatment at an outside institution. Time to recurrence was computed from date of end of treatment to date of last follow-up or date of clinically detectable recurrent cancer (local, regional, and distant). Participants who were recurrence free or lost to follow-up were considered censored. Overall survival was defined as the time from first appointment to death from any cause or date of last follow-up. Participants who were alive at the end of the study period or lost to follow-up were considered censored. Disease-specific survival was defined as the time from first appointment to death from disease or date of last follow-up. Participants who were alive at the end of the study period or lost to follow-up were considered censored. The Kaplan-Meier method was used to compare survival among selected variables and different genotypes, and Cox proportional hazard models, including age, sex, ethnicity, smoking history, alcohol consumption, disease T and N stage, comorbidity, and treatment as covariates, were used to estimate the association of IL-10 variants and risk of death and recurrence. All tests were 2-sided, and a value of P < 0.05 was defined for statistical significance performed using Statistical Analysis System software (SAS 9.2; SAS Institute, Cary, NC, USA).
RESULTS
The demographic and clinical variables of 309 incident patients with SCCOP are shown in Table 1. Overall, patients were predominantly male (87.1%), non-Hispanic white (92.6%), and with late index tumor stage (75.1%) and N stage (88.7%), and ∼93% of patients had advanced disease (TNM stage III or IV) at presentation. Of these cases, 230 (74.4%) were HPV16+, and 79 (25.6%) were HPV16− for tumor DNA.
Table 1.
Distribution of selected variables in patients with SCCOP
| Variable | Total, N = 309 |
5-yr survival rate | P | |
|---|---|---|---|---|
| n | % | |||
| Age | 0.040 | |||
| ≤54 yr | 167 | 54.0 | 81.0 | |
| >54 yr | 142 | 46.0 | 68.0 | |
| Sex | 0.273 | |||
| Male | 269 | 87.1 | 78.0 | |
| Female | 40 | 12.9 | 75.0 | |
| Ethnicity | 0.118 | |||
| Non-Hispanic white | 286 | 92.6 | 77.0 | |
| Other | 23 | 7.4 | 69.0 | |
| Tobacco smoking | 0.001 | |||
| Ever | 175 | 56.6 | 70.0 | |
| Never | 134 | 43.4 | 81.0 | |
| Alcohol drinking | 0.069 | |||
| Ever | 240 | 77.7 | 72.0 | |
| Never | 69 | 22.3 | 81.0 | |
| T stage | <0.001 | |||
| I–II | 232 | 75.1 | 85.0 | |
| III–IV | 77 | 24.9 | 49.0 | |
| N stage | 0.002 | |||
| 0 | 35 | 11.3 | 58.0 | |
| 1–3 | 274 | 88.7 | 78.0 | |
| Treatment | 0.100 | |||
| XRTa | 95 | 30.7 | 80.0 | |
| XRT + adjuvant TXb | 185 | 59.9 | 75.0 | |
| Otherc | 29 | 9.4 | n/a | |
| Comorbidity | 0.715 | |||
| 0–1 | 186 | 60.2 | 75.0 | |
| 2–3 | 123 | 39.8 | 70.0 | |
| Tumor HPV status | 0.112 | |||
| Positive | 230 | 74.4 | 74.0 | |
| Negative | 79 | 25.6 | 67.0 | |
P values are by log-rank test.
XRT: X-ray therapy (radiotherapy).
Adjuvant TX: surgery and/or chemotherapy.
Other: palliative treatment and/or treatment at outside institution.
We also observed significant differences in overall survival between the groups with regard to age (log-rank, P=0.040), smoking (log-rank, P=0.001), T stage (log-rank, P<0.001), and N stage (log-rank, P=0.002). However, such a significant difference was not found between HPV16+ and HPV− patients with SCCOP (log-rank, P=0.112).
The genotype distributions for the 3 IL-10 polymorphisms rs1800871, rs1800872, and rs1800896 are shown in Table 2. These 3 variants of IL-10 were not in linkage disequilibrium (LD) to each other (data not shown). The genotype distributions of IL-10 rs1800871 and rs1800872 polymorphisms indicated that HPV16+ patients were more likely to have the variant IL-10 rs1800871 CT/TT (50.9%) and rs1800872 CA/AA genotypes (52.6%) than the HPV16− patients (29.1% and 31.7%, respectively). The genotype distribution of IL-10 rs1800896 did not vary significantly between HPV16+ and HPV16− patients (P=0.483). The patients with IL-10 rs1800871 CT/TT and rs1800872 CA/AA genotypes were 2.6 and 2.4 times more likely to have HPV16+ tumors than the patients with IL-10 rs1800871 CC and rs1800872 CC genotypes, respectively (OR, 2.6; 95% CI, 1.5–4.5 for IL-10 rs1800871; and OR, 2.4; 95% CI, 1.4–4.2 for IL-10 rs1800872).
Table 2.
Association of IL-10 genotypes with tumor HPV16 status among patients with SCCOP
| IL-10 genotype | HPV16+ patients, N = 230 |
HPV16− patients, N = 79 |
P | Adjusted OR and 95% CI | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| IL-10 rs1800871 | 0.001 | |||||
| CCa | 113 | 49.1 | 56 | 70.9 | 1.0 | |
| CT + TT | 117 | 50.9 | 23 | 29.1 | 2.6 (1.5–4.5) | |
| IL-10 rs1800872 | 0.001 | |||||
| CCa | 109 | 47.4 | 54 | 68.4 | 1.0 | |
| CA + AA | 121 | 52.6 | 25 | 31.7 | 2.4 (1.4–4.2) | |
| IL-10 rs1800896 | 0.483 | |||||
| AAa | 83 | 36.1 | 32 | 40.5 | 1.0 | |
| GA + GG | 147 | 63.9 | 47 | 59.5 | 1.0 (0.6–1.8) | |
P values for χ2 test for genotype distribution. OR and 95% CI were adjusted for age, sex, ethnicity, and tobacco smoking and alcohol consumption status in a logistic regression model.
Reference group.
Because HPV16+ patients with SCCOP were the majority of the study cases and because HPV16− patients with SCCOP had few outcome events in this cohort of 309 patients, our analysis of effect of IL-10 genetic variants on survival was restricted to HPV16+ cases with respect to the death from all causes [overall survival (OS)], death from SCCOP [disease-specific survival (DSS)], and occurrence of recurrence [disease-free survival (DFS)]. Of the 230 HPV16+ patients with SCCOP, 215 patients were treated with radiation or radiation with adjuvant treatment for curative intent at our institution, and 15 patients were excluded from the analysis because of palliative treatment and/or treatment at an outside institution. Twenty-eight deaths occurred, with 16 deaths from SCCOP, and 23 patients experienced disease relapse, with a median follow-up duration of 26.0 mo. As shown in Fig. 1, the Kaplan-Meier univariate analysis on survival demonstrated that patients with SCCOP carrying the IL-10 rs1800871 and rs1800872 CC genotypes had a significantly better OS, DSS, and DFS than the patients carrying the corresponding variant genotypes, respectively, while the similarly significant differences were not observed for IL-10 rs1800896 polymorphism. Table 3 shows the multivariable analysis of associations between IL-10 polymorphisms with OS, DSS, and DFS among 215 HPV16+ patients with SCCOP. Estimates of an association were adjusted for potential confounders, including age, sex, ethnicity, smoking and alcohol status, disease T and N stage, comorbidity, and treatment. Compared with patients with SCCOP having IL-10 rs1800871 CT/TT and rs1800872 CA/AA variant genotypes, the patients with IL-10 rs1800871 and rs1800872 wild-type homogenous CC genotypes had significantly reduced risk of overall death, death from SCCOP, and recurrence (Table 3). For the IL-10 rs1800896 polymorphism, no significant associations were observed.
Figure 1.
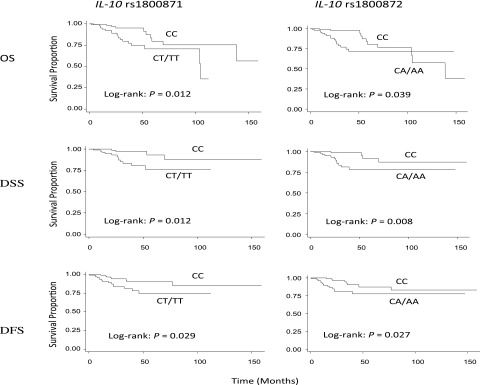
Survival by IL-10 rs1800871 and rs1800872 genotypes in 215 tumor HPV16+ patients with SCCOP.
Table 3.
Multivariable analysis of survivals associated with genotypes of IL-10 genetic variants among 215 tumor HPV16+ patients with SCCOP
| MicroRNA genotype | Total patients, N = 215 | OS |
DSS |
DFS |
|||
|---|---|---|---|---|---|---|---|
| Overall deaths | Adjusted HR and 95% CI | Deaths with SCCOP | Adjusted HR and 95% CI | Rec. | Adjusted HR and 95% CI | ||
| IL-10 rs1800871 | |||||||
| CT + TT | 112 | 10 | Ref. | 4 | Ref. | 8 | Ref. |
| CC | 103 | 18 | 0.3 (0.1–0.8) | 12 | 0.2 (0.1–0.8) | 15 | 0.3 (0.1–0.8) |
| IL-10 rs1800872 | |||||||
| CA + AA | 107 | 11 | Ref. | 4 | Ref. | 8 | Ref. |
| CC | 108 | 17 | 0.4 (0.2–0.9) | 12 | 0.2 (0.1–0.7) | 15 | 0.3 (0.1–0.8) |
| IL-10 rs1800896 | |||||||
| GA + GG | 137 | 19 | Ref. | 11 | Ref. | 13 | Ref. |
| AA | 78 | 9 | 2.0 (0.8–4.6) | 5 | 1.8 (0.5–6.0) | 10 | 1.0 (0.3–1.8) |
HR, hazard ratio; Rec., recovered; Ref., reference group. HR and 95% CI were adjusted for age, sex, ethnicity, tobacco smoking and alcohol consumption status, T and N stage, treatment, and comorbidity in a Cox regression model.
To further characterize the potentially functional relevance of the 3 polymorphisms in IL-10 promoter, we determined serum expression levels of IL-10 in another set of 180 incident patients with SCCOP, who were recently recruited and whose serum samples were available. We conducted a correlation analysis between tumor HPV16 status and genotypes of the three promoter polymorphisms and the circulating expression levels of IL-10. As shown in Table 4, we found that the expression of IL-10 was significantly higher in tumor HPV16+ patients than the HPV16− cases. Furthermore, the expression of IL-10 was significantly higher in the patients with variant genotypes of IL-10 rs1800871 (CT+TT) and rs1800872 (CA+AA) than the patients with the corresponding wild-type homogenous CC genotypes, while the similarly significant difference was not found for IL-10 rs1800896 (Table 4).
Table 4.
Correlation of IL-10 expression levels in serum with tumor HPV16 status and different genotypes of IL-10 genetic variants in 180 patients with SCCOP
| Variable | Patients (n) | Serum IL-10 level (pg/ml) | P |
|---|---|---|---|
| Tumor HPV16 status | 0.049 | ||
| Negative | 46 | 9.1 ± 8.3 | |
| Positive | 134 | 13.1 ± 12.8 | |
| IL-10 rs1800871 | 0.038 | ||
| CC | 99 | 9.6 ± 8.9 | |
| CT + TT | 81 | 12.8 ± 11.6 | |
| IL-10 rs1800872 | 0.037 | ||
| CC | 96 | 10.1 ± 8.6 | |
| CA + AA | 84 | 13.2 ± 11.1 | |
| IL-10 rs1800896 | 0.262 | ||
| AA | 68 | 10.7 ± 9.5 | |
| GA + GG | 112 | 12.6 ± 11.8 |
Serum level values are means ± sd. P values are by unpaired t test.
DISCUSSION
Previous epidemiological studies have demonstrated that HPV16 infection is one of the main etiologic risk factors for SCCOP and contributes to the increased incidence of SCCOP despite the declining overall incidence of SCCHN (19). HPV is a ubiquitous, nonenveloped, double-stranded DNA virus, which can be found in the upper aerodigestive tract in >10% of the general population (20). The characteristic of cryptic invaginations of the oropharynx allows for greater exposure of basal epithelial cells in the epithelium to HPV infection, (19), but only a small percentage of those infected develop SCCOP, probably because of lowered immune response and HPV clearance due to interindividual genetic variations that result in persistent HPV infection.
IL-10 is a quite complex cytokine with many diverse functions in different types of neoplasia and immune states. In addition to its anti-inflammation and immunosuppression activities, IL-10 is also recognized as an anticancer factor (11, 21). It is notable that IL-10 can enhance immune function and decrease the rate of tumor cell escape from immune surveillance by stimulating proliferation, activation, and chemotaxis, and it can also stimulate the proliferation and activity of natural killer cells, thus increasing the host's ability to clear cancerous cells (22, 23). Furthermore, IL-10 can interact with other cytokines, such as vascular endothelial growth factor (VEGF), IL-1β, tumor necrosis factor α (TNF-α), and IL-6, to regulate cellular activities (24–26). Therefore, the IL-10 polymorphisms may involve in the complex interplay of potential cancer promotion and cancer inhibition, causing the function of IL-10 to be different in different cancer types (24–27). It has been reported that 50–75% of the observed variability of IL-10 can be attributed to genetic polymorphisms (13).
The three common IL-10 promoter polymorphisms that we studied, rs1800896, rs1800871, and rs1800872, may affect the level and activity of IL-10 production and subsequently modulate the role of IL-10 in carcinogenesis (12). IL-10 rs1800896 has been found to be associated with the risk of oral cancer, small cell lung cancer, and diffuse large B-cell lymphoma, (28–30), but no association has been found with cervical or prostate cancer (31, 32). An association between IL-10 rs1800872 and cervical cancer has been found, and no association with prostate cancer was detected for IL-10 rs1800871 (31, 32). Thus far, only one other study has evaluated the association of IL-10 polymorphisms with risk of head and neck cancers in a Korean population, but no association was detected between the three main polymorphisms and the risk of head and neck cancers (33).
Several studies have demonstrated that IL-10 polymorphisms are associated with HPV clearance. Farzaneh et al. (14) studied the IL-10 rs1800896 in cases of HPV-associated cervical cancer and reported that higher levels of IL-10 may prevent cervical cancer by eliminating HPV, whereas Ivansson et al. (34) found that the IL-10 rs1800872 was not associated with cervical cancer in Caucasians. In immunosuppressed adolescents, the haplotype GCC in the IL-10 promoter has been associated with an increase in IL-10 production levels and decreased clearance of high-risk-type HPV infection (35). However, the results from our study demonstrated that IL-10 rs1800871 and rs1800872 polymorphisms were significantly associated with HPV16+ SCCOP tumors, but this significant association was absent for the rs1800896 polymorphism. These data indicate that IL-10 promoter polymorphisms may change certain immune functions and thus affect HPV16 clearance capacity. The conflicting results from these studies suggest that the function of IL-10 is very complex and that IL-10 promoter polymorphisms in different sites, in addition to other factors, such as environmental risk, genetic heterogeneity, and differences in populations, may lead to different changes in tissue-specific functions. It is also likely that other inflammatory cytokines (e.g., TGF-β1) and/or other molecular pathways (e.g., cell cycle control) may play important roles in HPV-related carcinogenesis.
Several studies have evaluated the association of IL-10 variants with survival of patients with various types of cancers, including breast cancer, lymphoid neoplasms, and advanced melanoma (36, 37). They found that IL-10 rs1800872 CC genotype and IL-10 ATA haplotype carriers of these 3 variants were independent favorable prognostic factors for survival. Our current study showed that IL-10 rs1800871 CC and IL-10 rs1800872 CC genotypes were significantly associated with HPV16+ tumors and modified the prognoses of HPV16+ patients with SCCOP, while such association was not found for IL-10 rs1800896. Although we do not know how these IL-10 variants influence the HPV16+ tumors and survival of HPV16+ patients with SCCOP, it is biologically plausible that these variants may be either functional or in linkage disequilibrium with other functional variants of IL-10, thereby altering the function of IL-10, or with alleles at other nearby susceptibility loci. Such functional variants could increase or reduce IL-10 expression levels and thus affect the regulation of the immune and inflammation, as well as apoptotic responses. For example, IL-10 rs1800871 CC and IL-10 rs1800872 CC genotypes might alter regulation in these pathways which might not enable many HPV-infected cells to escape or counterattack against the immune system and might enhance apoptotic response, leading to less likely HPV+ tumors and subsequently better response to chemoradiotherapy.
So far, no studies on functional relevance of these IL-10 promoter polymorphisms have been reported. Since increased expression of IL-10, an anti-inflammatory cytokine, is correlated with HPV clearance and these polymorphisms are within the functional regions of the gene's promoter of IL-10, we speculated that these IL-10 genetic variants may have potentially functional effect on expression levels of IL-10 by altering the efficiency of translational initiation, leading to interindividual differences in susceptibility to HPV16-associated SCCOP and treatment response. Indeed, in this study, we found that the variant genotypes of the two polymorphisms (IL-10 rs1800871 and rs1800872) are significantly correlated with increased expression of IL-10 in serum. While the functional relevance of these two polymorphisms has not yet been elucidated, our results might partially suggest a functional correlation between the two polymorphisms and expression of IL-10, which may provide preliminary evidence of biological plausibility for the observed association in the current study.
Our study has some inherent limitations. Because >90% of the SCCOP cases in our study were non-Hispanic white patients, the generalizability of our results to other ethnic populations may be limited. Since the outcome event rates in death or recurrence in this study was lower than expected, the statistically significant results could be by chance due to small number of outcome events and a limited short duration of follow-up. Furthermore, for stratified analysis, although the findings from several stratum analyses were significant, the fairly small subgroup numbers may have limited the interpretation of our findings. Finally, our results could be biased due to selection bias from the hospital-based nature of the study, as well as other unknown confounding factors, such as sexual behavior characteristics, infection with other high-risk HPV types (although unlikely). To confirm our findings, larger, well-designed prospective studies may be necessary.
In summary, our findings indicate that IL-10 promoter polymorphisms might serve as a susceptibility marker for and prognosis of HPV16+ patients with SCCOP. Since IL-10 variants in promoter were significantly associated with survival of HPV16+ SCCOP, particularly in smokers, IL-10 polymorphisms, together with other significant markers, may help stratify HPV16+ patients with SCCOP for appropriate treatment strategies and prognostic value. However, further larger studies are required to carefully assess the clinical validity and utility before implementation, as well as exploration of the molecular mechanisms underlying the observed associations.
Acknowledgments
The authors gratefully thank Ms. Dawn Chalaire for article editing, and Dr. Chong Zhao and Ms. Yingdong Li for laboratory support.
This study was supported by U.S. National Institute of Environmental Health Sciences grant R01 ES-11740 (to Q.W.) and U.S. National Institutes of Health grants CA 135679 and CA133099 (to G.L.)
The authors declare no conflicts of interest.
Footnotes
- CI
- confidence interval
- DFS
- disease-free survival
- DSS
- disease-specific survival
- HPV
- human papillomavirus
- IL-10
- interleukin-10
- OR
- odds ratio
- OS
- overall survival
- PCR
- polymerase chain reaction
- SCCHN
- squamous cell carcinoma of the head and neck
- SCCOP
- squamous cell carcinoma of the oropharynx
REFERENCES
- 1. Sturgis E. M., Cinciripini P. M. (2007) Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer 110, 1429–1435 [DOI] [PubMed] [Google Scholar]
- 2. Siegel R., Naishadham D., Jemal A. (2012) Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 [DOI] [PubMed] [Google Scholar]
- 3. Vineis P., Alavanja M., Buffler P., Fontham E., Franceschi S., Gao Y. T., Gupta P. C., Hackshaw A., Matos E., Samet J., Sitas F., Smith J., Stayner L., Straif K., Thun M. J., Wichmann H. E., Wu A. H., Zaridze D., Peto R., Doll R. (2004) Tobacco and cancer: recent epidemiological evidence. J. Natl. Cancer Inst. 96, 99–106 [DOI] [PubMed] [Google Scholar]
- 4. Dahlstrom K. R., Adler-Storthz K., Etzel C. J., Liu Z., Dillon L., El-Naggar A. K., Spitz M. R., Schiller J. T., Wei Q., Sturgis E. M. (2003) Human papillomavirus type 16 infection and squamous cell carcinoma of the head and neck in never-smokers: a matched pair analysis. Clin. Cancer Res. 9, 2620–2626 [PubMed] [Google Scholar]
- 5. Gillison M. L., Koch W. M., Capone R. B., Spafford M., Westra W. H., Wu L., Zahurak M. L., Daniel R. W., Viglione M., Symer D. E., Shah K. V., Sidransky D. (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 92, 709–720 [DOI] [PubMed] [Google Scholar]
- 6. Marur S., D'Souza G., Westra W. H., Forastiere A. A. (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 11, 781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hammarstedt L., Lindquist D., Dahlstrand H., Romanitan M., Dahlgren L. O., Joneberg J., Creson N., Lindholm J., Ye W., Dalianis T., Munck-Wikland E. (2006) Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int. J. Cancer 119, 2620–2623 [DOI] [PubMed] [Google Scholar]
- 8. Sturgis E. M., Wei Q. (2002) Genetic susceptibility–molecular epidemiology of head and neck cancer. Curr. Opin. Oncol. 14, 310–317 [DOI] [PubMed] [Google Scholar]
- 9. Ritchie J. M., Smith E. M., Summersgill K. F., Hoffman H. T., Wang D., Klussmann J. P., Turek L. P., Haugen T. H. (2003) Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int. J. Cancer 104, 336–344 [DOI] [PubMed] [Google Scholar]
- 10. Mellin H., Friesland S., Lewensohn R., Dalianis T., Munck-Wikland E. (2000) Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int. J. Cancer 89, 300–304 [PubMed] [Google Scholar]
- 11. Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. (1990) Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 248, 1230–1234 [DOI] [PubMed] [Google Scholar]
- 12. Turner D. M., Williams D. M., Sankaran D., Lazarus M., Sinnott P. J., Hutchinson I. V. (1997) An investigation of polymorphism in the interleukin-10 gene promoter. Eur. J. Immunogenet. 24, 1–8 [DOI] [PubMed] [Google Scholar]
- 13. Westendorp R. G., Langermans J. A., Huizinga T. W., Elouali A. H., Verweij C. L., Boomsma D. I., Vandenbroucke J. P. (1997) Genetic influence on cytokine production and fatal meningococcal disease. Lancet 349, 170–173 [DOI] [PubMed] [Google Scholar]
- 14. Farzaneh F., Roberts S., Mandal D., Ollier B., Winters U., Kitchener H. C., Brabin L. (2006) The IL-10-1082G polymorphism is associated with clearance of HPV infection. BJOG 113, 961–964 [DOI] [PubMed] [Google Scholar]
- 15. Bolpetti A., Silva J. S., Villa L. L., Lepique A. P. (2010) Interleukin-10 production by tumor infiltrating macrophages plays a role in human papillomavirus 16 tumor growth. BMC Immunol. 11, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan X., Sturgis E. M., Lei D., Liu Z., Dahlstrom K. R., Wei Q., Li G. (2010) Association of TGF-β1 genetic variants with HPV16-positive oropharyngeal cancer. Clin. Cancer Res. 16, 1416–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park J. S., Kim E. J., Lee J. Y., Sin H. S., Namkoong S. E., Um S. J. (2001) Functional inactivation of p73, a homolog of p53 tumor suppressor protein, by human papillomavirus E6 proteins. Int. J. Cancer 91, 822–827 [DOI] [PubMed] [Google Scholar]
- 18. Ji X., Sturgis E. M., Zhao C., Etzel C. J., Wei Q., Li G. (2009) Association of p73 G4C14-to-A4T14 polymorphism with human papillomavirus type 16 status in squamous cell carcinoma of the head and neck in non-Hispanic whites. Cancer 115, 1660–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller D. L., Puricelli M. D., Stack M. S. (2012) Virology and molecular pathogenesis of HPV (human papillomavirus)-associated oropharyngeal squamous cell carcinoma. Biochem. J. 443, 339–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller C. S., Johnstone B. M. (2001) Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982–1997. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 91, 622–635 [DOI] [PubMed] [Google Scholar]
- 21. De Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. (1991) Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174, 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kundu N., Beaty T. L., Jackson M. J., Fulton A. M. (1996) Antimetastatic and antitumor activities of interleukin 10 in a murine model of breast cancer. J. Natl. Cancer Inst. 88, 536–541 [DOI] [PubMed] [Google Scholar]
- 23. Jinquan T., Larsen C. G., Gesser B., Matsushima K., Thestrup-Pedersen K. (1993) Human IL-10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocyte migration. J. Immunol. 151, 4545–4551 [PubMed] [Google Scholar]
- 24. Te Velde A. A., de Waal Malefijt R., Huijbens R. J., de Vries J. E., Figdor C. G. (1992) IL-10 stimulates monocyte Fc gamma R surface expression and cytotoxic activity. Distinct regulation of antibody-dependent cellular cytotoxicity by IFN-gamma, IL-4, and IL-10. J. Immunol. 149, 4048–4052 [PubMed] [Google Scholar]
- 25. Rousset F., Garcia E., Defrance T., Peronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. (1992) Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 89, 1890–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang S., Ullrich S. E., Bar-Eli M. (1999) Regulation of tumor growth and metastasis by interleukin-10: the melanoma experience. J. Interferon Cytokine Res. 19, 697–703 [DOI] [PubMed] [Google Scholar]
- 27. Fortis C., Foppoli M., Gianotti L., Galli L., Citterio G., Consogno G., Gentilini O., Braga M. (1996) Increased interleukin-10 serum levels in patients with solid tumours. Cancer Lett. 104, 1–5 [DOI] [PubMed] [Google Scholar]
- 28. Lech-Maranda E., Baseggio L., Bienvenu J., Charlot C., Berger F., Rigal D., Warzocha K., Coiffier B., Salles G. (2004) Interleukin-10 gene promoter polymorphisms influence the clinical outcome of diffuse large B-cell lymphoma. Blood 103, 3529–3534 [DOI] [PubMed] [Google Scholar]
- 29. Seifart C., Plagens A., Dempfle A., Clostermann U., Vogelmeier C., von Wichert P., Seifart U. (2005) TNF-alpha, TNF-β, IL-6, and IL-10 polymorphisms in patients with lung cancer. Dis. Markers 21, 157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yao J. G., Gao L. B., Liu Y. G., Li J., Pang G. F. (2008) Genetic variation in interleukin-10 gene and risk of oral cancer. Clin. Chim. Acta. 388, 84–88 [DOI] [PubMed] [Google Scholar]
- 31. Michaud D. S., Daugherty S. E., Berndt S. I., Platz E. A., Yeager M., Crawford E. D., Hsing A., Huang W. Y., Hayes R. B. (2006) Genetic polymorphisms of interleukin-1β (IL-1β), IL-6, IL-8, and IL-10 and risk of prostate cancer. Cancer Res. 66, 4525–4530 [DOI] [PubMed] [Google Scholar]
- 32. Zoodsma M., Nolte I. M., Schipper M., Oosterom E., van der Steege G., de Vries E. G., Te Meerman G. J., van der Zee A. G. (2005) Interleukin-10 and Fas polymorphisms and susceptibility for (pre)neoplastic cervical disease. Int. J. Gynecol. Cancer 15, Suppl. 3, 282–290 [DOI] [PubMed] [Google Scholar]
- 33. Jeong S. W., Tae K., Lee S. H., Kim K. R., Park C. W., Park B. L., Shin H. D. (2010) Cox-2 and IL-10 polymorphisms and association with squamous cell carcinoma of the head and neck in a Korean sample J. Korean Med. Sci. 25, 1024–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ivansson E. L., Gustavsson I. M., Magnusson J. J., Steiner L. L., Magnusson P. K., Erlich H. A., Gyllensten U. B. (2007) Variants of chemokine receptor 2 and interleukin 4 receptor, but not interleukin 10 or Fas ligand, increase risk of cervical cancer. Int. J. Cancer 121, 2451–2457 [DOI] [PubMed] [Google Scholar]
- 35. Shrestha S., Wang C., Aissani B., Wilson C. M., Tang J., Kaslow R. A. (2007) Interleukin-10 gene (IL10) polymorphisms and human papillomavirus clearance among immunosuppressed adolescents. Cancer Epidemiol. Biomarkers Prev. 16, 1626–1632 [DOI] [PubMed] [Google Scholar]
- 36. Gerger A., Renner W., Langsenlehner T., Hofmann G., Knechtel G., Szkandera J., Samonigg H., Krippl P., Langsenlehner U. (2010) Association of interleukin-10 gene variation with breast cancer prognosis. Breast Cancer Res. Treat. 119, 701–705 [DOI] [PubMed] [Google Scholar]
- 37. Vuoristo M. S. (2007) The polymorphisms of interleukin-10 gene influence the prognosis of patients with advanced melanoma. Cancer Genet. Cytogenet. 176, 54–57 [DOI] [PubMed] [Google Scholar]


