Abstract
One possible way to address both water and energy shortage issues, the two of major global challenges, is to recover energy and water resource from wastewater. Herein, a novel electrochemical membrane bioreactor (EMBR) was developed to recover energy from wastewater and meantime harvest clean water for reuse. With the help of the microorganisms in the biocatalysis and biodegradation process, net electricity could be recovered from a low-strength synthetic wastewater after estimating total energy consumption of this system. In addition, high-quality clean water was obtained for reuse. The results clearly demonstrate that, under the optimized operating conditions, it is possible to recover net energy from wastewater, while at the same time to harvest high-quality effluent for reuse with this novel wastewater treatment system.
Water and energy shortage are two major global challenges1,2,3,4. One possible way to address both issues is to recover energy from wastewater and meantime harvest clean water for reuse5,6. In this regard, wastewater should been regarded more as a resource than as a waste, a resource for both water and energy1,6. However, current municipal wastewater treatment systems are energy intensive7, produce large quantities of residuals5, and fail to recover the potential resources available in wastewater8. For example, in the US, about 0.5 kWh electrical energy is consumed for treating per m3 municipal wastewater using the conventional activated sludge treatment1,9. As a result, approximately 3% of the total electricity consumption is consumed for wastewater treatment in USA1. Thus, it is meaningful to develop new processes to capture energy and recover clean water for reuse from municipal wastewater, and to do so with little offsetting energy expenditure and costs5.
Anaerobic digestion process has shown a great promise for energy recovery from high-strength wastewater, and has been extensively studied and applied for decades10,11. It is characterized by slow biomass growth, low energy consumption, and most of all, high methane production12. However, there are several limitations for anaerobic digestion process. For example, methane, if not properly collected, may escape to the atmosphere and aggravate the global warming13. In addition, anaerobic treatment alone is generally insufficient to meet the stringent discharge standards for chemical oxygen demand (COD), suspended solids, and nutrients (especially nitrogen)1,14. Although the use of anaerobic membrane bioreactors allows a high effluent quality14,15, difficulties still remain in membrane fouling16, biogas collection and ammonia removal1.
Recently, microbial fuel cells (MFCs) have emerged as a new technology to directly extract energy from wastewater9,17. This technology has several advantages over the anaerobic process in terms of conversion efficiency and environmental footprint18. However, for practical implementation, MFCs usually have poor effluent quality and a low treatment efficiency because of limited biomass retention19. To sort out this problem, the filtration technique was successfully applied to MFC to reduce biomass washout20. However, in this system, the catholyte was aerated to provide dissolved oxygen, and more energy was consumed, rather than recovered from wastewater. To enable a sustainable operation, the energy consumption of such a system should be further lowered and the energy recovery should be improved. Fortunately, air-cathode MFCs, which use free air to supply oxygen to cathode, will avoid the energy consumption on aeration and may partially resolve this problem21. However, there are still other bottlenecks to their scale-up and practical application, such as high costs of membrane materials18, membrane resistance in the proton transport process22, accumulation of inorganic salts deposits on cathode23 and severe water leaking through cathode24. Furthermore, like the anaerobic process, the effluent often requires post-treatment before it can be discharged to achieve satisfactory levels for municipal wastewater treatment7,19. This would limit the wide application of this process for water reuse. To make this technology more practically viable, the configuration and operation of the system need to be further optimized.
In light of the above reason, exploration of a facile and low-cost wastewater treatment plant, which could be used to replace the conventional biological wastewater treatment system, to recover energy from municipal wastewater and meantime harvest clean water for reuse is of great meaning to address the challenges of energy and water shortage. Herein, we report a novel electrochemical membrane bioreactor (EMBR) without aeration for energy recovery and wastewater treatment. The treated water flew directly through the separator and cathode for filtration. With the help of the microorganisms in the system, net electricity could be recovered from the wastewater and high-quality effluent was obtained. Therefore, it is possible that this novel EMBR system might become a net energy producer, rather than a consumer for clean water harvest from wastewater.
Results
EMBR operation and electricity generation
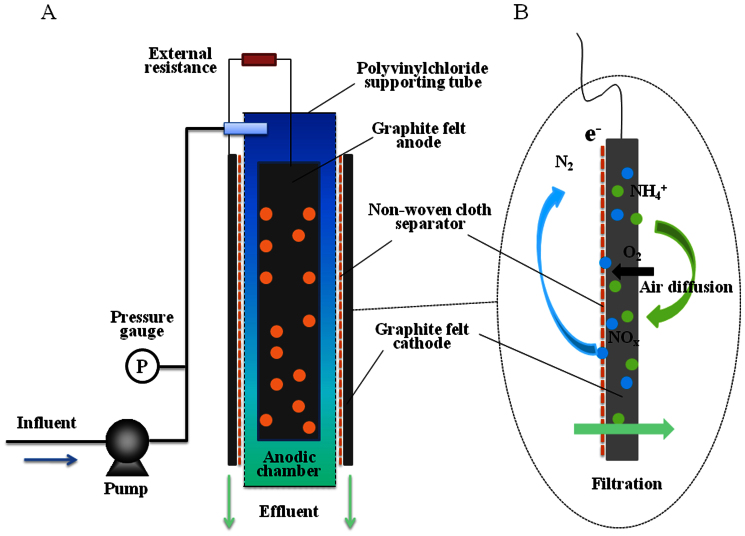
The setup of the EMBR is illustrated in Figure 1. The reactor was composed of an anodic chamber, in which the graphite felt was filled to sever as anode electrode, and a cathode surrounded the reactor. In our study, the non-woven cloth without any pretreatment was served as the separator and filter in the EMBR, which have been approved that it could be used as a separator for MFC25,26 and as a filter for MBR27,28 with high efficiency. It was used to separate the anode and cathode electrodes to avoid short circuit (Figure 1A). A synthetic municipal wastewater was continuously fed into the anodic chamber, and the substrate in the wastewater was oxidized. At the same time, electrons and protons were produced by the bacteria attached on the anode. The treated water then flowed directly through the separator and the cathode for filtration, which ensured high-quality effluent (Figure 1B). The produced electrons were transferred to the electrode and then to the cathode through the external circuit. The protons diffused from the anodic chamber to the cathode were combined with the electrons and oxygen diffused from the air (internal circuit). As a result, electricity was generated from the oxidation of the substrate in the wastewater.
Figure 1. Schematic diagrams of: (A) the EMBR system; and (B) the reaction in the cathode.
Hydraulic retention time (HRT) is one of the important parameters for wastewater treatment process, as it significantly affects the power generation and nutrient removal of this system. The system performance at various HRTs was evaluated, and the operating parameters are summarized in Table 1. The electricity generation of the system at varied HRTs is shown in Figure 2. The current density and the corresponding power density changed slightly at HRTs of 14.5 h–3.6 h (Runs 1–4), but decreased significantly at an HRT of 1.6 h (Run 5). Meanwhile, the Coulombic efficiency (CE), which reflected the energy recovery efficiency from substrate in wastewater to electricity, was found to decrease from 36% to 1.8% with the decrease in HRT (Table 2).
Table 1. Operation parameters of the EMBR system.
| Run | Operation time (day) | HRT (h) | Inflow COD (mg/L) | Inflow nitrogen (mg/L) | Organic loading rate kg/(m3 d) | Nitrogen loading rate kg/(m3 d) |
|---|---|---|---|---|---|---|
| 1 | 1–11 | 14.5 | 287.0 (23.8) | 28.5 (1.5) | 0.48 | 0.047 |
| 2 | 12–26 | 9.1 | 292.7 (20.1) | 30.1 (5.0) | 0.77 | 0.080 |
| 3 | 27–40 | 5.2 | 292.0 (11.3) | 29.8 (5.5) | 1.35 | 0.138 |
| 4 | 41–58 | 3.6 | 296.4 (17.5) | 29.6 (1.4) | 1.95 | 0.195 |
| 5 | 58–76 | 1.6 | 275.6 (25.3) | 29.8 (2.3) | 4.26 | 0.460 |
Figure 2. Electricity generation of the EMBR during the long-term operation.
Table 2. System performance in different runs.
| Run | COD removal % | NH4+-N removal % | TN removal % | Current density A/m3 | CE % | Power density W/m3 | Maximum power density W/m3 | Energy recovery kWh/m3 | Maximum energy recovery kWh/m3 | Energy consumption* kWh/m3 | Net energy production kWh/m3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 87.4 (1.9) | 97.6 (2.3) | 23.1 (10.0) | 10.2 (1.5) | 36 | 2.73 (0.80) | 7.4 | 0.081 | 0.22 | 0.0044 | 0.0766 |
| 2 | 91.2 (2.7) | 96.9 (1.5) | 55.0 (17.3) | 9.2 (1.4) | 19.1 | 2.19 (0.63) | 7.6 | 0.041 | 0.142 | 0.0049 | 0.0361 |
| 3 | 88.7 (3.3) | 89.3 (5.5) | 55.0 (11.0) | 11.3 (0.6) | 13.9 | 3.27 (0.32) | 6 | 0.035 | 0.063 | 0.0056 | 0.0294 |
| 4 | 88.9 (3.1) | 79.6 (8.3) | 57.2 (5.2) | 9.8 (1.1) | 8.3 | 2.48 (0.57) | 4.2 | 0.019 | 0.032 | 0.0059 | 0.0131 |
| 5 | 88.0 (2.9) | 69.5 (4.6) | 54.9 (5.3) | 4.7 (2.2) | 1.8 | 0.68 (0.81) | 1.2 | 0.002 | 0.004 | 0.0066 | −0.0046 |
Note: energy for incubator is not included.
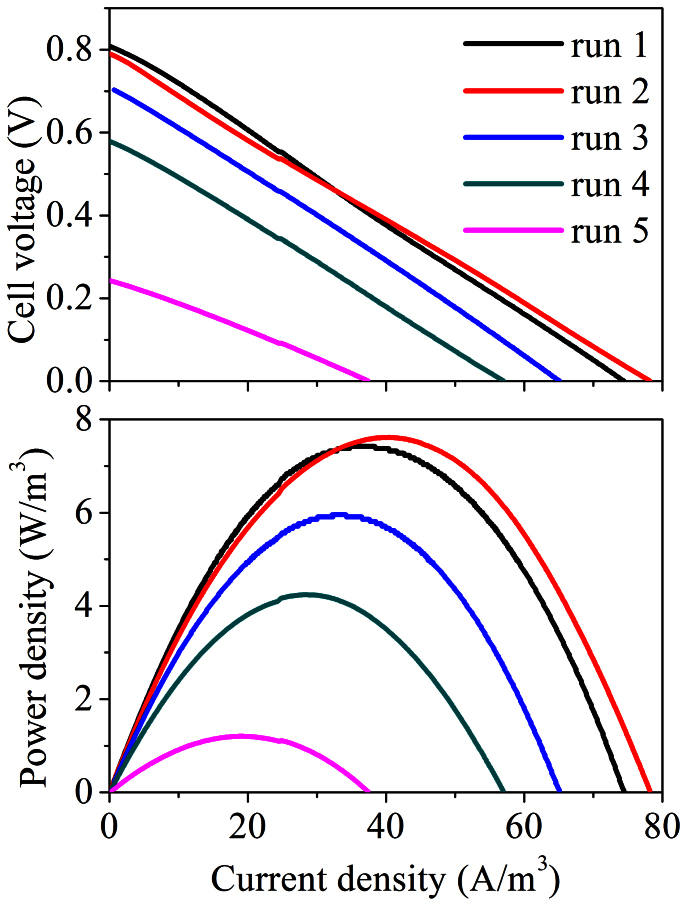
To evaluate the electricity producing ability of the system, the polarization curves were measured at the end of each run to determine the maximum power densities at various HRTs. As shown in Figure 3, the maximum power density and open circuit voltage (OCV) varied slightly when the HRT was decreased from 14.5 h to 9.1 h in Runs 1 and 2. However, as the HRT was further decreased in Runs 3–5, the power density decreased obviously from 7.6 W/m3 to 1.2 W/m3, while the corresponding OCV decreased from 790 mV to 243 mV.
Figure 3. Power output (A) and polarization curves (B) of the EMBR under different operating conditions.
The electrode potentials of anode and cathode were also affected by HRT (Figure S1 in Supplementary Information). If the HRT was too short, the oxygen reduction reaction became a limiting step, resulting in a low cathode potential. On the contrary, at a long HRT the substrate for electricity-producing microbes became insufficient, which resulted in a high anode potential. Thus, an appropriate range of HRT should be maintained for the effective electricity recovery.
COD and nitrogen removal
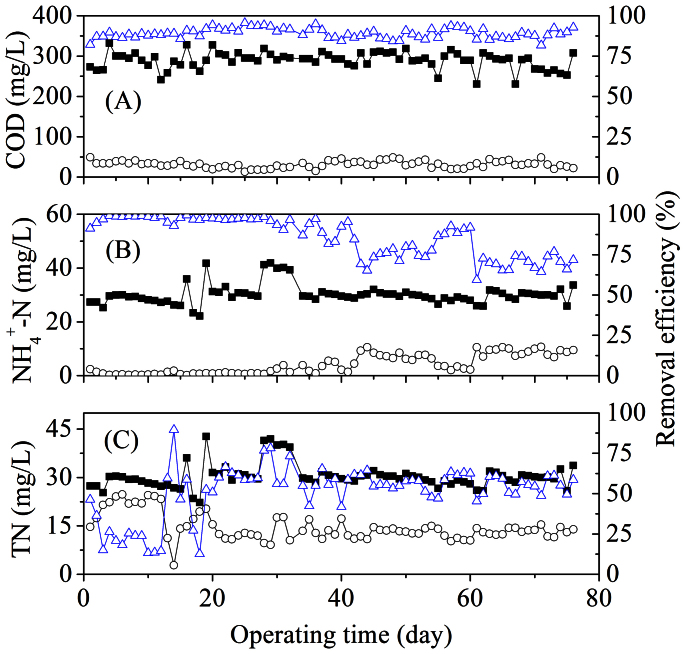
The COD and nutrient removal of the EMBR system are illustrated in Figure 4. Low effluent COD concentrations (31.3 ± 8.6 mg/L) and high COD removal efficiencies (averaged at 89.1%) were achieved during the 76-day operation at various HRTs without aeration. It might be due to that the low-strength synthetic wastewater with acetate as substrate was used in this study, which could be easily degraded by the anodic microorganisms. The cathodic biofilm also had a considerable contribution to the COD removal, evidenced by the low CE at a low HRT (Table 2).
Figure 4. Performance of the EMBR: (A) COD; (B) ammonia; and (C) TN.
( ) Influent concentration, (
) Influent concentration, ( ) Effluent concentration, and (
) Effluent concentration, and ( ) Removal efficiency.
) Removal efficiency.
Meanwhile, the NH4+-N and TN removal efficiencies were 69.5–97.6% (86% in average) and 23.1–57.2% (51.3% in average), respectively. The removal efficiencies of NH4+-N and TN were dependent upon the operating parameters, and a high nitrogen removal could be achieved under optimized conditions.
The COD, NH4+-N and TN removal efficiencies at different HRTs during the operation are summarized in Table 2. The COD removal was stable, but the NH4+-N removal efficiency decreased from 97.6% to 69.5% as the HRT was decreased from 14.5 to 1.6 h. The TN removal was also affected by HRT significantly. A comparison of nitrogen removal in Runs 1 and 5 shows that a longer HRT (e.g., 14.5 h in Run 1) resulted in NO3−-N accumulation, while a shorter HRT (e.g., 1.6 h in Run 5) led to poor NH4+-N removal. Too long or short HRTs would decrease the TN removal efficiency.
The microbes in the cathodic biofilm played important roles in nutrient removal and oxygen reduction. The morphology of the biofilm on the cathodic graphite felt is shown in Figure S2 (Supplementary Information). A dense biofilm could be clearly observed on the surface of graphite felt, with the bacteria embedded in extracellular polymeric matrix. Less bacteria were observed at the inner of the graphite felt, which might be attributed to the limited oxygen diffusion from the surface of cathode graphite felt to the inner. Microbial community analysis shows that both nitrifiers and denitrifiers were found in the cathodic biofilm (Figure S2), which benefited for the nitrogen removal. The presence of DO gradient within the cathodic biofilm and availability of electron donors (i.e., the residual acetate from the anode chamber and electrons from the cathode electrode) ensured the simultaneous nitrification and denitrification in the biofilm.
It is well known that oxygen governs both electricity generation and nitrification. The nitrification and oxygen reduction reactions at the cathode relied heavily on diffusion of oxygen through the cathode. Thus, the concentration and diffusion velocity of DO in the cathodic biofilm would significantly influence the extent of nitrification and denitrification and thus affect TN removal. If the HRT was too short, the DO concentration in the cathodic biofilm would be limited, resulting in insufficient nitrification (Run 5 in Table 2). On the contrary, if the HRT was too long, the DO concentration in the cathodic biofilm would be surplus, leading to insufficient denitrification and also causing a poor TN removal (Run 1 in Table 2). Thus, there existed an appropriate range of HRT for simultaneous nitrification and denitrification and maximized TN removal. Beyond this range, NH4+-N or NO3−-N would accumulate and TN removal efficiency would drop.
Effluent turbidity and water head-drop
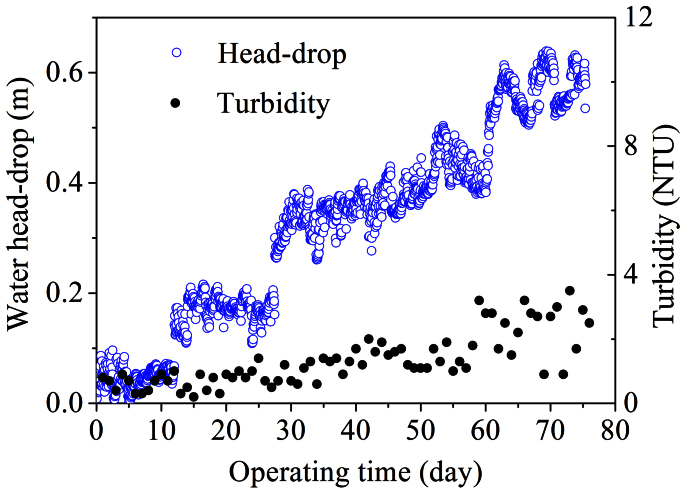
The rejection of the detached biomass by the cathode was assessed through measuring the effluent turbidity. The effluent turbidity remained at below 2 NTU at HRTs of 3.6–14.5 h (Figure 5). When the HRT was further decreased to 1.6 h, it fluctuated around 3 NTU. The low effluent turbidity indicated the good retention of the biomass in the reactor, which would ensure the good performance for wastewater treatment.
Figure 5. Profiles of the EMBR effluent turbidity and water head-drops over the operating time.
With a decrease in HRT, the total water head-drop of the influent pipelines, anodic chamber and cathodic filtration materials also increased due to the simultaneous increase in feed rate (Figure 5). Although the operation pressure in this study was slightly higher than that in other coarse mesh filteres29,30, no online or offline backwashing of the filtration materials was needed in the long time operation. This implied that no significant membrane fouling occurred in this study, which was mainly attributed to the reduced fouling rate by the attached growth of microorganisms on the graphite felt anode. It has been reported that by adding biofilm carriers into a conventional MBR, the membrane fouling could be effectively controlled due to the reduced suspending biomass and the sludge extracellular polymeric substances content31,32. In addition, a small membrane flux (3.2–29.6 L/m2/h) was enabled by the large membrane filtration area of the tubular-structured EMBR, which also benefited for a low membrane fouling.
Discussion
MFC with air cathode is a promising configuration for scaled-up application. However, the use of high-cost membranes, noble metal catalysts and Nafion (or polytetrafluoroethylene)24 binder in air-cathode MFCs all present bottlenecks to their practical application33. Although exciting progresses have been made in air-cathode MFC construction and power density has been improved significantly, challenges such as membrane pH gradient34, water leaking through cathode24 and accumulation of inorganic salts deposits on cathode23,35, still need to be overcome for the long-term sustainable operation of MFCs.
In our EMBR, graphite felt served as the cathode, and non-woven cloth as the electrolyte separator. In the operation, wastewater was filtrated through the non-woven cloth, graphite felt in sequence and was finally discharged. This unique configuration confers it several advantages for wastewater treatment: (1) Problems commonly encountered in air-cathode MFCs, such as fluid leakage through the cathode, pH gradient, and accumulation of inorganic salts deposits on the cathode, could be circumvented or overcome. For example, in this EMBR system, the pH gradient was decreased attributed to a direct flow of the anolyte to the cathode side that effectively neutralized the alkalinity at the cathode. During the long time operation, the solution pH only changed slightly from 7.5 ± 0.1 (influent) to 7.8 ± 0.1 (effluent); (2) Simultaneous oxygen reduction, nitrification and denitrification reactions occurred at the cathode without the need of aeration; (3) High quality effluent was obtained due to the filtration of the non-woven cloth and graphite felt cathode; and (4) Small sludge yield could be achieved. During the long-term operation of the EMBR, no excess sludge was discharged, except for few biomass draining with the effluent under low HRT conditions (Figure 5). This was possible because of the low sludge yield in our EMBR system, which has been reported in MFCs19. The biomass rejection by the separator and the cathode increased the solid retention time in the anode chamber, which further decreased the sludge production yield36. All these advantages suggest a promising future of the EMBR for sustainable wastewater treatment and energy recovery.
As summarized in Table 2, the energy balance was completed through analyzing energy production and consumption in the EMBR. Electrical energy gain from the EMBR system was calculated according to the electric power production. It was found that about 7.5% of the energy in the wastewater could be obtained as electrical energy in this EMBR system in Run 1 (details in Supplementary Information). A further increase in the energy recovery could be expected after optimization of the system operation. The polarization curve in Run 1 indicates that a maximum power density of 7.4 W/m3 could be achieved, accounting for 20.4% of the total energy recovery from the wastewater.
The theoretical power requirement for the EMBR system was estimated according to a previous study14 (more details in Supplementary Information). The theoretical net energy production, En was calculated by subtracting energy consumption (Ec) from the electrical energy gain from the EMBR system (Eg). It increased from −0.0046 kWh/m3 to 0.0766 kWh/m3 when the HRT was increased from 1.6 to 14.5 h. Thus, a maximum theoretical net energy of 0.0766 kWh/m3 could be produced, although the operational parameters were not optimized in the present work. Therefore, it is feasible that this novel EMBR system might become a net energy producer, rather than a consumer. Since there was no aeration energy consumption in this system, more net energy could be recovered from this EMBR system, compared with the aerated MFC37,38. Compared with other air-cathode MFCs reported previously39,40, similar energy output but much better effluent quality was obtained here, attributed to the configuration design in this EMBR system. No further treatment is needed, which can reduce the energy consumption compared with the conventional MFCs.
It is reported that about 28% of the energy potential in biodegradable organics in wastewater could be converted into electricity energy in a complete anaerobic municipal wastewater treatment system1. However, a comparable energy conversion efficiency was obtained from the EMBR system. Furthermore, some drawbacks in anaerobic wastewater treatment systems, such as installation of CH4 collection system and poor nutrient (especially nitrogen) removal efficiency, could be overcome in this system. Thus, as an energy-producing wastewater treatment process, this EMBR system could efficiently capture the energy potential of substrate in wastewater and meanwhile harvest high-quality effluent. If such a strategy is adopted for a wastewater treatment plant with a capacity of 50,000 m3/day, a net power of about 3850 kWh could be produced per day. At the same time, high-quality reclaimed water will be produced.
However, for application of this new technology, the long term operation stability of the system should be tested, and the electricity recovery and nutrient removal should be further enhanced. Although much work should been done before the application of this process for real wastewater treatment, our system may provide an effective and sustainable energy-recovering solution to upgrade the existing wastewater treatment plants.
Methods
EMBR assembly
The cathode and the tubular anodic chamber were separated by non-woven cloth (70 g/m2). The non-woven cloth with a thickness of 0.2 mm, 75% porosity and 50 μm pore size was supported by a perforated polyvinylchloride tube, which had a total pore area of 27 cm2 to facilitate wastewater flow. The anodic chamber (height 20 cm, diameter 4.5 cm) was filled with graphite felt with a 3-mm thickness and a 530 cm2 projected surface area (Sanye Carbon Co., China). The total volume and working volume of the anodic chamber were 254 mL and 124 mL, respectively. A graphite felt with a projected surface area of 294 cm2 was used as the cathode without pretreatment. The electrodes were connected to the circuit with titanium wires across a 100-Ω external resistor. The reactor was operated in a constant-temperature incubator (25°C) and in a continuous-flow mode.
Inoculation and operation conditions
The anodic chamber was inoculated with 100-mL effluent from a laboratory-scale MFC. The graphite cloth cathode was immerged in a laboratory-scale MBR for 5 min to inoculate nitrifiers and dinitrifiers. A synthetic wastewater was continuously fed into the anodic chamber through a peristaltic pump (Lange Co., China). The wastewater composition was: CH3COONa·3H2O, 0.64 g/L; NH4Cl, 115 mg/L; K2HPO4·3H2O, 44 mg/L; CaCl2, 11.5 mg/L; MgSO4 12 mg/L and 10 mL of trace element solution. The composition of the trace element solution (in μg/L) was: EDTA, 50, ZnSO4·7H2O, 22, CaCl2·2H2O, 8.2, MnCl2·4H2O, 5.1, FeSO4·7H2O, 5.0, (NH4)6Mo7O24·4H2O, 1.1, CuSO4·5H2O, 1.8, CoCl2·6H2O, 1.6. The effluent from the anodic chamber then penetrated through the non-woven cloth and graphite felt, and was finally discharged from the system. The experiments were carried out after starting up and the system was operated over 30 days, an anodic biofilm of electricity-producing microbes and cathodic biofilm were gradually formed. Then, the system performance at various HRTs was evaluated.
Analysis and calculations
COD, NH4+-N, TN and turbidity were measured following the Standard Methods41. During the operation, the hydraulic pressure (or water head, which reflects the transmembrane pressure, TMP) was monitored daily using a pressure transmitter (GB-3000E, Gangbei Ltd., China).
The voltage across the resistor was automatically record every 5 min using a data acquisition system (34970A, Agilent Co., USA). Linear sweep voltammetry was performed with an electrochemical workstation (CHI660C, Chenhua Co., China) to obtain the polarization curves, as reported elsewhere18,42. Firstly, the circuit of the EMBR was opened for 12 h to measure the OCV. Then, voltammetry scanning was performed using the anode as the working electrode, the cathode as the counter electrode and reference electrode, respectively. The voltage range was set between zero and the OCV. The scan rate was 1 mV/s. Current (I) was calculated according to I = V/R. Power (P) was obtained as P = IV. CE was calculated as CE = Cp/Cth × 100%, where Cp is the total coulombs calculated by integrating the current over time, and Cth is the theoretical amount of coulombs available based on the COD removed in the system. The HRT of the anodic chamber, organic loading rate and nutrient loading rate were calculated according to the net effective volume of the anodic chamber and the influent flow rate. The current density and power density were normalized to the total anodic chamber volume.
Author Contributions
Y.K.W. carried out the experiments, analyzed the data, and wrote the paper; G.P.S. designed the experiments, analyzed the data, and wrote the paper; B.J.S. carried out the experiments, W.W.L. and H.Q.Y. analyzed the data and wrote the paper.
Supplementary Material
Supplementary Information
Acknowledgments
The authors wish to thank the National Hi-Technology Development 863 Program of China (2011AA060907), the NSFC (51178443), the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China, and the Fundamental Research Funds for the Central Universities for the partial support of this study.
References
- McCarty P. L., Bae J. & Kim J. Domestic wastewater treatment as a net energy producer–can this be achieved? Environ. Sci. Technol. 45, 7100–7106 (2011). [DOI] [PubMed] [Google Scholar]
- Gleeson T., Wada Y., Bierkens M. F. & van Beek L. P. Water balance of global aquifers revealed by groundwater footprint. Nature 488, 197–200 (2012). [DOI] [PubMed] [Google Scholar]
- Scanlon B. R. et al. Groundwater depletion and sustainability of irrigation in the US High Plains and Central Valley. Proc. Natl. Acad. Sci. USA 109, 9320–9325 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorosmarty C. J. et al. Global threats to human water security and river biodiversity. Nature 467, 555–561 (2010). [DOI] [PubMed] [Google Scholar]
- Shannon M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008). [DOI] [PubMed] [Google Scholar]
- Grant S. B. et al. Taking the “waste” out of “wastewater” for human water security and ecosystem sustainability. Science 337, 681–686 (2012). [DOI] [PubMed] [Google Scholar]
- Foley J. M., Rozendal R. A., Hertle C. K., Lant P. A. & Rabaey K. Life cycle assessment of high-rate anaerobic treatment, microbial fuel cells, and microbial electrolysis cells. Environ. Sci. Technol. 44, 3629–3637 (2010). [DOI] [PubMed] [Google Scholar]
- Guest J. S. et al. A new planning and design paradigm to achieve sustainable resource recovery from wastewater. Environ. Sci. Technol. 43, 6126–6130 (2009). [DOI] [PubMed] [Google Scholar]
- Logan B. E. & Rabaey K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 337, 686–690 (2012). [DOI] [PubMed] [Google Scholar]
- Klass D. L. Methane from anaerobic fermentation. Science 223, 1021–1028 (1984). [DOI] [PubMed] [Google Scholar]
- Jeganathan J., Nakhla G. & Bassi A. Long-term performance of high-rate anaerobic reactors for the treatment of oily wastewater. Environ. Sci. Technol. 40, 6466–6472 (2006). [DOI] [PubMed] [Google Scholar]
- Liao B. Q., Kraemer J. T. & Bagley D. M. Anaerobic membrane bioreactors: applications and research directions. Crit. Rev. Environ. Sci. Technol. 36, 489–530 (2006). [Google Scholar]
- Daelman M. R. J., van Voorthuizen E. M., van Dongen U., Volcke E. I. P. & van Loosdrecht M. C. M. Methane emission during municipal wastewater treatment. Water Res. 46, 3657–3670 (2012). [DOI] [PubMed] [Google Scholar]
- Kim J. et al. Anaerobic fluidized bed membrane bioreactor for wastewater treatment. Environ. Sci. Technol. 45, 576–581 (2011). [DOI] [PubMed] [Google Scholar]
- Bandara W. M. et al. Anaerobic treatment of municipal wastewater at ambient temperature: Analysis of archaeal community structure and recovery of dissolved methane. Water Res. 46, 5756–5764 (2012). [DOI] [PubMed] [Google Scholar]
- Charfi A., Ben Amar N. & Harmand J. Analysis of fouling mechanisms in anaerobic membrane bioreactors. Water Res. 46, 2637–2650 (2012). [DOI] [PubMed] [Google Scholar]
- Lovley D. R. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 4, 497–508 (2006). [DOI] [PubMed] [Google Scholar]
- Logan B. E. et al. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 40, 5181–5192 (2006). [DOI] [PubMed] [Google Scholar]
- Logan B. E. Microbial Fuel Cells. John Wiley & Sons, Inc. (2008). [Google Scholar]
- Wang Y. K. et al. Development of a novel bioelectrochemical membrane reactor for wastewater treatment. Environ. Sci. Technol. 45, 9256–9261 (2011). [DOI] [PubMed] [Google Scholar]
- Liu H. & Logan B. E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 38, 4040–4046 (2004). [DOI] [PubMed] [Google Scholar]
- Oh S. T. et al. Sustainable wastewater treatment: how might microbial fuel cells contribute. Biotechnol. Adv. 28, 871–881 (2010). [DOI] [PubMed] [Google Scholar]
- Chung K., Fujiki I. & Okabe S. Effect of formation of biofilms and chemical scale on the cathode electrode on the performance of a continuous two-chamber microbial fuel cell. Bioresour. Technol. 102, 355–360 (2011). [DOI] [PubMed] [Google Scholar]
- Cheng S., Liu H. & Logan B. E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 8, 489–494 (2006). [Google Scholar]
- Logan B. E., Zhang X. Y., Cheng S. A., Wang X. & Huang X. Separator Characteristics for Increasing Performance of Microbial Fuel Cells. Environ. Sci. Technol. 43, 8456–8461 (2009). [DOI] [PubMed] [Google Scholar]
- Fan Y. Z., Han S. K. & Liu H. Improved performance of CEA microbial fuel cells with increased reactor size. Energ. Environ. Sci. 5, 8273–8280 (2012). [Google Scholar]
- Horng R. Y., Shao H., Chang W. K. & Chang M. C. The feasibility study of using non-woven MBR for reduction of hydrolysed biosolids. Water Sci. Technol. 54, 85–90 (2006). [DOI] [PubMed] [Google Scholar]
- Chuang S. H., Lin P. K. & Chang W. C. Dynamic fouling behaviors of submerged nonwoven bioreactor for filtration of activated sludge with different SRT. Bioresour. Technol. 102, 7768–7776 (2011). [DOI] [PubMed] [Google Scholar]
- Fan B. & Huang X. Characteristics of a self-forming dynamic membrane coupled with a bioreactor for municipal wastewater treatment. Environ. Sci. Technol. 36, 5245–5251 (2002). [DOI] [PubMed] [Google Scholar]
- Chu H. Q., Cao D. W., Jin W. & Dong B. Z. Characteristics of bio-diatomite dynamic membrane process for municipal wastewater treatment. J. Membr. Sci. 325, 271–276 (2008). [Google Scholar]
- Jamal Khan S., Zohaib Ur R., Visvanathan C. & Jegatheesan V. Influence of biofilm carriers on membrane fouling propensity in moving biofilm membrane bioreactor. Bioresour. Technol. 113, 161–164 (2012). [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu Z., Zhang A., Chen Y. & Wang X. The role of EPS concentration on membrane fouling control: Comparison analysis of hybrid membrane bioreactor and conventional membrane bioreactor. Desalination 305, 38–43 (2012). [Google Scholar]
- Dong H., Yu H., Wang X., Zhou Q. & Feng J. A novel structure of scalable air-cathode without Nafion and Pt by rolling activated carbon and PTFE as catalyst layer in microbial fuel cells. Water Res. 46, 5777–5787 (2012). [DOI] [PubMed] [Google Scholar]
- Zhuang L., Zhou S., Li Y. & Yuan Y. Enhanced performance of air-cathode two-chamber microbial fuel cells with high-pH anode and low-pH cathode. Bioresour. Technol. 101, 3514–3519 (2010). [DOI] [PubMed] [Google Scholar]
- Chae K. J. et al. Mass transport through a proton exchange membrane (Nafion) in microbial fuel cells. Energ. Fuel. 22, 169–176 (2007). [Google Scholar]
- Ghyoot W. & Verstraete W. Reduced sludge production in a two-stage membrane-assisted bioreactor. Water Res. 34, 205–215 (2000). [Google Scholar]
- Cha J., Choi S., Yu H., Kim H. & Kim C. Directly applicable microbial fuel cells in aeration tank for wastewater treatment. Bioelectrochemistry 78, 72–79 (2010). [DOI] [PubMed] [Google Scholar]
- Virdis B., Rabaey K., Rozendal R. A., Yuan Z. & Keller J. Simultaneous nitrification, denitrification and carbon removal in microbial fuel cells. Water Res. 44, 2970–2980 (2010). [DOI] [PubMed] [Google Scholar]
- Clauwaert P. et al. Open air biocathode enables effective electricity generation with microbial fuel cells. Environ. Sci. Technol. 41, 7564–7569 (2007). [DOI] [PubMed] [Google Scholar]
- Yan H., Saito T. & Regan J. M. Nitrogen removal in a single-chamber microbial fuel cell with nitrifying biofilm enriched at the air cathode. Water Res. 46, 2215–2224 (2012). [DOI] [PubMed] [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater. 20 edn, American Public Health Association (1998). [Google Scholar]
- Verstraete W., Aelterman P., Rabaey K., Pham H. T. & Boon N. Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ. Sci. Technol. 40, 3388–3394 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information