Abstract
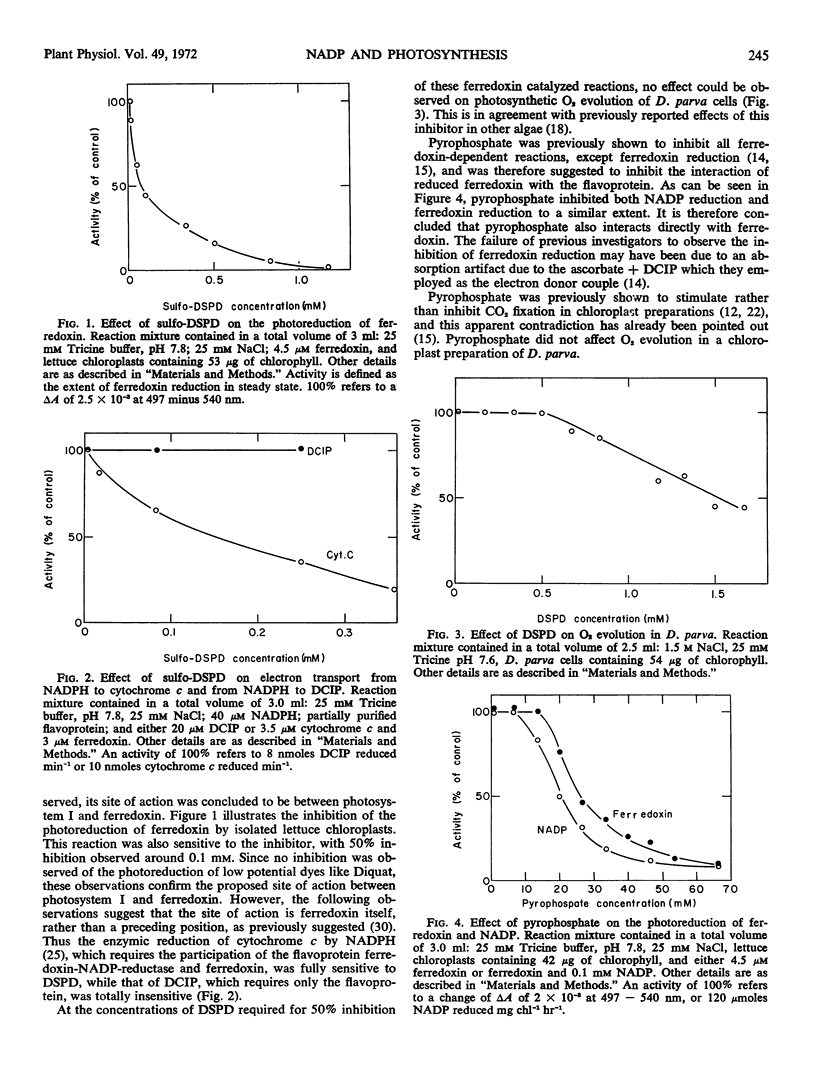
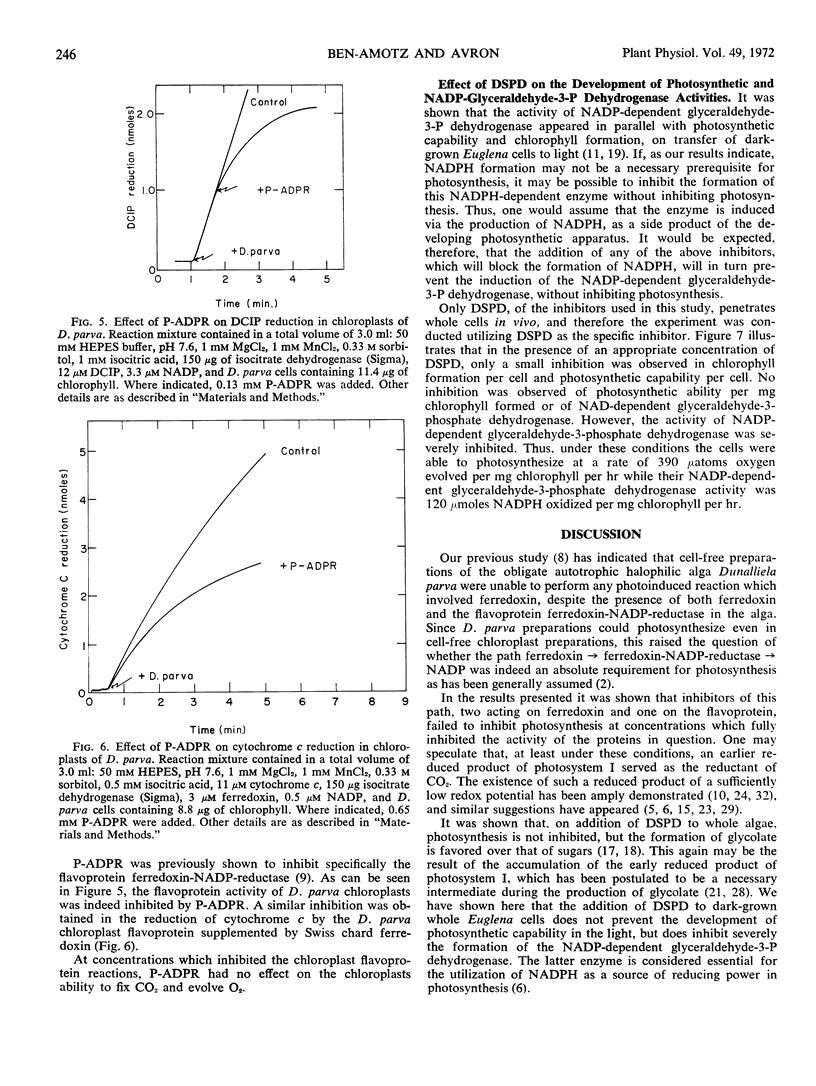
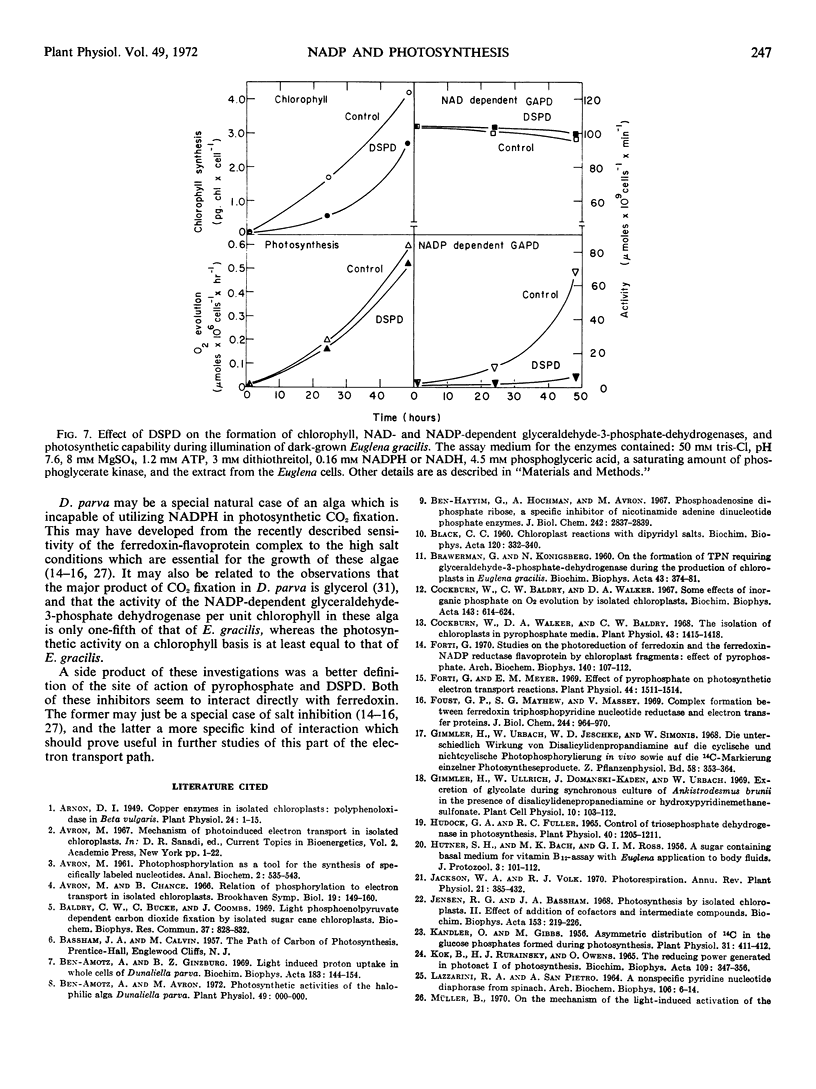
The site of action of the inhibitors disalicylidenepropanediamine and pyrophosphate was more closely defined as acting on ferredoxin. Three inhibitors which act on the electron transport path between ferredoxin and NADP: disalicylidenepropanediamine, pyrophosphate, and phosphoadenosinediphosphate ribose, had no effect on photosynthesis in cell free preparations of Dunaliela parva at concentrations which completely inhibited the enzymic activity on which each inhibitor acts. The addition of disalicylidenepropanediamine to dark-grown Euglena gracilis cells prevented the light-induced formation of NADP-dependent glyceraldehyde-3-phosphate dehydrogenase, but not of photosynthesis, chlorophyll synthesis, or NAD-dependent glyceraldehyde-3-phosphate dehydrogenase.
The above results are interpreted as indicating that, at least under some conditions, a reduced product of photosystem I preceding ferredoxin in the electron transport path can serve as the reductant of CO2 in photosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation as a tool for the synthesis of specifically labeled nucleotides. Anal Biochem. 1961 Dec;2:535–543. doi: 10.1016/0003-2697(61)90021-5. [DOI] [PubMed] [Google Scholar]
- Avron M., Chance B. Relation of phosphorylation to electron transport in isolated chloroplasts. Brookhaven Symp Biol. 1966;19:149–160. [PubMed] [Google Scholar]
- Baldry C. W., Bucke C., Coombs J. Light-phosphoenolpyruvate dependent carbodioxide fixation by isolated sugar cane chloroplasts. Biochem Biophys Res Commun. 1969 Nov 20;37(5):828–832. doi: 10.1016/0006-291x(69)90966-8. [DOI] [PubMed] [Google Scholar]
- Ben-Amotz A., Ginzburg B. Z. Light-induced proton uptake in whole cells of Dunaliella parva. Biochim Biophys Acta. 1969 Jun 3;183(1):144–154. doi: 10.1016/0005-2736(69)90138-2. [DOI] [PubMed] [Google Scholar]
- Ben-Hayyim G., Hochman A., Avron M. Phosphoadenosine diphosphate ribose, a specific inhibitor of nicotinamide adenine dinucleotide phosphate enzymes. J Biol Chem. 1967 Jun 25;242(12):2837–2839. [PubMed] [Google Scholar]
- Black C. C., Jr Chloroplast reactions with dipyridyl salts. Biochim Biophys Acta. 1966 Jul 13;120(3):332–340. doi: 10.1016/0926-6585(66)90300-1. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Some effects of inorganic phosphate on O2 evolution by isolated chloroplasts. Biochim Biophys Acta. 1967;143(3):614–624. doi: 10.1016/0005-2728(67)90067-9. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. The isolation of spinach chloroplasts in pyrophosphate media. Plant Physiol. 1968 Sep;43(9):1415–1418. doi: 10.1104/pp.43.9.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti G., Melandri B. A., San Pietro A. Studies on the photoreduction of ferredoxin and the ferredoxin-NADP reductase flavoprotein by chlorplasts fragments: effect of pyrophosphate. Arch Biochem Biophys. 1970 Sep;140(1):107–112. doi: 10.1016/0003-9861(70)90014-7. [DOI] [PubMed] [Google Scholar]
- Forti G., Meyer E. M. Effect of pyrophosphate on photosynthetic electron transport reactions. Plant Physiol. 1969 Nov;44(11):1511–1514. doi: 10.1104/pp.44.11.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust G. P., Mayhew S. G., Massey V. Complex formation between ferredoxin triphosphopyridine nucleotide reductase and electron transfer proteins. J Biol Chem. 1969 Feb 10;244(3):964–970. [PubMed] [Google Scholar]
- Hudock G. A., Fuller R. C. Control of Triosephosphate Dehydrogenase in Photosynthesis. Plant Physiol. 1965 Nov;40(6):1205–1211. doi: 10.1104/pp.40.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. II. Effects of addition of cofactors and intermediate compounds. Biochim Biophys Acta. 1968 Jan 15;153(1):219–226. doi: 10.1016/0005-2728(68)90163-1. [DOI] [PubMed] [Google Scholar]
- Kandler O., Gibbs M. Asymmetric Distribution of C in the Glucose Phosphates Formed During Photosynthesis. Plant Physiol. 1956 Sep;31(5):411–412. doi: 10.1104/pp.31.5.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Rurainski H. J., Owens O. V. The reducing power generated in photoact I of photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):347–356. doi: 10.1016/0926-6585(65)90162-7. [DOI] [PubMed] [Google Scholar]
- LAZZARINI R. A., SANPIETRO A. A NONSPECIFIC PYRIDINE NUCLEOTIDE DIAPHORASE FROM SPINACH. Arch Biochem Biophys. 1964 Jul 20;106:6–14. doi: 10.1016/0003-9861(64)90151-1. [DOI] [PubMed] [Google Scholar]
- Plaut Z., Gibbs M. Glycolate formation in intact spinach chloroplasts. Plant Physiol. 1970 Apr;45(4):470–474. doi: 10.1104/pp.45.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZWEIG G., AVRON M. ON THE OXIDATION-REDUCTION POTENTIAL OF THE PHOTOPRODUCED REDUCTANT OF ISOLATED CHLOROPLASTS. Biochem Biophys Res Commun. 1965 May 3;19:397–400. doi: 10.1016/0006-291x(65)90135-x. [DOI] [PubMed] [Google Scholar]


