Abstract
Background
B-lymphocyte depletion with rituximab has been shown to benefit patients with various autoimmune diseases. We have previously demonstrated that this benefit is also apparent in patients with newly diagnosed type 1 diabetes.
Objectives
The effect of rituximab on in vivo antibody responses, particularly during the period of B-lymphocyte depletion, is incompletely determined. This study was designed to assess this knowledge void.
Methods
In patients with recent-onset type 1 diabetes treated with rituximab (n = 46) or placebo (n = 29), antibody responses to neoantigen phiX174 during B-lymphocyte depletion and with hepatitis A (as a second neoantigen) and tetanus/diphtheria (as recall antigens) after B-lymphocyte recovery were studied. Anti- tetanus, diphtheria, mumps, measles, and rubella titers were measured before and after treatment by means of ELISA. Antibody titers and percentage IgM versus percentage IgG to phiX174 were measured by means of phage neutralization. B-lymphocyte subsets were determined by means of flow cytometry.
Results
No change occurred in preexisting antibody titers. Tetanus/diphtheria and hepatitis A immunization responses were protective in the rituximab-treated subjects, although significantly blunted compared with those seen in the controls subjects, when immunized at the time of B-lymphocyte recovery. Anti-phiX174 responses were severely reduced during the period of B-lymphocyte depletion, but with B-lymphocyte recovery, anti-phiX174 responses were within the normal range.
Conclusions
During the time of B-lymphocyte depletion, rituximab recipients had a decreased antibody response to neoantigens and significantly lower titers after recall immunization with diphtheria and tetanus toxoid. With recovery, immune responses return toward normal. Immunization during the time of B-lymphocyte depletion, although ineffective, does not preclude a subsequent response to the antigen.
Keywords: B lymphocytes, human, diabetes, antibodies, immunization, CD20
We completed a trial using a 4-dose course of rituximab (Rituxan; Genentech [South San Francisco, Calif] and Biogen IDEC, Inc [Cambridge, Mass]) for the treatment of recent-onset type 1 diabetes (T1D).1-5 At the 1-year primary end point, we demonstrated significant preservation of β-cell function with a lower insulin dose and better percentage of glycosylated hemoglobin. Because T1D frequently occurs in children and young adults, a period of life in which selected primary and booster immunizations are recommended, clinical use of rituximab must address its effect on protective immune responses. Previous reports of the effect of rituximab on immune responses were conducted in patients taking other immunomodulatory drugs.6,7
In our patients, with rituximab as the only immunomodulatory agent, we assessed the effect of B-lymphocyte depletion on in vivo primary and recall antibody responses. We particularly evaluated the possibility that antigen exposure during B-lymphocyte depletion would preclude subsequent response to the immunogen, a question of critical safety and mechanistic importance. By studying antibody amplification and isotype switching to phiX174, we evaluated maturation of the humoral immune response, including class-switch recombination.
Several potential antigens are available to analyze recall and de novo immune responses. Given the potential immunosuppressive activity of rituximab, such an antigen should not be infectious or replicate in human beings. For recall responses, tetanus is the most commonly studied antigen because prior immunization is ubiquitous and booster immunizations are standard medical care. For de novo responses, potential antigens include hepatitis A vaccine and the T lymphocyte–dependent antigen bacteriophage phiX174.8-10 It has been used to test immune responsiveness in patients with primary and secondary immunodeficiency.10-21
Methods
Study design and patient selection
The design, demographics, and primary outcome of the study have been reported.5 Subjects could not receive other immunomodulatory drugs or corticosteroids. Patients were randomized 2:1 to 4 weekly infusions of either rituximab (375 mg/m2) or placebo. Because we wished to analyze the effect of a complete 4-dose course of treatment on immunization, 75 subjects who received all 4 rituximab infusions or 3 or more placebo infusions form the basis of this report. For certain analyses, subjects were excluded (eg, having been previously immunized to a particular antigen). Subjects were masked to study drug assignment.
Responder definition
The within-subject coefficient of variation of 2-hour area under the curve (AUC) mean C-peptide level after a mixed meal tolerance test was calculated.22 A subject was classified as a treatment responder if the AUC mean increased from baseline to 6 months or if the AUC decreased but the within-subject coefficient of variation was less than 0.097.
Measles, mumps, and rubella serology
Subjects had been immunized during routine care as children and received no additional immunizations with these antigens during the study. At baseline (ie, before dosing with study medication) and at weeks 52 and 56, sera were obtained to determine antibody titers to measles, mumps, and rubella (MMR). Antigen-specific IgG concentrations were measured in duplicate by using commercial ELISA immunoassay kits (Diamedix/IVAX Diagnostics, Miami, Fla: Rubella IgG ELISA kit, catalog no. 720-360; Mumps IgG ELISA kit, catalog no. 720-540; Measles IgG ELISA kit, catalog no. 720-520). Results were accepted if controls performed within the expected range and values of duplicate samples had less than 2-fold differences.
Tetanus/diphtheria
Serum samples were obtained at baseline, before dosing with study medication, and at 52 weeks (before tetanus/diphtheria [Td] immunization). At 12 months (ie, 44 weeks after the last dose of study medication), subjects were immunized intramuscularly with 5 Lf of alum-precipitated tetanus toxoid and 2 Lf of diphtheria toxoid in a total volume of 0.5 mL (DECAVAC; Aventis Pasteur, Inc, Paris, France). One month after immunization, titers to Td were measured with an ELISA (Immuno-Biological Laboratories, Minneapolis, Minn: Tetanus Toxoid IgG ELISA kit, catalog no. IB79282; Diphtheria Toxoid IgG ELISA kit, catalog no. IB79219). Results are expressed in international units per milliliter.
Hepatitis A
At 12 months, subjects were immunized intramuscularly with 1 mL (1440 ELU) of hepatitis Aviral antigen (HAVRIX; Hepatitis-AVaccine, Inactivated; GlaxoSmithKline Biologicals, Research Triangle Park, NC). Subjects with a known history of hepatitis A or of hepatitis A immunization were not reimmunized. At the time of but before immunization and again 1 month after immunization, serum samples were obtained to determine titers to hepatitis A (Mediagnost/Immuno-Biological laboratories: Anti-HAV ELISA kit, catalog no. E10). A titer of greater than 20 mIU/mL was considered positive.23
phiX174
phiX174 immunization was given only to a subset of the study population because of the additional effort required for this immunization (ie, additional study visits and blood sampling). Baseline characteristics for the group immunized with phiX174 versus those who did not consent to phiX174 immunization were similar (including age) except that more female subjects participated in phiX174 immunization (50% [19/38] of the immunized subgroup were female subjects vs 29% [14/49] of those not participating, P = .048). phiX174 lot no. 11-99 (Hans D. Ochs, University of Washington, Seattle, Wash)9-17 was filter sterilized and stored at −80°C at 1 × 1011 plaque-forming units (PFU)/mL.
Subjects were immunized 4 times with phiX174 by means of intravenous injection of 2 × 109 PFU/kg body weight. The first immunization was given 2 weeks after the last dose of study medication (ie, week 6). A secondary immunization was injected 6 weeks after the primary immunization. A tertiary immunization was given at 52 weeks and a quaternary immunization was given at 58 weeks after the first dose of rituximab. Antibody activity to phiX174 was determined by means of the phage neutralization assay before and 1, 2, and 4 weeks after each immunization. Healthy volunteers (n = 52) of both sexes were immunized with phiX174 at the University of Washington by using the same standard protocol without rituximab infusion.
Concentrations of circulating phage were measured15 minutes after primary immunization to ensure intravenous delivery and 1 week later to determine phage clearance. Total phage-neutralizing antibody was determined as the rate of phage inactivation by using serially diluted serum and a standard formula expressed as the K value (Kv).9,10 A Kv of 0.01 is the lower limit of the phage inactivation assay sensitivity.9 Peak Kv was defined as the highest Kv obtained by a subject for each of the immunizations regardless of time after immunization. IgG-specific antibody to phage was determined by incubating serum with 2-mercaptoethanol, which destroys antibody of the IgM isotype.9,10
Peripheral blood lymphocyte phenotyping
Flow cytometry was performed by the Immune Tolerance Network (at Roswell Park Cancer Institute, Buffalo, NY) on fresh whole blood by using antibodies from BD Biosciences PharMingen (San Jose, Calif) with a FACSCanto (BD BioSciences) flow cytometer. Data were analyzed with WinList software.
Statistical analyses
A repeated-measures mixed model was used with adjustments for baseline titer, age, and sex and allowed for a treatment group-by-time interaction for MMR assays. Recall responses were analyzed as the percentage change from 12 to13months in an unadjusted model (Wilcoxon test). Spearman correlation was used to examine association with other factors. Analysis of B-lymphocyte flow cytometry used separate analysis of covariance models at each time point with adjustment for baseline value, age, and sex. For phiX174, any Kv value of less than 0.01 was truncated at 0.01, the lower limit of detection. Means and SDs were calculated by using the Kv natural log transformation and transformed back to the unit scale for reporting. All P values reported are 2-sided but not adjusted for multiple comparisons.
Results
Demographics
The overall demographics are shown in Table E1 (available in this article's Online Repository at http://www.jacionline.org). Thirty-five of the phiX174 group received all 4 immunizations. There were no adverse events related to any of the immunizations, including phiX174. The healthy control volunteers for phiX174 were greater than 18 years of age, with 31 female and 21 male subjects for the primary/secondary responses and 12 female and 7 male subjects for the tertiary responses. There were no healthy control subjects who had been immunized 4 times.
Effect of rituximab on B-lymphocyte populations
Rituximab depleted total circulating CD19+ B lymphocytes (Table I), which remained depleted at 12 months (P < .0001). Although the naive B-lymphocyte population had recovered (P = .33) by 12 months, the switched-memory B-lymphocyte population remained depleted by more than 50% (P < .0001, Table I). There were no changes in lymphocyte populations after immunization at month 13 compared with month 12.
Table I. B-lymphocyte populations determined by means of flow cytometry in the rituximab versus placebo control groups.
| B-lymphocyte population | Week | Rituximab group (cells/μL) | Control group (cells/μL) | P value |
|---|---|---|---|---|
| Total CD19+ B lymphocytes | 0 | 249.81 (216.79 to 287.84) | NA | |
| 5 | 1.4 (1.1 to 1.7), n = 43 | 238.2 (196.8 to 288.2), n = 23 | <.0001 | |
| 12 | 1.22 (0.9 to 1.6), n = 44 | 279.7 (229.6 to 340.6), n = 27 | <.0001 | |
| 26 | 5.4 (3.3 to 8.4), n = 43 | 216.3 (133.0 to 351.4), n = 27 | <.0001 | |
| 52 | 118.7 (97.7 to 144.3), n = 39 | 229.9 (182.2 to 290.1), n = 27 | <.0001 | |
| 56 | 109.9 (86.8 to 139.0), n = 38 | 205.3 (152.6 to 276.1), n = 24 | <.0001 | |
| CD1c+/−, IgD−, CD27+, CD19+, IgM−/+ switch memory B lymphocytes | 0 | 65.1 (54.44 to 77.76) | NA | |
| 5 | 0.9 (0.6 to 1.2), n = 39 | 53.9 (42.4 to 68.6), n = 20 | <.0001 | |
| 12 | 0.9 (0.5 to 1.5), n = 40 | 47.9 (35.3 to 64.8), n = 25 | <.0001 | |
| 26 | 2.2 (1.3 to 3.4), n = 42 | 25.8 (17.1 to 38.8), n = 26 | <.0001 | |
| 52 | 16.3 (12.8 to 20.1), n = 38 | 40.0 (30.3 to 52.8), n = 26 | <.0001 | |
| 56 | 13.4 (10.2 to 17.4), n = 36 | 37.8 (27.0 to 52.8), n = 21 | <.0001 | |
| (CD24+, IgD+, CD38−, CD19+, CD10−) naive B lymphocytes | 0 | 94.13 (75.15 to 111.92) | NA | |
| 5 | 0.7 (−0.05 to 0.2), n = 41 | 64.11 (54.6 to 75.2), n = 22 | <.0001 | |
| 12 | 2.31 (1.24 to 3.88), n = 43 | 76.2 (45.8 to 126.1), n = 26 | <.0001 | |
| 26 | 2.31 (1.24 to 3.88), n = 43 | 76.2 (45.8 to 126.1), n = 26 | <.0001 | |
| 52 | 61.82 (47.0 to 81.21), n = 38 | 73.5 (52.5 to 102.9), n = 25 | .426 | |
| 56 | 49.96 (37.3 to 66.8), n = 37 | 65.8 (45.9 to 94.1), n = 24 | .236 | |
Absolute cell counts (in cells per microliter) are expressed as geometric means with 95% confidence limits. Separate analysis of covariance models were conducted at each time point with adjustment for the baseline value, age, and sex. For treatment group comparisons of B lymphocytes over the 13-month period, a repeated-measures model with group and time was used adjusted for the baseline value, age, and sex to assess overall group change averaged over time. The log(x+1) transformation was used to account for zero values. Least-squares means and 95% confidence limits estimated by the model were transformed back to the normal scale for reporting.
NA, Not applicable.
Preservation of protective antibody titers
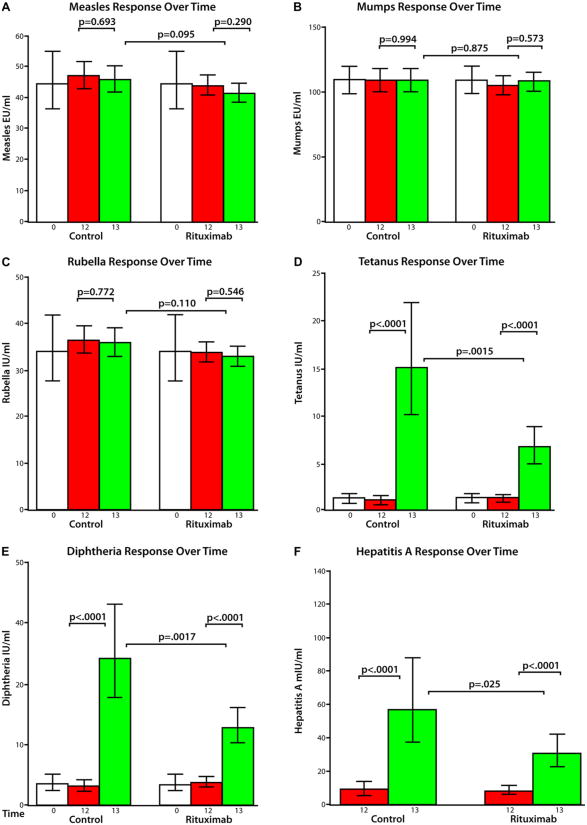
There were no changes in Td or MMR titers from baseline to 12 months (Fig 1, A-E). There was no increase in month 13 MMR titers after heterologous immunization at 12 months (Fig 1, A-C).
Fig 1.
Mean titers (and 95% confidence limits) for measles (A), mumps (B), rubella (C), tetanus (D), diphtheria (E), and hepatitis A (F) from a repeated-measures model with adjustments for baseline titer (except for hepatitis A because its baseline titer was not measured), age, and sex. Two-sided unadjusted P values are reported. Log transformation of antigen titer was used for all but mumps. Log values were transformed to normal scale for the figure.
Tetanus and diphtheria immunization
Subjects in both groups responded to tetanus (Fig 1, D) and diphtheria (Fig 1, E; <0.0001 for each group). A similar percentage of subjects within each group had a clinical response to immunization defined as greater than a 2- fold increase in ELISA titer. Eighty-three percent and 76% in the placebo control group and 67% and 69% in the rituximab-treated group mounted a tetanus or diphtheria recall response, respectively. The percentage of subjects with a clinical response did not differ between treatment groups (P = .25 for tetanus and P =.77 for diphtheria).
Hepatitis A immunization
Fifteen subjects with previous hepatitis A immunization or with anti–hepatitis A antibodies (ie, titer >20 mIU/mL) in the 12-month preimmunization sera (n = 4) were excluded from this analysis. Hepatitis A–naive subjects (n = 56) had an increase in titer (P < .0001; Fig 1, F). The response in the rituximab-treated subjects was less than in the placebo group (P =.025). A similar proportion of placebo-treated (12/18 [67% ]) and rituximab-treated (17/36 [47% ]) subjects had a protective antibody (P = .25).
phiX174 immunization
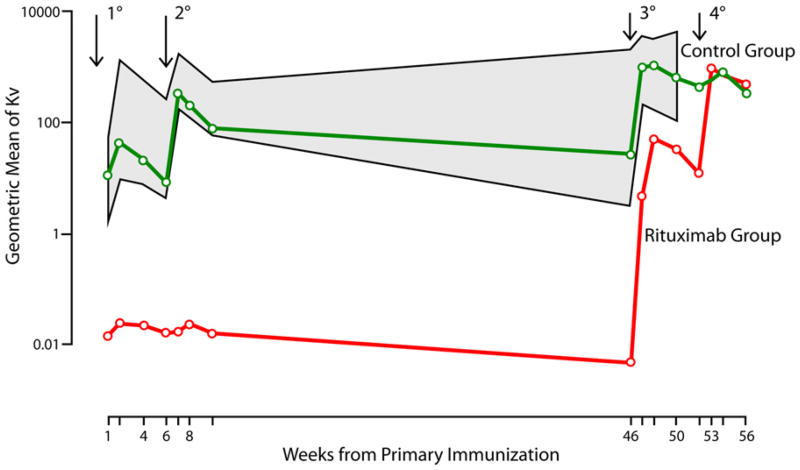
Fifteen minutes after the first immunization, all subjects had circulating phiX174 (2-4 × 107 PFU/mL serum), but no subject had circulating phiX174 at day 7, indicating an immune response sufficient to clear the phiX174. phiX174-neutralizing antibody titers are summarized (Table II, Fig 2, and Table E2 in this article's Online Repository at http://www.jacionline.org). Patients with T1D treated with placebo had anti-phage antibody responses within the range for healthy control subjects (Fig 2, shaded area). Rituximab severely inhibited anti-phiX174 after primary and secondary immunizations compared with that seen in placebo-treated subjects (P < .0001) and healthy control subjects (P < .0001, Fig 2). When rituximab-treated subjects were reimmunized (third and fourth immunizations) at 52 and 58 weeks, an antiphage response developed that qualitatively and quantitatively mirrored the primary and secondary responses seen in the placebo-treated subjects. Despite the level of response to the primary and secondary immunizations, all subjects had a tertiary response that augmented further after the quaternary immunization (see Fig E1 in this article's Online Repository at http://www.jacionline.org).
Table II. Anti-phiX174 antibody responses after phiX174 immunizations expressed as geometric mean of Kv with 95% confidence limits.
| Days after immunization | Primary response | Secondary response | ||||
|---|---|---|---|---|---|---|
| Rituximab (n = 20) | Placebo control (n = 15) | Healthy subjects (n = 52) | Rituximab (n = 20) | Placebo Control (n = 15) | Healthy subjects (n = 52) | |
| 7 | 0.02 (0-0.53) | 10 (2-49) | 9 (1.5-50) | 0.02 (0-0.27) | 325 (34-3152) | 550 (165-1827) |
| 14 | 0.03 (0-1.2) | 37 (2-577) | 114 (9-1461) | 0.03 (0-0.62) | 187§ (15-2272) | 357 (113-1126) |
| 28 | 0.03 (0-0.53) | 17 (0.76-400) | 65 (9-565) | 0.02 (0-0.18) | 69 (5-953) | 183 (60-555) |
| P ≤ .0001* | P ≤ .0001* | |||||
| P = .0186† | P = .0155† | |||||
| P ≤ .0001‡ | P ≤ .0001‡ | |||||
| Tertiary response | Quaternary response | |||||
| Days after immunization | Rituximab (n = 16) | Placebo control (n = 15) | Healthy subjects (n = 19) | Rituximab (n = 13) | Placebo control (n = 15) | Healthy subjects (NA) |
| 7 | 4.74 (0.18-123) | 926 (200-4293) | 878 (214-3603) | 902 (186-4378) | 555 (91-3389) | NA |
| 14 | 51 (3.5-754) | 1022‖ (255-4103) | 704 (156-3171) | 768 (181-3267) | 687¶ (128-3693) | NA |
| 28 | 32 (0.92-643) | 579 (123-2715) | 664 (103-4285) | 338 (85-1346) | 450 (89-2286) | NA |
| P ≤ .0001* | P = .8740* | |||||
| P = .7423† | ||||||
| P ≤ .0001‡ | ||||||
P values are from a repeated-measures mixed model using the log-transformed Kv as the dependent variable adjusting for a time component only. A model was computed separately for primary, secondary, tertiary, and quaternary responses:
P < .0001 for 2 weeks after secondary response in rituximab group versus control group;
P < .0001 for 2 weeks after tertiary response in rituximab group versus control group;
P = .7318 for 2 weeks after quaternary response in rituximab group versus control group.
NA, Not applicable.
P value for rituximab versus placebo control comparison.
P value for placebo control versus healthy subjects comparison.
P value for rituximab versus healthy subjects comparison.
Fig 2.
Antibody response to phiX174 immunization over time in rituximab-treated and placebo-treated subjects and healthy control subjects (gray hatched area). Immunization times are indicated by arrows. Time 0 is before rituximab treatment. Healthy control subjects were only immunized 3 times. Geometric means were calculated by using natural logarithms and transformed back by means of exponentiation. P values are from a repeated-measures model.
Placebo-treated subjects demonstrated normal isotype switching from predominantly IgM after the first immunization to predominantly IgG after subsequent immunizations (Table III). The very weak early antiphage response in rituximab-treated subjects was all IgM and showed no evidence of switching to IgG after secondary immunization. At the time of B-lymphocyte recovery, rituximab-treated subjects had an isotype switch, although there was a lower percentage of IgG compared with that seen in the placebo-treated subjects at the time of secondary, tertiary, and quaternary responses (Table III).
Table III. Percentage of IgG anti-phiX174 antibody after phiX174 immunization (least-squares mean with 95% mean confidence limits).
| Time point | Rituximab group | Control group | P value |
|---|---|---|---|
| 2 wk after secondary | 1.3 (−6.1 to 8.7), n = 20 | 46.6 (38.3 to 55.0), n = 16 | <.0001 |
| 2 wk after tertiary | 0.00 (−8.0 to 8.0), n = 17 | 88.3 (79.9 to 96.6), n = 16 | <.0001 |
| 2 wk after quaternary | 29.6 (20.5 to 38.8), n = 13 | 90.3 (81.9 to 98.6), n = 16 | <.0001 |
The ratio of IgM/IgG activities against phiX174 was determined by using the in vitro treatment of serum with 2-mercaptoethanol (to eliminate IgM) for 30 minutes before the phage neutralization assay and is expressed as a percentage of IgG.9,10 Analysis used a repeated-measures model with a treatment group-by-time interaction adjusting for age and sex.
Correlation of immune responses to glucose control
There was no correlation of antibody titers between rituximab-treated therapeutic responders and nonresponders: for tetanus, 56% of nonresponders had a recall response versus 76% of responders (P =.20); for diphtheria, 65% of nonresponders had a recall response versus 74% of responders (P =.73); and there was no correlation between the percentage of glycosylated hemoglobin at 12 months and responses to immunization for tetanus (r = −0.019, P = .09), diphtheria (r = −0.136, P = .41), hepatitis A (r = −0.117, P =.50), or phiX174 (r = 0.131, P =.63).
Effect of age on immune response
There was no association between age and responses to immunization for tetanus (rituximab, P = .39; placebo, P = .23), diphtheria (rituximab, P = .77; placebo, P = .68), hepatitis A (rituximab, P =.22; placebo, P =.16), or phiX174 (rituximab, P = .50; placebo, P = .86).
Effect of B-lymphocyte recovery on immune responses
We hypothesized that the degree of recovery of the various types (eg, memory vs naive) could have an effect on the immune responses to immunization at 12 months. However, there was no correlation between the absolute number of lymphocyte subpopulations at 12 months and the response at 56 weeks for total CD19 B lymphocytes (tetanus, P =.68; diphtheria, P =.64; hepatitis A, P =.57; phiX174, P = .86), naive B lymphocytes (tetanus, P = .57; diphtheria, P = .64; hepatitis A, P = .30; phiX174, P = .83), or switch memory B lymphocytes (tetanus, P =.56; diphtheria, P =.71; hepatitis A, P =.70; phiX174, P = .61).
Discussion
This study presents the most detailed analysis to date of in vivo humoral immune responses in subjects after rituximab treatment, including responses to both recall antigens and neoantigens, particularly during the period of maximal B-lymphocyte depletion. Of significance, because the subjects had not received prior or concurrent immunomodulatory drugs that might affect general immune responses or B-lymphocyte function, the data represent an uncontaminated test of rituximab's effect. Such data provide important safety information and needed guidance for immunizations in patients receiving treatment with rituximab. Our study confirms the maintenance of protective antibody titers and the successful (albeit slightly depressed) recall and de novo responses after B-lymphocyte recovery and, more importantly, that antigen exposure during B-lymphocyte depletion did not preclude subsequent responses.
As others and we have reported previously, B lymphocytes recover over a period of 1 year after rituximab treatment.24-26 The recovery is not uniform because naive B lymphocytes completely recover by 1 year, whereas memory B lymphocytes remain depleted.25,26 In the current study the memory B-lymphocyte population was initially depleted to the same degree as total CD19 B lymphocytes and naive B lymphocytes. However, memory B lymphocytes were much slower to recover. Moreover, we did not observe an increase in the memory B-lymphocyte population 1 month after the immunizations given at month 12.
Rituximab has little effect on circulating antibody titers to previous immunizations in either adults5,27 or children.5,28 Although rituximab results in a reduction in IgM levels, it does not affect serum IgG levels.5,26,29 IgG antibodies are made by long-lived plasma cells that do not express CD20 on their surface and thus are not eliminated with rituximab. In healthy adults the half-life of anti-MMR titers after live virus vaccine have been estimated to be greater than the life of a person,30 and we saw no change in titers in our study.
Several studies have evaluated the responses to various antigens after treatment with rituximab. Recall responses to tetanus are substantially reduced when immunization is given early (4 weeks)6 or late (6 and 9 months)31 after completing rituximab. Recall responses to diphtheria immunization after rituximab treatment have not been reported previously. In our study the response to Td after rituximab was diminished compared with that seen in the placebo group, but adequate protection was achieved. Although it has been reported in patients with long-standing T1D that responses to diphtheria toxoid are reduced, we saw no such effect (the anti-diphtheria geometric mean titer after booster vaccination of 4.84 IU/mL [95% CI, 3.53-6.63 IU/mL; range, 0.52-11.72 IU/mL] was in the normal range).32
Although response to Pneumococcus species is often used in evaluation of immunodeficiency, we did not include it because polysaccharide vaccines are associated with hyporesponsiveness, and their use could potentially jeopardize the recall response of the recipients when re-exposed to the same antigen.
The de novo response to hepatitis A immunization has been studied in patients treated with rituximab for malignant diseases, but the results were ambiguous.6 Patients with T1D have been reported to have an attenuated anti–hepatitis A response (ELISA titer: 53 IU/L in patients with T1D vs 212 IU/L in healthy control subjects, P = .017).32 In our placebo group the anti–hepatitis A response was 57 mIU/mL (95% CI, 37-88 mIU/mL; range, 7-269 mIU/mL); 14 of 18 achieved a protective titer of greater than 20 mIU/mL. We saw a significant increase in hepatitis A responses after recovery from rituximab, although to a lower degree than in the control group.
In healthy subjects phiX174 circulates for 3 to 4 days after primary intravenous immunization until the induced IgM antibody response neutralizes and eliminates the phage before day 7. Because no phiX174 was detected at 7 days after initial immunization, even the very low amount of IgM produced by the rituximab-treated subjects was sufficient to neutralize and remove this antigen from the circulation. Thus the rituximab-induced B-lymphocyte defect is not as profound as in patients with X-linked agammaglobulinemia because of mutation of Bruton tyrosine kinase or in those with T-B severe combined immunodeficiency in whom clearance of phage can be delayed for weeks9,10 and resembles that seen in patients with X-linked agammaglobulinemia with some residual function of Bruton tyrosine kinase, children with adenosine deaminase deficiency, patients with Wiskott-Aldrich syndrome,13 or a subset of patients with common variable immune deficiency.9-12 A possible source of this IgM could be marginal zone B lymphocytes that, despite expressing CD20, have been suggested to be resistant to rituximab depletion.33 Germinal center B lymphocytes, the site of isotype switching, are sensitive to rituximab and thus could explain the lack of an IgG response while rituximab is present in the circulation.33
An important aspect of our study is the analysis of a recall antibody response to an antigen first seen during the period of B-lymphocyte depletion and again when B lymphocytes have recovered. It was hypothesized that such immunization precludes a subsequent response to the antigen at the time of subsequent immunizations similar to what has been seen in patients with HIV infection.18,19 However, the response of rituximab-treated subjects to a tertiary and quaternary immunization with phiX174 recapitulates the pattern seen in the placebo-treated subjects and healthy control subjects after primary and secondary immunization. This indicates that in the presence of rituximab, a memory B-lymphocyte response was not generated. Rituximab has been shown to persist in the blood up to 9 months after the first dose of rituximab.5 Because CD27+ memory B lymphocytes express CD20, they are expected to be lysed as soon as they begin to appear.
DiLillo et al34 reported an extensive analysis of immunizations in mice that were depleted with an anti-murine CD20 antibody. Although their results are similar to ours in some ways, there are some differences as well, perhaps related to differences in physiology between murine and human responses.
Heterologous (ie, bystander) immune responses have been postulated as a mechanism of antibody persistence.35 Bernasconi et al35 hypothesized “that during an antigen-specific response, the increased availability of activated T cells will lead to increased production of plasma cells of unrelated specificities.” They reported that tetanus immunization increased both tetanus-specific and antigen-nonspecific circulating plasma cells. A corollary to this finding would be an increase in detectable levels of nonspecific antibody, but they did not report data for nonspecific antibodies. Di Genova et al36 found that an anti-tetanus antibody response was not associated with changes in antibodies to Candida species or purified protein derivative. Amanna et al30 saw no increase in antibody titers to 7 independent antigens in healthy subjects followed for 60 days after a vaccinia virus booster immunization. We, too, did not see any change in nonspecific antibody titers to MMR 1 month after multiple immunizations with Td, hepatitis A, and phiX174. These 3 independent assessments bring into question the bystander hypothesis for antibody persistence long after antigen exposure.
In summary, treatment with rituximab has a profound effect on immunization given during the time of B-lymphocyte depletion. With recovery, the immune response returns toward normal. Immunization during the time of B-lymphocyte depletion, although ineffective, does not preclude a subsequent response to the antigen.
Supplementary Material
Clinical implications.
Rituximab profoundly depletes antibody responses. With recovery of B lymphocytes, the response normalizes. Immunization during the time of B-lymphocyte depletion, although ineffective, does not induce tolerance to the antigen.
Acknowledgments
The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. TrialNet is a clinical trials network funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Research Resources; the Juvenile Diabetes Research Foundation International; and the American Diabetes Association.
Abbreviations used
- AUC
Area under the curve
- Kv
K value
- MMR
Measles, mumps, and rubella
- PFU
Plaque-forming units
- Td
Tetanus/diphtheria
- T1D
Type 1 diabetes
Footnotes
Disclosure of potential conflict of interest: T R. Torgerson has consultant arrangements with Baxter Biosciences. J. M. Lachin has consultant arrangements with Genentech, Bayhill Therapeutics, GlaxoSmithKline, and TolerRx. C. Greenbaum receives research support from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, the Juvenile Diabetes Research Foundation International, and the National Institutes of Health/National Institute of Allergy and Infectious Diseases. J. S. Skyler is on the Board of Directors for Amylin Pharmaceuticals and DexCom Inc, has consultant arrangements with SanofiAventis and BD Technologies, and has received research support from Bayhill Therapeutics, Halozyme Inc, and Osiris Therapeutics. The rest of the authors have declared that they have no conflict of interest.
References
- 1.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–32. [PubMed] [Google Scholar]
- 2.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 3.Tobinai K, Kobayashi Y, Narabayashi M, Ogura M, Kagami Y, Morishima Y, et al. Feasibility and pharmacokinetic study of a chimeric anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab) in relapsed B-cell lymphoma The IDEC-C2B8 Study Group. Ann Oncol. 1998;9:527–34. doi: 10.1023/a:1008265313133. [DOI] [PubMed] [Google Scholar]
- 4.Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–66. [PubMed] [Google Scholar]
- 5.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–52. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Kolk LE, Baars JW, Prins MH, van Oers MHJ. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100:2257–9. [PubMed] [Google Scholar]
- 7.Gonzalez-Stawinski GV, Yu PB, Love SD, Parker W, Davis RD., Jr Hapten-induced primary and memory humoral responses are inhibited by the infusion of anti-CD20 monoclonal antibody (IDEC-C2B8, Rituximab) Clin Immunol. 2001;98:175–9. doi: 10.1006/clim.2000.4980. [DOI] [PubMed] [Google Scholar]
- 8.Fudenberg H, Good RA, Goodman HC, Hitzig W, Kunkel HG, Roitt IM, et al. Primary immunodeficiencies. Report of a World Health Organization Committee Pediatrics. 1971;47:927–46. [PubMed] [Google Scholar]
- 9.Wedgwood RJ, Ochs HD, Davis SD. The recognition and classification of immunodeficiency diseases with bacteriophage phiChi 174. Birth Defects Orig Artic Ser. 1975;11:331–8. [PubMed] [Google Scholar]
- 10.Ochs HD, Davis SD, Wedgwood RJ. Immunologic responses to bacteriophage phi-X 174 in immunodeficiency diseases. J Clin Invest. 1971;50:2559–68. doi: 10.1172/JCI106756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochs HD, Buckley RH, Kobayashi RH, Kobayashi AL, Sorensen RU, Douglas SD, et al. Antibody responses to bacteriophage phi X174 in patients with adenosine deaminase deficiency. Blood. 1992;80:1163–71. [PubMed] [Google Scholar]
- 12.Seyama K, Nonoyama S, Gangsaas I, Hollenbaugh D, Pabst HF, Aruffo A, et al. Mutations of the CD40 ligand gene and its effect on CD40 ligand expression in patients with X-linked hyper IgM syndrome. Blood. 1998;92:2421–34. [PubMed] [Google Scholar]
- 13.Ochs HD, Slichter SJ, Harker LA, Von Behrens WE, Clark RA, Wedgwood RJ. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. 1980;55:243–52. [PubMed] [Google Scholar]
- 14.Fass L, Ochs HD, Thomas ED, Mickelson E, Storb R, Fefer A. Studies of immunological reactivity following syngeneic or allogeneic marrow grafts in man. Transplantation. 1973;16:630–40. doi: 10.1097/00007890-197312000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Witherspoon RP, Kopecky K, Storb RF, Flournoy N, Sullivan KM, Sosa R, et al. Immunological recovery in 48 patients following syngeneic marrow transplantation or hematological malignancy. Transplantation. 1982;33:143–9. doi: 10.1097/00007890-198202000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein LJ, Ochs HD, Wedgwood RJ, Rubinstein A. Defective humoral immunity in pediatric acquired immune deficiency syndrome. J Pediatr. 1985;107:352–7. doi: 10.1016/s0022-3476(85)80505-9. [DOI] [PubMed] [Google Scholar]
- 17.Ochs HD, Junker AK, Collier AC, Virant FS, Handsfield HH, Wedgwood RJ. Abnormal antibody responses in patients with persistent generalized lymphadenopathy. J Clin Immunol. 1988;8:57–63. doi: 10.1007/BF00915157. [DOI] [PubMed] [Google Scholar]
- 18.Rubinstein A, Mizrachi Y, Bernstein L, Shliozberg J, Golodner M, Liu GQ, et al. Progressive specific immune attrition after primary, secondary and tertiary immunizations with bacteriophage phi X174 in asymptomatic HIV-1 infected patients. AIDS. 2000;14:F55–62. doi: 10.1097/00002030-200003100-00004. [DOI] [PubMed] [Google Scholar]
- 19.Fogelman I, Davey V, Ochs HD, Elashoff M, Feinberg MB, Mican J, et al. Evaluation of CD4+ T cell function In vivo in HIV-infected patients as measured by bacteriophage phiX174 immunization. J Infect Dis. 2000;182:435–41. doi: 10.1086/315739. [DOI] [PubMed] [Google Scholar]
- 20.Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–52. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bearden CM, Agarwal A, Book BK, Vieira CA, Sidner RA, Ochs HD, et al. Rituximab inhibits the in vivo primary and secondary antibody response to a neoantigen, bacteriophage phiX174. Am J Transplant. 2005;5:50–7. doi: 10.1111/j.1600-6143.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- 22.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, Battelino T, Haastert B, Ludvigsson J, et al. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31:1966–71. doi: 10.2337/dc07-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiedermann G, Ambrosch F, Kollaritsch H, Hofmann H, Kunz C, D'Hondt E, et al. Safety and immunogenicity of an inactivated hepatitis A candidate vaccine in healthy adult volunteers. Vaccine. 1990;8:581–4. doi: 10.1016/0264-410x(90)90013-c. [DOI] [PubMed] [Google Scholar]
- 24.Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8 in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–66. [PubMed] [Google Scholar]
- 25.Sidner RA, Book BK, Agarwal A, Bearden CM, Vieira CA, Pescovitz MD. In vivo human B-cell subset recovery after in vivo depletion with rituximab, anti-human CD20 monoclonal antibody. Hum Antibodies. 2004;13:55–62. [PubMed] [Google Scholar]
- 26.Bingham CO, 3rd, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62:64–74. doi: 10.1002/art.25034. [DOI] [PubMed] [Google Scholar]
- 27.Cambridge G, Leandro MJ, Teodorescu M, Manson J, Rahman A, Isenberg DA, et al. B cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profiles. Arthritis Rheum. 2006;54:3612–22. doi: 10.1002/art.22211. [DOI] [PubMed] [Google Scholar]
- 28.Rao A, Kelly M, Musselman M, Ramadas J, Wilson D, Grossman W, et al. Safety, efficacy, and immune reconstitution after rituximab therapy in pediatric patients with chronic or refractory hematologic autoimmune cytopenias. Pediatr Blood Cancer. 2008;50:822–5. doi: 10.1002/pbc.21264. [DOI] [PubMed] [Google Scholar]
- 29.Vieira CA, Agarwal A, Book BK, Sidner RA, Bearden CM, Gebel HM, et al. Rituximab for reduction of anti-HLA antibodies in patients awaiting renal transplantation: 1. Safety, pharmacodynamics, and pharmacokinetics. Transplantation. 2004;77:542–8. doi: 10.1097/01.tp.0000112934.12622.2b. [DOI] [PubMed] [Google Scholar]
- 30.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz SM, Negrin RS, Blume KG, Breslin S, Stuart MJ, Stockerl-Goldstein KE, et al. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood. 2004;103:777–83. doi: 10.1182/blood-2003-04-1257. [DOI] [PubMed] [Google Scholar]
- 32.Eibl N, Spatz M, Fischer GF, Mayr WR, Samstag A, Wolf HM, et al. Impaired primary immune response in type-1 diabetes: results from a controlled vaccination study. Clin Immunol. 2002;103:249–59. doi: 10.1006/clim.2002.5220. [DOI] [PubMed] [Google Scholar]
- 33.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–26. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 34.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–71. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 35.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 36.Di Genova G, Roddick J, McNicholl F, Stevenson FK. Vaccination of human subjects expands both specific and bystander memory T cells but antibody production remains vaccine specific. Blood. 2006;107:2806–13. doi: 10.1182/blood-2005-08-3255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.