Abstract
Study Objectives:
Upper airway inflammation and oxidative stress have been implicated in the pathogenesis of obstructive sleep apnea (OSA) and may be linked to cardiovascular consequences. We prospectively examined fraction of exhaled nitric oxide (FENO), a surrogate marker of upper airway inflammation using a portable nitric oxide analyzer (NIOX MINO).
Design:
In consecutive adult nonsmokers with suspected OSA, FENO was measured immediately before and after polysomnographic studies, and within 1-3 months following continuous positive airway pressure (CPAP) therapy.
Measurement and Results:
FENO levels were increased in the 75 patients with OSA compared to the 29 controls, both before sleep (13.4 ± 6.5 ppb vs. 6.5 ± 3.5; p < 0.001) and after sleep (19.0 ± 7.7 ppb vs. 6.9 ± 3.7; p < 0.001). Furthermore, in patients with OSA, FENO levels were significantly higher post-sleep than pre-sleep (19.0 ± 7.7 ppb vs. 13.4 ± 6.5; p < 0.001), while there was no significant overnight change in patients without OSA. The rise in FENO correlated with the apnea-hypopnea index (r = 0.65, p < 0.001), nadir oxygen saturation (r = 0.54, p < 0.001), and arousal index (r = 0.52, p < 0.001). Thirty-seven of these patients underwent CPAP titration and treatment. Successful titration was associated with a lower overnight increase in FENO (7.2 ± 3.3 vs. 11.0 ± 4.3, p = 0.02). FENO levels declined after 1-3 months of CPAP therapy (11.7 ± 4.4 ppb, p < 0.001).
Conclusions:
FENO levels are elevated in OSA, correlate with severity, and decrease after positive pressure therapy. This study supports the role of upper airway inflammation in OSA pathogenesis and a possible role for FENO in monitoring CPAP therapy.
Citation:
Chua AP; Aboussouan LS; Minai OA; Paschke K; Laskowski D; Dweik RA. Long-term continuous positive airway pressure therapy normalizes high exhaled nitric oxide levels in obstructive sleep apnea. J Clin Sleep Med 2013;9(6):529-535.
Keywords: Sleep disordered breathing, endogenous nitrate vasodilator, positive pressure ventilation
Obstructive sleep apnea (OSA) is a sleep disorder characterized by recurrent episodes of upper airway collapse leading to significant hypoxemia and disturbed sleep with increased arousals and sleep fragmentation. The etiology of obstruction of the upper airway in OSA is postulated to be multifactorial, including anatomic narrowing due to structural changes, neurologic, vascular, and upper airway inflammation (UAWI).1–4 Mechanical stress on the mucosa caused by intermittent airway closure and reopening as well as ischemia-reperfusion injury from intermittent nocturnal hypoxemia produces oxygen free radicals which can lead to increased UAWI. However the extent to which the presence of UAWI plays a role in the pathogenesis of sleep disordered breathing is not clear. The fraction of exhaled nitric oxide (FENO) has been proposed as a marker of airway inflammation (AWI) and can be easily measured in exhaled breath.5,6
Prior studies that have evaluated the FENO levels in OSA have shown inconsistent results.7–17 Specifically, studies have shown no difference,8,10 mild increase,13 or significant increases in FENO7,9,11,12,14,16,17 compared to controls. Post- vs. pre-sleep FENO was significantly increased in one study7 but unchanged in another.9 The FENO correlated with the apnea-hypopnea index (AHI) in one study11 but not in another.8 Finally, few studies have assessed the impact of CPAP on FENO, generally showing a decrease in FENO with CPAP.12,17
BRIEF SUMMARY
Current Knowledge/Study Rationale: We perform this study to examine FENO levels in patients with OSA and the impact on these levels by CPAP therapy. Thus we hope to establish the role of FENO measurement in the assessment and control of sleep apnea.
Study Impact: This scientific work adds to the increasing knowledge on the role of upper airway inflammation in the pathophysiology and treatment of obstructive sleep apnea. It also provides further insight into the role of exhaled nitric oxide gas measurement in the diagnosis and monitoring of this common sleep condition.
We therefore sought to address these issues and hypothesized that patients with OSA would have elevated levels of exhaled NO at baseline and immediately after sleep, and that the levels would be reduced by nasal continuous positive airway pressure (CPAP), the current standard recommended treatment for OSA. In this study, we set out to demonstrate that FENO is elevated in OSA; to assess whether increase in FENO level correlates with body mass index (BMI) and polysomnographic parameters such as AHI, arousal indices, pulse oximetry oxyhemoglobin saturation (SpO2), and percentage of total sleep time (TST) with recorded SpO2 < 90% (%TST SpO2 < 90); and to determine whether FENO levels are reduced after short-term (overnight) or long-term (1-3 months) CPAP treatment.
METHODS
This was a prospective 1-year follow up study of consecutive adults ≥ 18 years of age recruited from September 2009 to June 2010 at a single sleep disorder center in a tertiary care facility. Patients were selected during their outpatient office visit or when they came for their diagnostic overnight polysomnography in the sleep laboratory (Figure 1). Subject exclusion criteria included current smoking; history of chronic airway or lung disease, atopy, nasal allergy, or polyps; active pulmonary infections; autoimmune conditions; liver diseases or systemic infections; current use of immunosuppressive medications including corticosteroids or leukotriene modifiers; non-communicative patients; and absence of informed consent. Patients who underwent subsequent CPAP titration on the same night as diagnostic PSG, i.e., split-night studies, were also excluded. The protocol was approved by the institutional review board. All patients signed a written informed consent before participation.
Figure 1. Flow diagram summarizing study enrollment.

Polysomnography (PSG) was performed in accordance to the methods outlined by the American Academy of Sleep Medicine (AASM).18 Sleep stage and arousals were scored manually in 30-sec epochs and respiratory events were identified, both according to the standard criteria of the AASM.19 The alternative rule in the AASM manual19 for scoring hypopneas was used. The Epworth Sleepiness Scale (ESS) was used to evaluate subjective daytime sleepiness, with a score ≥ 12 denoting diurnal hypersomnia.
Subjects diagnosed with OSA and planned for CPAP treatment subsequently returned for a full-night CPAP titration study in accordance to AASM recommendations.20 Preliminary education and habituation for CPAP was performed 30 min prior to the start of the CPAP titration. An optimal titration study was defined as one in which an optimal CPAP pressure was reached that normalizes the AHI and eliminates snoring, desaturation, and arousals, and restores a normal flow contour.20 Patients were prescribed CPAP treatment at the optimized titrated pressure. A face-to-face reevaluation during an office visit was performed in accordance with CPAP Medicare guidelines between 30 to 90 days after initiating therapy.21
Adherence to the CPAP was assessed on the follow-up visit by reviewing the download information from the CPAP device. Adherence was defined as CPAP use for an average of 4 h on ≥ 70% of the nights.21
FENO measurements were performed with the portable NIOX MINO analyzer (Aerocrine, Sweden) according to international recommendations,22 using the on-line standardized single-breath technique at a constant mouth flow rate of 50 mL/s. We measured FeNO levels within 1 h before (between 21:00 and 23:00) starting PSG (prePSGno) or CPAP titration (preCPAPno), and within 30 min (06:00-08:00) after completing the PSG (postPSGno) or CPAP titration study (postCPAPno). A final FENO level (Tno) was measured during a scheduled follow-up office visit. An outline of the study work flow is shown in Figures 1 and 2.
Figure 2. Reasons for dropping out of study.
Demographic, anthropometric, and polysomnographic data were analyzed. Comparisons between groups were performed using Student t-test for normally distributed data and the Wilcoxon rank sum test for non-normally distributed data. The relationship between the continuous variables was explored using Pearson correlation coefficient for normally distributed data, or Spearman correlation test if data were not normally distributed. Potential correlations were identified using linear regression analysis. Descriptive statistics for patient clinical characteristics and polysomnographic parameters were expressed as mean ± standard deviation (SD) for normally distributed data and median (25th, 75th percentiles) if data were not normally distributed. Statistical analysis was performed using SPSS 13.0 software (SPSS Inc. Chicago, IL, USA). Significance level was set at p-value < 0.05.
The study was powered to detect a 5 ppb mean difference between the post-PSG FENO and the post-treatment FENO. We also adopted a conservatively elevated standard deviation of 15 for the difference in the response of matched pairs. Other assumptions included a type I error probability for a 2-sided test of 0.05 (α), and a power of 0.80 of correctly rejecting the null hypothesis of equal population means. These assumptions yielded a sample size of 73 with a goal to enroll 100 patients to accommodate loss to PAP follow-up and other attrition factors.
RESULTS
One hundred four patients who fulfilled the inclusion criteria were recruited for the study: 75 had OSA with an overall AHI (AHItotal) ≥ 5, and 29 had no OSA (non-OSA group). Table 1 summarizes the characteristics of both groups. Although there was a trend for increased age, greater representation of the male gender, and increased BMI and neck circumference in the OSA compared to the non-OSA group, those differences were not statistically significant. Symptoms and polysomnographic abnormalities were significantly more prevalent in the OSA than the non-OSA group.
Table 1.
Characteristics of OSA and non-OSA patients
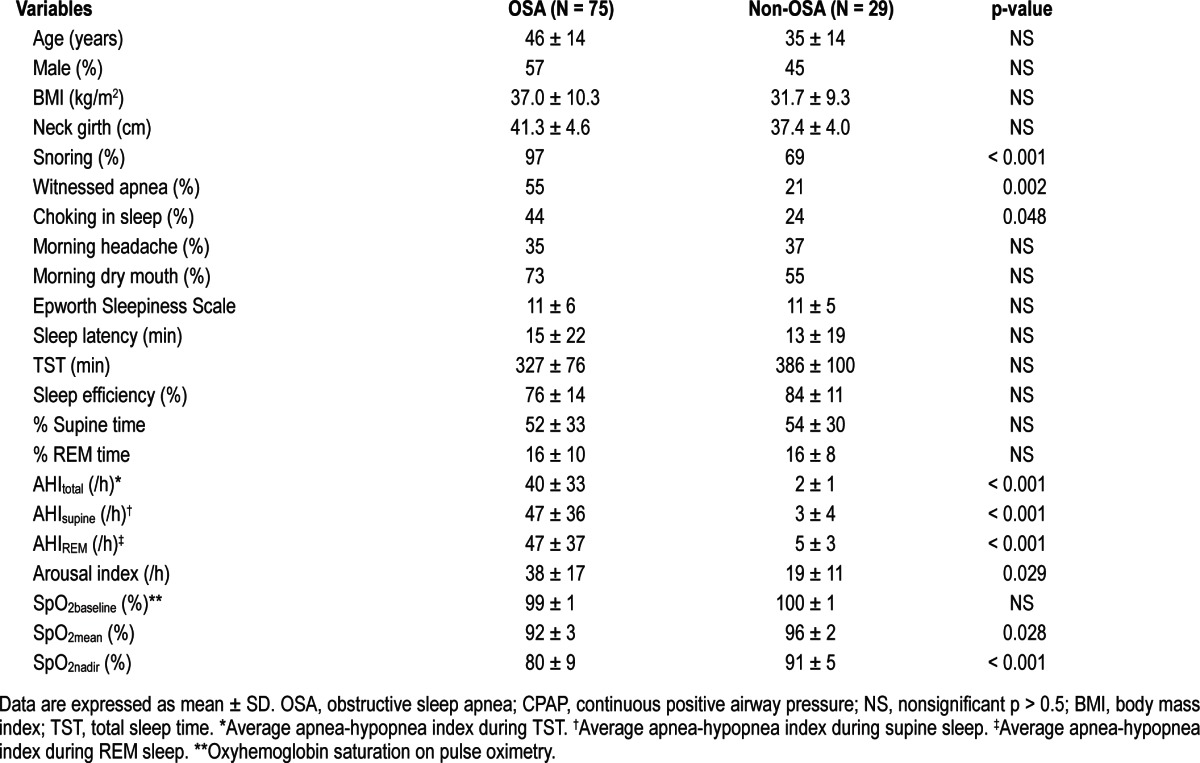
Sleep apnea patients had significantly higher pre-sleep FENO (prePSGno) than non-OSA patients (13.4 ± 6.5 ppb versus 6.5 ± 3.5 ppb, p < 0.001), who had normal FENO levels. They also had significantly higher post-sleep FENO (postPSGno) levels than non-OSA patients (19.0 ± 7.7 ppb versus 6.9 ± 3.7 ppb, p < 0.001; Figure 3). There was a signifi-cant rise in FENO levels following sleep (ΔFENOpre-postPSG) in OSA, which was not seen in non-OSA patients (6.0 ± 2.9 ppb versus 0.4 ± 0.8 ppb, p < 0.001). The prePSGno was significantly correlated with AHItotal on PSG (r = 0.73, p < 0.001) but was not significantly correlated with any of the demographic or anthropometric parameters, nor with the other polysomno-graphic data such as arousal index (AI), average SpO2 during sleep (SpO2mean), lowest SpO2 recorded during sleep (SpO2nadir), and %TST SpO2 < 90. Similarly, postPSGno was also significantly correlated with AHItotal on PSG (r = 0.79, p < 0.001) but was not associated with any of the demographic or anthropo-metric, nor with the polysomnographic parameters. In linear regression analysis, ΔFENOpre-postPSG was positively correlated with AHItotal (r = 0.65, p < 0.001; Figure 4) and AI (r = 0.52, p < 0.001), and negatively correlated to SpO2nadir (r = 0.54, p < 0.001). Incorporating these 3 variables in a multiple linear regression model, only AHItotal remained significantly correlated to ΔFENOpre-postPSG (p < 0.001).
Figure 3. FENO levels before and after sleep in OSA and non-OSA subjects.
Pre-sleep FENO (prePSGno) and post-sleep FENO (postPSGno) levels during 1 night of diagnostic PSG study in OSA (N = 75) and non-OSA subjects (N = 29). OSA patients had significantly higher prePSGno and postPSGno than non-OSA patients. *, mean ± SD.
Figure 4. Relationship between ΔFENOpre-postPSG levels and AHI.

Rise in FENO levels following sleep (ΔFENOpre-postPSG) in our study cohort (N = 104) increased with OSA severity, as indicated by AHI. There was a significant positive correlation between ΔFENOpre-postPSG and AHItotal (r = 0.65, p < 0.001).
Of the 75 OSA patients, 37 subsequently underwent CPAP titration with FENO levels measured before and after sleep as outlined in the methodology section. Table 2 summarizes the characteristics of these 37 study subjects. Mean TST during titration was 337.4 ± 65.0 min, and mean percentage of TST during which AHI was < 5/h (%TST AHI < 5) was 77% ± 29%. After a night of CPAP titration, FENO levels (postCPAPno) taken immediately following sleep were higher than pre-sleep levels (preCPAPno) (22.7 ± 7.7 ppb versus 14.7 ± 6.6 ppb, p < 0.001). This trend was similar to that observed after sleep without CPAP application during diagnostic PSG. Mean rise in FENO level post-sleep following CPAP titration (ΔFENOpre-postCPAP) was 8.0 ± 3.7 ppb. Seventy-three percent (N = 27) of the subjects underwent an optimal titration study. ΔFENOpre-postCPAP was significant lower in patients who achieved an optimal titration than those who did not (7.2 ± 3.3 vs. 11.0 ± 4.3, respectively; p = 0.02). Information on whether CPAP was started prior to the CPAP titration to allow acclimatization to the device was available in 45 patients. There was no difference in postCPAPno, postCPAPno, or ΔFENOpre-postCPAP between those who received and those who did not receive an acclimatization device. There was no association between postCPAPno or ΔFENOpre-postCPAP levels and any of the demographic or anthropometric parameters.
Table 2.
Characteristics of 37 OSA patients undergoing CPAP titration and CPAP treatment
Comparing FENO levels obtained during CPAP titration night and that of diagnostic PSG, there was no significant difference between mean preCPAPno and prePSGno levels (14.7 ± 6.6 ppb versus 13.4 ± 6.5 ppb). However mean postCPAPno was slightly higher than mean postPSGno level (22.7 ± 7.7 ppb versus 19.0 ± 7.7 ppb, p < 0.001).
All 37 patients were successfully initiated on CPAP and returned for their first office visit with repeat FENO measurements between 1 to 3 months of starting CPAP treatment. The mean duration of CPAP treatment among these 37 subjects was 9.0 ± 3.2 weeks at a mean pressure level of 12 ± 6 cm H2O for an average of 6.2 ± 2.1 h per night (Table 2). All the patients were adherent to the CPAP therapy. Figure 5 shows the FENO levels at various timelines in the study in these patients (N = 37). We saw a reduction in the mean FENO level performed at the post-CPAP treatment office visit, Tno, compared to their baseline mean pre-sleep FENO levels, prePSGno, and preCPAPno (11.7 ± 4.6 ppb versus 13.4 ± 6.5 or 14.7 ± 6.6 ppb, respectively; both p < 0.001; Figure 5).
Figure 5. FENO levels at various study stages in OSA patients on CPAP therapy.
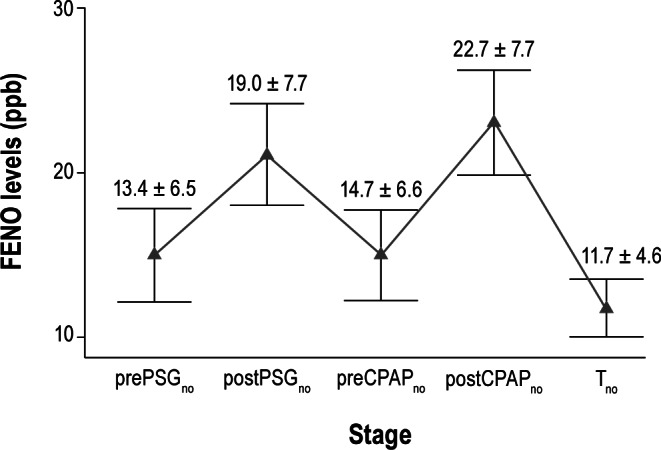
This graph shows the trending of FENO values (mean ± 95% confidence intervals) taken before and after sleep during PSG study (prePSGno and postPSGno), before and after sleep during CPAP titration night (preCPAPno and postCPAPno), and following 1-3 months of CPAP therapy (Tno) in the OSA patients (N = 37) who were started on CPAP therapy and had undergone all 5 sets of NO measurements. Mean FENO levels increased following sleep during both nights of PSG and CPAP titration studies. Mean FENO level, Tno, dropped to a level lower than baseline pre-sleep values after regular long-term use of CPAP therapy.
Of the 75 subjects with sleep apnea, 38 did not complete the study. Figure 2 summarizes the reasons for non-completion of the study. Twenty-six of them received non-CPAP forms of therapy because of the nature and severity of their sleep apnea, comorbidities (morbid obesity, retrognathia and otolaryngological pathology, e.g., enlarged tonsils, adenoids, and turbinates, deviated nasal septum), and/or as a result of patient's treatment preference. The remaining 12 patients were advised CPAP treatment but did not undergo CPAP titration and follow-up for various reasons (Figure 2). Of these 12 patients, 5 were lost to follow-up; we were unable to ascertain the reason as they were not available via mail or phone.
DISCUSSION
Our study conclusively demonstrates that baseline pre-sleep FENO levels are elevated in untreated OSA patients and rise further after overnight sleep. The overnight increase in FENO levels correlates with AHI, hypoxia, and sleep fragmentation. The overnight rise in FENO is not affected by a single-night CPAP application, but declines significantly to below baseline levels after long-term regular use of CPAP.
Nasal, tonsillar, and oropharyngeal tissues are potential inflammatory sites in patients with OSA.4,23–24 The results we observed in our study are consistent with the current evidence showing elevated cytokines and inflammatory markers in exhaled breath as well as upper airway tissues of OSA patients.4,9–17,23,25–28 Salerno et al. and Devouassoux et al. detected increased neutrophils and reduced macrophages in the induced sputum of OSA patients.27,28 The latter study also observed a concurrent higher interleukin-8 concentration in the induced sputum specimen of these patients.28 Petrosyan et al., using samples of exhaled breath condensate demonstrated increased levels of NO, carbon monoxide (CO), 8-isoprostane, leukotriene B4 (LTB4), nitrates, and hydrogen peroxide (H2O2) in OSA patients compared to control subjects; they found AHI to be positively correlated with nitrates, H2O2, LTB4, and 8-isoprostane. They also concluded that AWI and oxidative stress are present in OSA patients.17
Previous reports on FENO measurements in OSA revealed inconsistent results.7–17 Part of the problem could have been patient selection or the use of old technology and methodology. With the latest generation portable FENO analyzer, we demonstrated a rise in FENO levels following sleep in adult OSA patients reflecting increased UAWI that were not present in healthy controls with normal airways. Airway inflammation in this patient group could indicate physical injury to the mucosal lining due to repetitive airway closure and reopening. Oxidative stress from recurrent nocturnal oxygen desaturation may be contributive. In our study, the correlation between FENO levels and markers of OSA severity such as AHI, degree of nocturnal oxygen desaturations, and arousals suggests more severe OSA patients have an increased amount of UAWI. Przybyłowski et al.9 found a weak but statistically significant correlation between post-sleep FENO with SpO2mean, SpO2nadir during sleep, and %TST SpO2 < 90. Similarly Fortuna et al.12 reported a significant correlation between post-sleep FENO levels with AHI, SpO2mean, and %TST SpO2 < 90. In the study by Petrosyan et al.,17 AHI was positively correlated with exhaled breath markers such as 8-isoprostane, LTB4, nitrates, and H2O2 that were measured in the morning. In another experiment by Verhulst et al.16 involving the pediatric population, habitual snoring and age were the only variables associated with raised FENO levels in the morning and afternoon, leading the authors to conclude that snoring is more important than the actual obstructive respiratory events in leading to increased UAWI, with the mechanism being that snoring induced vibration of the soft tissues which caused damage to these tissues contributing to the pathogenesis of OSA.
It is interesting to note that the increase in FENO during the CPAP titration night was much lower in patients for whom an optimal CPAP titration was achieved. Moreover, the long-term use of CPAP resulted in a significant decline to below baseline pre-sleep levels. CPAP treatment has been associated with anatomical change of the nasal mucosa and increased resistance of the nasal passageway.29,30 It can trigger or exacerbate nasal mucosal inflammation due to continuous compression stimulation, giving rise to increased nasal symptoms of sneezing, congestion, and rhinorrhea.31–33 In the study by Devouassoux et al.,28 one week of CPAP use in OSA patients was found to induce increased airway hyperresponsiveness to methacholine with increased NO levels in exhaled breath and neutrophil concentration in induced sputum. In the study by Foresi et al.,10 increased alveolar NO concentrations were normalized after two nights of CPAP treatment, suggestive of restoration of endothelial function in OSA patients, although FENO remained unchanged following the two nights of CPAP therapy. However, we note that in that study, the baseline FENO levels taken in the morning were not different from the healthy controls, and 18 of their subjects had underlying arterial hypertension. In another study by Petrosyan et al.,17 a significant reduction in FENO levels was seen with one month of CPAP use. Similarly in the latest study by Fortuna et al.,12 three months of CPAP therapy saw a significant decline in FENO levels at the same restored depressed alveolar NO levels to normal. The uniform use of CPAP in our study, as objectively documented from CPAP compliance data downloads, was at odds with the usual 45% to 80% adherence rate34 and may reflect the close follow-up the patients received as study participants.
One possible limitation to our interpretation of the post-CPAP titration measurement data is the washout of nitric oxide from the upper airway by the overnight CPAP treatment. We are not aware of any evidence for this possibility in the literature and thus did not consider this in our study plans. Based on our knowledge and experience with the use of FENO, it equilibrates rather quickly within minutes of any changes in flow. As such, we think the stated concern is at most a theoretical limitation.
Elevated FENO levels seen in OSA patients, and falls in the levels only after a period of compliant and effective CPAP therapy, may confer a role to this measurement as an adjunct to current device-based adherence tracking. The role of NO analyzers in that regard remains to be determined, but with their increasing portability, it may be feasible to expand the role of FENO to the monitoring of AWI and follow-up on CPAP therapy in otherwise healthy OSA patient, similar to their use in the diagnosis and management of bronchial asthma.6
CONCLUSIONS
Our study supports the emerging concept that UAWI is present in OSA, is directly related to its severity, responds to treatment, and likely contributes to its pathogenesis. In addition, our findings suggest that parallel to its established role in the evaluation and management of asthma, FENO may have a role in the assessment and control of sleep apnea. Measuring FENO levels with a portable analyzer may thus be a simple way to monitor AWI in OSA patients.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors express gratitude to Miss Subhra Chakrabarti, Miss Alquam Mashir, and Dr. Zahr Alsheikhtaha for their help in providing the technical and logistic support for this project. This work was supported with Cleveland Clinic Foundation grant RPC 2009-1052 and BRCP 08-049 Third Frontier Program grant from the Ohio Department of Development (ODOD). Dr. Dweik is also supported by the following grants: HL081064, HL107147, HL095181, and RR026231 from the National Institutes of Health (NIH). The work was performed at Fairhill Sleep Disorder Center, Cleveland Clinic, Cleveland, OH. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafted the article and/or revised it critically for important intellectual content; approved the final version of the article to be published and; are guarantors of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
REFERENCES
- 1.Berger G, Gilbey P, Hammel I, Ophir D. Histopathology of the uvula and the soft palate in patients with mild, moderate, and severe obstructive sleep apnea. Laryngoscope. 2002;112:357–63. doi: 10.1097/00005537-200202000-00028. [DOI] [PubMed] [Google Scholar]
- 2.Paulsen FP, Steven P, Tsokos M, et al. Upper airway epithelial structural changes in obstructive sleep-disordered breathing. Am J Respir Crit Care Med. 2002;166:501–9. doi: 10.1164/rccm.2109099. [DOI] [PubMed] [Google Scholar]
- 3.Zakkar M, Sekosan M, Wenig B, Olopade CO, Rubinstein I. Decrease in immunoreactive neutral endopeptidase in uvula epithelium of patients with obstructive sleep apnea. Ann Otol Rhinol Laryngol. 1997;106:474–7. doi: 10.1177/000348949710600606. [DOI] [PubMed] [Google Scholar]
- 4.Sekosan M, Zakkar M, Wenig BL, Olopade CO, Rubinstein I. Inflammation in the uvula mucosa of patients with obstructive sleep apnea. Laryngoscope. 1996;106:1018–20. doi: 10.1097/00005537-199608000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 6.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olopade CO, Christon JA, Zakkar M, et al. Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest. 1997;111:1500–4. doi: 10.1378/chest.111.6.1500. [DOI] [PubMed] [Google Scholar]
- 8.Agusti AG, Barbe F, Togores B. Exhaled nitric oxide in patients with sleep apnea. Sleep. 1999;22:231–5. [PubMed] [Google Scholar]
- 9.Przybylowski T, Bielicki P, Kumor M, et al. [Exhaled nitric oxide in patients with obstructive sleep apnea syndrome] Pneumonol Alergol Pol. 2006;74:21–5. [PubMed] [Google Scholar]
- 10.Foresi A, Leone C, Olivieri D, Cremona G. Alveolar-derived exhaled nitric oxide is reduced in obstructive sleep apnea syndrome. Chest. 2007;132:860–7. doi: 10.1378/chest.06-3124. [DOI] [PubMed] [Google Scholar]
- 11.Depalo A, Carpagnano GE, Spanevello A, et al. Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med. 2008;263:70–8. doi: 10.1111/j.1365-2796.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 12.Fortuna AM, Miralda R, Calaf N, Gonzalez M, Casan P, Mayos M. Airway and alveolar nitric oxide measurements in obstructive sleep apnea syndrome. Respir Med. 2011;105:630–6. doi: 10.1016/j.rmed.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Culla B, Guida G, Brussino L, et al. Increased oral nitric oxide in obstructive sleep apnoea. Respir Med. 2010;104:316–20. doi: 10.1016/j.rmed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–92. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 15.Carpagnano GE, Spanevello A, Sabato R, Depalo A, Turchiarelli V, Foschino Barbaro MP. Exhaled pH, exhaled nitric oxide, and induced sputum cellularity in obese patients with obstructive sleep apnea syndrome. Transl Res. 2008;151:45–50. doi: 10.1016/j.trsl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Verhulst SL, Aerts L, Jacobs S, et al. Sleep-disordered breathing, obesity, and airway inflammation in children and adolescents. Chest. 2008;134:1169–75. doi: 10.1378/chest.08-0535. [DOI] [PubMed] [Google Scholar]
- 17.Petrosyan M, Perraki E, Simoes D, et al. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath. 2008;12:207–15. doi: 10.1007/s11325-007-0160-8. [DOI] [PubMed] [Google Scholar]
- 18.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 20.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–71. [PMC free article] [PubMed] [Google Scholar]
- 21. http://www.nationwidemedical.com/wp-content/uploads/2010/06/LCD-for-Positive-Airway-Pressure-doc-region-d.pdf Medicare coverage policy 2010.
- 22.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 23.Rubinstein I. Nasal inflammation in patients with obstructive sleep apnea. Laryngoscope. 1995;105:175–7. doi: 10.1288/00005537-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Goldbart AD, Goldman JL, Li RC, Brittian KR, Tauman R, Gozal D. Differential expression of cysteinyl leukotriene receptors 1 and 2 in tonsils of children with obstructive sleep apnea syndrome or recurrent infection. Chest. 2004;126:13–8. doi: 10.1378/chest.126.1.13. [DOI] [PubMed] [Google Scholar]
- 25.Li AM, Hung E, Tsang T, et al. Induced sputum inflammatory measures correlate with disease severity in children with obstructive sleep apnoea. Thorax. 2007;62:75–9. doi: 10.1136/thx.2006.060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldbart AD, Krishna J, Li RC, Serpero LD, Gozal D. Inflammatory mediators in exhaled breath condensate of children with obstructive sleep apnea syndrome. Chest. 2006;130:143–8. doi: 10.1378/chest.130.1.143. [DOI] [PubMed] [Google Scholar]
- 27.Salerno FG, Carpagnano E, Guido P, et al. Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med. 2004;98:25–8. doi: 10.1016/j.rmed.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Devouassoux G, Levy P, Rossini E, et al. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol. 2007;119:597–603. doi: 10.1016/j.jaci.2006.11.638. [DOI] [PubMed] [Google Scholar]
- 29.Constantinidis J, Knobber D, Steinhart H, Kuhn J, Iro H. Fine-structural investigations of the effect of nCPAP-mask application on the nasal mucosa. Acta Otolaryngol. 2000;120:432–7. doi: 10.1080/000164800750000694. [DOI] [PubMed] [Google Scholar]
- 30.Richards GN, Cistulli PA, Ungar RG, Berthon-Jones M, Sullivan CE. Mouth leak with nasal continuous positive airway pressure increases nasal airway resistance. Am J Respir Crit Care Med. 1996;154:182–6. doi: 10.1164/ajrccm.154.1.8680678. [DOI] [PubMed] [Google Scholar]
- 31.Pepin JL, Leger P, Veale D, Langevin B, Robert D, Levy P. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome. Study of 193 patients in two French sleep centers. Chest. 1995;107:375–81. doi: 10.1378/chest.107.2.375. [DOI] [PubMed] [Google Scholar]
- 32.Brander PE, Soirinsuo M, Lohela P. Nasopharyngeal symptoms in patients with obstructive sleep apnea syndrome. Effect of nasal CPAP treatment. Respiration. 1999;66:128–35. doi: 10.1159/000029354. [DOI] [PubMed] [Google Scholar]
- 33.Skoczynski S, Ograbek-Krol M, Tazbirek M, Semik-Orzech A, Pierzchala W. Short-term CPAP treatment induces a mild increase in inflammatory cells in patients with sleep apnoea syndrome. Rhinology. 2008;46:144–50. [PubMed] [Google Scholar]
- 34.Pepin JL, Krieger J, Rodenstein D, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999;160:1124–9. doi: 10.1164/ajrccm.160.4.9802027. [DOI] [PubMed] [Google Scholar]



