Abstract
Purpose
Preclinical studies suggest that inhibition of vascular endothelial growth factor (VEGF) improves glioma response to radiotherapy. Bevacizumab, a monoclonal antibody against VEGF, has shown promise in recurrent gliomas, but the safety and efficacy of the concurrent use of bevacizumab with brain irradiation has not been extensively studied. The objectives of this study were to determine the safety and activity of this combination in malignant gliomas.
Methods and Materials
After prior treatment with standard radiation therapy patients with recurrent glioblastoma (GBM) and anaplastic gliomas (AG) received bevacizumab (10 mg/kg IV) every 2 weeks of 28-day cycles until tumor progression. Patients also received 30 Gy of hypofractionated stereotactic radiotherapy (HFSRT) in 5 fractions after the first cycle of bevacizumab.
Results
Twenty-five patients (20 GBM and 5 AG) median age of 56 years (range, 30 to 80) and median KPS 90 (range, 70 to 100) received a median of 7 cycles of bevacizumab. One patient did not undergo HFSRT because overlap with prior radiotherapy would exceed the safe dose allowed to the optic chiasm. Three patients discontinued treatment due to: grade 3 CNS intratumoral hemorrhage, wound dehiscence and bowel perforation. Other non-hematologic and hematologic toxicities were transient. No radiation necrosis was seen in these previously-irradiated patients. For the GBM cohort, overall response rate was 50%, 6-month progression free survival was 65%; median overall survival was 12.5 months and 1-year survival was 54%.
Discussion
Bevacizumab in combination with HFSRT is safe and well tolerated. Radiographic responses, duration of disease control and survival suggest that this regimen is active in recurrent malignant glioma.
Keywords: malignant gliomas, glioblastoma, bevacizumab, anti-angiogenesis, intensity-modulated radiation therapy
INTRODUCTION
Radiotherapy (RT) has been shown to be the most effective adjuvant treatment for malignant gliomas but has only limited benefit in these tumors because of intrinsic radioresistance and the limited radiation tolerance of surrounding normal brain (1, 2). Attempts to improve the therapeutic index of brain tumor irradiation by localized dose escalation, altered fractionation, and radiosensitization have failed to significantly affect survival of patients with malignant gliomas (3).
Malignant gliomas are innately hypoxic tumors with strong endogenous expression of hypoxia-inducible factor -1 (HIF-1), VEGF, and VEGF receptors and consequently demonstrate vigorous angiogenesis (4–7). Tumor xenografts, including U87 gliomas, induce vascular endothelial growth factor (VEGF) expression in response to irradiation, which may serve to protect their endothelium.(8, 9) Furthermore, adding VEGF to cultures of human umbilical endothelial cells enhances radioresistance (8).
Bevacizumab, a humanized monoclonal antibody to VEGF, has been used with safety and clinical success with concomitant chemotherapy in solid tumors (10–12). In recurrent malignant gliomas it has been used alone and in combination with irinotecan (13–15). Bevacizumab has been successfully used in patients undergoing radiotherapy and chemotherapy for solid tumors, including glioblastomas (16–19). Reasons to combine bevacizumab and RT include the ability of antiangiogenic agents to sensitize tumor endothelium to RT by depletion of VEGF and reduction of its pro-survival signaling (8, 20). It is known that blockade of the VEGF receptor-2 by the monoclonal antibody DC101 can lower the dose of radiation needed to control 50% of tumor xenografts, including the glioblastoma U87 (21). Recent evidence points to a population of radioresistant glioma stem cells residing within vascular niches. These stem cells may be a nidus for regrowth following RT but, promisingly, this niche can be disrupted by bevacizumab in xenograft brain tumor models (22, 23). Garcia-Barros et al have found that at a single dose threshold of approximately 8–10 Gy, the endothelium in tumor xenografts undergoes apoptosis, legitimizing tumor endothelium as an additional target for radiotherapy (24). Early clinical trials have shown efficacy of stereotactic high dose fraction irradiation for paraspinal and brain metastases, lung cancer, pancreatic cancer and renal cancer (25–32). The threshold for endothelial apoptosis in glioblastoma endothelium is not clear, therefore, we chose an aggressive fractionation scheme to optimize the antiendothelial impact, particularly since bevacizumab could maximize the effects of radiation on this target (8).
We hypothesized that a combined approach of hypofractionated stereotactic radiotherapy (HFSRT) with VEGF inhibition would be an effective strategy for malignant glioma. The study was performed in previously-irradiated patients with recurrences as a pilot to assess the safety of bevacizumab used during RT for glioma treatment in general and also the potential of this regimen in particular for patients at all stages of disease. Correlative markers of response to anti-angiogenic therapy are being actively sought (33); perfusion MRI imaging was done in some patients in order to assess changes in tumor perfusion after bevacizumab administration.
METHODS AND MATERIALS
Patient eligibility
Patients were recruited from March 2006 to February 2008. Adult patients (≥ 18 years of age) with histopathologic confirmation of malignant glioma who had recurrent or progressive tumor and had failed prior RT were eligible. Brain MRI needed to show a circumscribed enhancing tumor ≤ 3.5 cm in its largest diameter; surgery for recurrent malignant glioma could be offered prior to enrollment in this protocol but at least four weeks had to elapse between the surgery and first dose of bevacizumab. Additional eligibility criteria included KPS ≥70, adequate bone marrow function (hemoglobin ≥ 10g/dL, absoluteneutrophil count ≥ 1,500/mm3, platelet count ≥ 100,000/mm3), adequate liver function (bilirubin <1.5 times the upper limit of normal (ULN), AST and ALT ≤ 3 times the ULN, alkaline phosphatase ≤ 2 times the ULN), adequate renal function (BUN and creatinine <1.5 times the ULN), and life expectancy ≥12 weeks. At least four weeks must have elapsed from major surgery, open biopsy or significant traumatic injury, one week from minor surgical procedures, six weeks from RT, four weeks from prior cytotoxic therapy and one week from non-cytotoxic agents; patients must have recovered from all toxicities of prior therapies. Patients who received prior treatment with bevacizumab, or had a history of hypertensive crisis or hypertensive encephalopathy, stroke or transient ischemic attack, symptomatic peripheral vascular disease, grade 2 congestive heart failure, unstable angina or myocardial infarction within 12 months of enrollment, peptic ulcer, abdominal fistula, gastrointestinal perforation or intra-abdominal abscess within six months of enrollment were excluded. Additional exclusion criteria included blood pressure >150/100 mmHg, ongoing use of anticoagulant or antiplatelet agents, pregnant or nursing women, urine protein:creatinine ratio ≥1.0 at baseline, non-healing wound, ulcer or bone fracture and prior spontaneous CNS hemorrhage as determined from clinical history or preoperative CT or MRI scan. Agreement to use an acceptable method of birth control was required for men and women with reproductive potential.
Study design
The study was approved by the Memorial Sloan-Kettering Cancer Center Institutional Review Board and written informed consent was obtained from all patients. Baseline evaluation included gadolinium-enhanced brain MRI with gradient echo sequence and perfusion, complete physical and neurological examination, blood and urine tests within two weeks prior to treatment. Patients received bevacizumab 10 mg/kg every 14 days on days 1 and 15 of 28-day cycles until treatment failure. After completion of the first cycle, patients underwent a physical and neurological examination and a repeat brain MRI with gradient echo sequence, perfusion for RT planning. If patients had a response or had stable findings on MRI they proceeded to radiation therapy.
Radiation technique
Patients underwent an MRI with 1.5mm slices within 1 week of a treatment planning CT with 3mm slices. MRI was fused with the treatment planning CT and a gross tumor volume (GTV) was designed based on the contrast enhancing lesion. The planning treatment volume (PTV) typically was defined as the GTV plus a 5mm margin. At the time of the treatment planning CT an immobilization device was created for each patient. The first 11 patients were immobilized with a Gil-Thomas-Cosman (GTC) frame and the subsequent 13 patients were immobilized with a 7 point face mask system. Treatment planning was performed with either the BrainLAB system for the patients with a GTC frame or the MSKCC treatment planning system for patients treated with a facemask. A single isocenter plan was utilized for each patient and IMRT with a sliding window technique was used.
A total dose of 30 Gy (6 Gy x 5 fractions) was prescribed to the 100% isodose line for all plans. HFSRT started on day 7–10 of cycle 2 and was delivered over a two and half week period. A median number of 9 beams (range 2 to 11 beams) were used. The median planning target volume was 34 cm3 (range 2 to 62 cm3). Brain MRI was repeated every two cycles after cycle 2.
Response and Toxicity Evaluation
Response to treatment was evaluated by brain MRI and neurological status according to the Macdonald criteria (34). Toxicity was evaluated using the National Cancer Institute’s Common Toxicity Criteria, version 3.0, throughout the clinical trial until 30 days after removal from the protocol.
Perfusion MRI and Analysis
In a subset of patients, perfusion MRI, a non-invasive method of assessing cerebral microvascular environment, was performed at baseline and after the first cycle of bevacizumab before starting HFSRT. Dynamic contrast enhanced (DCE) MRI, a technique that provides quantitative measure of the cerebral microvasculature such as permeability represented by Ktrans (volume transfer constant) and fractional blood volumes (fBV) (35) and dynamic susceptibility contrast (DSC) MRI, a method that provides information on the relative cerebral blood volume (rCBV) were used.
The MRI scans were all acquired during single imaging sessions using 1.5 Tesla TwinSpeed scanners (GE Medical Systems, Milwaukee, WI) equipped with a standard quadrature birdcage head coil. The sequences were obtained in the following order: pre-contrast anatomic, DCE perfusion, post-contrast anatomic, and finally DSC perfusion using a second bolus of contrast agent. The contrast agent, gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ), at a dose of 0.1 mmol per kilogram of body weight and rate of 3 ml/s was administered through the antecubital vein using a power injector. The DSC perfusion imaging was done last using a second, identical bolus of contrast which was advantageous due to presaturation and extravasation correction from the first bolus.
The DCE maps, Ktrans and fBV, were obtained using software with in-house modifications. The mean time courses for regions of interest (ROI) in the tumors were fitted by applying the Tofts two compartmental model to obtain the bolus wash-in slopes (1/minute) (35).
Statistics
The primary endpoint was safety of bevacizumab given in combination with HFSRT for recurrent malignant glioma. Safety was measured by the occurrence of grade 3 or higher non-hematologic toxicity; an early stopping rule was included to halt accrual if five treatment-related toxicities of grade 3 or higher were observed. Because the toxicity of greatest concern was CNS hemorrhage, if two or more grade 2 or higher symptomatic CNS hemorrhages were observed, the trial would have been terminated. The study planned to accrue 25 patients with the expectation that at least 20 patients would receive the proposed treatment with bevacizumab and HFSRT. Secondary endpoints included 6-month progression-free survival (PFS), response rate and overall survival (OS).
Demographic, safety, laboratory data and treatment response were analyzed using descriptive statistics; survival analyses were based on Kaplan-Meier estimates. For all patients, PFS was measured from enrollment in the study to disease progression, removal from study due to toxicity, last contact or death. OS was measured from study entry to death or last follow-up. Survival analyses were performed in an intent-to-treat fashion. Follow-up extended thru June 2008. Mean and standard deviation for the wash-in slopes and the ratios of Ktrans, fBV and rCBV pre and post-cycle 1 of bevacizumab were calculated and a paired t-test was performed. A p value of less than 0.05 was considered significant.
RESULTS
Patients Characteristics
Twenty-five patients (14 men, 11 women) with histologically confirmed malignant glioma (20 GBM, 5 anaplastic gliomas [AG]) were enrolled in this trial (Table 1). The median age was 56 years (range, 30 to 80) and median Karnofsky performance scale (KPS) was 80 (range, 70 to 100). The median elapsed time between prior RT and study enrollment was 15 months and 23 patients (92%) had completed RT at least 6 months prior to enrollment. Two patients were enrolled two and four months after completing RT but after receiving adjuvant temozolomide; the patient who enrolled two months after RT had a hypermetabolic lesion on a fluorodeoxyglucose–positron emission tomography (FDG-PET) scan. Patients completed a median of seven cycles of bevacizumab (range, 2 to 30 cycles); one patient did not undergo HFSRT because overlap with prior RT would exceed the safe dose allowed to the optic chiasm. Of the 24 patients who received radiation therapy all had re-irradiation to the same region that was previously treated to 60 Gy except for one patient who had a recurrence outside of their initial field. For the patients who were treated using the BrainLAB software system the PTV median Dmax was 106% (range: 104–113%) and the PTV median Dmin was 97% (range 100–44%). One patient had a Dmin of 44% because the PTV overlapped the chiasm. For the patients who were treated with IMRT using the MSKCC software program the PTV median Dmax was 106% (range: 103–111%) and the PTV median D05 was 103% (range 101–107%). In these patients (treated with IMRT) 95% or more of the PTV received 100% of the dose.
Table 1.
Patient characteristics
| Characteristic | Patients (n=25) |
|---|---|
| Gender | |
| Men | 14 (56%) |
| Women | 11 (44%) |
| Median age (range) | 56 (30–80) |
| Karnofsky performance scale | |
| 70–80 | 13 (52%) |
| 90–100 | 12 (48%) |
| Prior low grade glioma | 3 (12%) |
| Histological diagnosis | |
| Glioblastoma | 20 (80%) |
| Anaplastic astrocytoma | 4 (16%) |
| Anaplastic oligodendroglioma | 1 (4%) |
| Median No. of prior recurrences (range) | 1 (1 to 4) |
| Median time from initial malignant glioma diagnosis (range) | 14.5 mos (6 to 135) |
| Median dose of prior radiotherapy (range) | 5940 cGy (5400 to 6120) |
| Median elapsed time between prior radiotherapy and study enrollment (range) | 15 mos (2.2 to 292) |
Response to treatment
Two patients underwent surgical resection for recurrent malignant glioma prior to enrollment on this trial; all 25 patients were assessable for response because there was still residual enhancing tumor on the immediate post-operative scan of these two patients. Thirteen patients (52%, 95% confidence interval [CI], 28 to 89%) had an objective radiographic response (ORR), including complete response in five and partial response in eight (Figure 1). Fifty percent of patients with GBM and 60% of patients with AG had an ORR. Twelve patients (48%) had stable disease for a median of 4.6 months (range, 2.3 to 11.5 months). Six patients required dexamethasone at the time of enrollment in the study (median dose 5 mg/daily, range: 4 to 16 mg); after three months on the clinical trial, three patients discontinued dexamethasone and three had reduction in the dose.
Figure 1.
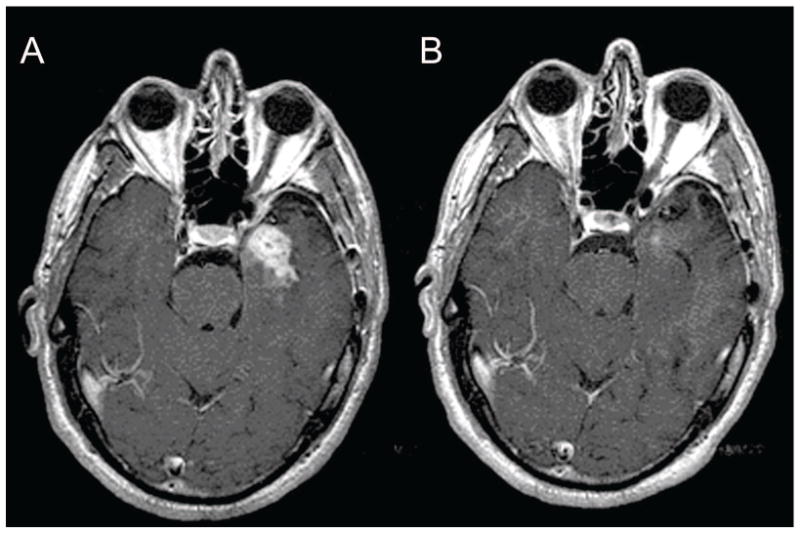
Baseline (A) and post-treatment (B) gadolinium enhanced brain MRI in a patient with glioblastoma showing a partial response.
Progression-free and overall survival
The 6-month PFS for all patients was 64% (95% CI, 42 to 79%) (Figure 2). The 6-month PFS was 65% (95% CI, 40 to 82%) for GBM and 60% (95% CI, 13 to 88%) for AG patients. The median PFS was 7.3 months (95% CI, 4.4 to 8.9 months) for GBM and 7.5 months (95% CI, 3 to not reached) for AG patients. To date, among the 25 patients enrolled, 20 patients were removed from the study (17 due to tumor progression and three due to toxicity) and 15 (60%) had died. The median follow-up among survivors was 6.6 months. Median OS was 12.5 months (95% CI, 6.9 to 22.8 months) for GBM and 16.5 months (95% CI, 8 to not reached) for the AG patients.
Figure 2.
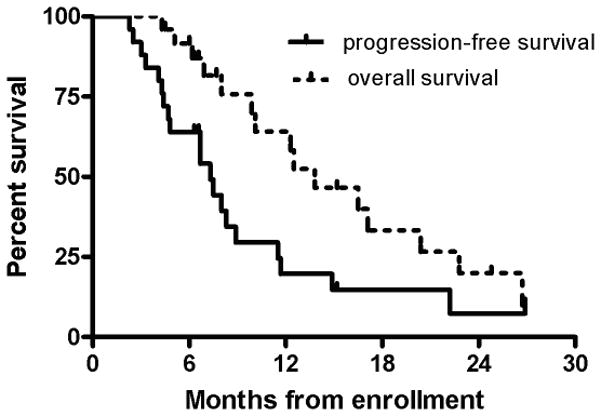
Progression-free and overall survival curves for all patients (n=25).
Safety
Grade 3 and 4 toxicity is shown in Table 2; hematological toxicity was transient and six patients had hyponatremia. Three patients discontinued treatment due to toxicity; one patient had a grade 3 CNS intratumoral hemorrhage after four cycles of bevacizumab, one had bowel perforation in the setting of chronic dexamethasone use six weeks after the last dose of bevacizumab, and one patient who had undergone craniotomy for tumor resection four weeks prior to starting bevacizumab developed wound dehiscence that required debridement, scalp rotation flap and skin graft. One patient developed lower gastrointestinal bleeding three weeks after coming off study for tumor progression. There was no clinical or radiographic radiation necrosis; moreover, no significant radiation necrosis was seen on the pathological examination of three patients who required re-operation and two patients who underwent autopsy.
Table 2.
Grade 3 and 4 toxicity.
| Number of patients (%)
|
||
|---|---|---|
| Toxicity | Grade 3 | Grade 4 |
| Leukopenia | 2 (8%) | |
| Neutropenia | 2 (8%) | |
| Lymphopenia | 7 (28%) | 2 (8%) |
| Thrombocytopenia | 2 (8%) | 1 (4%) |
| Anemia | 3 (12%) | |
| Hyponatremia | 6 (24%) | |
| Fatigue | 1 (4%) | |
| Hypertension | 1 (4%) | |
| CNS hemorrhage | 1 (4%) | |
| Bowel perforation | 1 (4%) | |
| Wound healing complication | 1 (4%) | |
| Gastrointestinal bleeding | 1(4%) | |
Perfusion MRI
Baseline perfusion MRI ratios of Ktrans (n=13), fBV (n=13) and rCBV (n=17) were higher in the tumor compared to the contralateral hemisphere with average ratios of 1.85 ± 0.80, 1.83 ± 0.91 and 2.10 ± 1.02, respectively. Ten patients underwent both DCE and DSC perfusions studies; the mean bolus wash-in slope and the mean ratios of Ktrans, fBV, and rCBV decreased after one cycle of bevacizumab (Table 3). Figure 3 illustrates the decrease in the values of Ktrans, fBV, and rCBV in the tumor areas for a GBM patient before and after one cycle of bevacizumab. The mean ratios of rCBV of four patients who only underwent DSC perfusion imaging at baseline and after cycle one also decreased. In summary, all patients who underwent perfusion imaging demonstrated diminished perfusion parameters after cycle one. Twelve patients (86%) had matching radiographic findings (decreased enhancing tumoral disease); of these 12 patients, 2 (14%) had < 25% reduction, 3 (21%) had minor response, 6 (43%) had partial response and 1 patient had complete response. In 2 patients (14%), a similar match was not observed.
Table 3.
Bolus wash-in slopes, ratios of Ktrans, fBV, and rCBV before and after one cycle of bevacizumab (n= 10 patients).
| Perfusion MRI parameter | Baseline | After 1 cycle of bevacizumab | P |
|---|---|---|---|
| Bolus wash-in slopes | 1.79±1.82 | 0.58±0.37 | 0.02 |
| Ktrans* | 1.79±0.78 | 1.24±0.21 | 0.03 |
| fBV† | 1.83±0.80 | 1.34±0.68 | 0.01 |
| rCBV†† | 1.93±0.93 | 1.23±0.28 | 0.02 |
All measurements are shown as mean ± standard deviation.
Ktrans=transfer coefficient, a marker of microvascular permeability;
fBV=fractional blood volume;
rCBV=relative cerebral blood volume.
Figure 3.
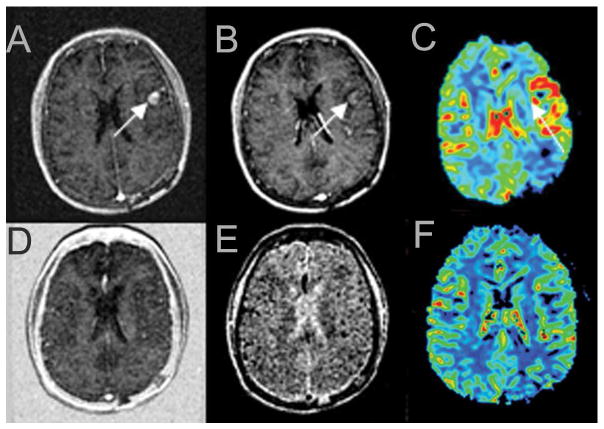
The upper panel shows pre-treatment perfusion MRI studies and the lower panel shows the perfusion MRI studies after one cycle of bevacizumab for a left frontal lobe glioblastoma patient. There are increased ratios of transfer coefficient (Ktrans) (A), fractional blood volume (fBV) (B) and relative cerebral blood volume (rCBV) (C) before treatment; Ktrans (D), fBV (E) and rCBV (F) normalized after the first cycle of bevacizumab.
DISCUSSION
Concomitant administration of bevacizumab and HFSRT in previously-irradiated malignant glioma recurrences was safe and effective in this pilot study. Overall toxicity in our study was in line with other reports of bevacizumab use in patients with malignant glioma.(13–15, 18). No significant adverse reactions were attributable to the interaction of bevacizumab with radiation, except possibly the single wound dehiscence. In fact, the combination appears to have improved the therapeutic ratio of radiation with a lower than expected side effect and toxicity profile in heavily pre-treated patients. There were no cases of radionecrosis and there was no need for additional corticosteroid use during or after radiation. A single CNS hemorrhage occurred in a patient who had tolerated the initial treatment with bevacizumab and HFSRT well, with a partial response, and received a total of four cycles of bevacizumab before developing sudden hemiparesis due to intra-tumoral bleeding. Lai et al and Narayana et al each treated 10 newly-diagnosed GBM patients with RT, temozolomide, and bevacizumab.(18, 19) Lai et al reported two cases of grade 3 wound breakdown including one in the RT phase and another in on week 21 (18). Moreover, one unexpected complication possibly attributed to the interaction of RT and bevacizumab was reported; a patient developed an optic neuropathy, felt to be due to depletion of VEGF, a possible protector of normal brain (18, 36).
The outcome of this trial compares favorably with the best prior experience with the treatment of recurrent malignant gliomas. Wong et al(37) reviewed the results of eight different chemotherapy trials for patients with recurrent glioblastomas and showed that the 6-month PFS was 15% and the median PFS was 9 weeks, with a 1-year OS of 21% compared to our 1-year OS of 54%. Improved benchmarks have been set by the introduction of bevacizumab into clinical trials for recurrent glioblastomas. Vredenburgh et al (14) reported on the treatment of 35 patients with the combination of bevacizumab and irinotecan; 6-month PFS was 46% and the 6-month overall survival was 77%. Twenty (57%) had at least a partial response (14). In a randomized trial the results in recurrent glioblastomas treated with bevacizumab with irinotecan are slightly better than with bevacizumab alone (15). Our results with HFSRT and bevacizumab compare favorably with those of either treatment group of recurrent GBM in the randomized trial.
In order to evaluate the effects of bevacizumab the results of our trial need also to be compared to those of hypofractionated re-irradiation alone for malignant glioma recurrences. We modeled our hypofractionation scheme for recurrent malignant gliomas on the report of Vordermark and his coworkers (38) who safely re-irradiated patients with recurrent malignant gliomas with hypofractionated regimens similar or identical to the one we adopted. The median OS in their study was 9.3 months from the time of HFSRT for the whole group and 7.9 months for glioblastomas (38). Our results combining bevacizumab with radiation are superior. Significantly, in the Vordermark trial the median tumor volume treated was 12.6 cm3, smaller than our median of 34 cm3. Both of these median volumes reflect selection of patients for re-irradiation as larger volumes and their consequently more complex geometries are inappropriate because of treatment planning constraints for this radiation modality and for fear of radiation injury of normal tissue. Obviously, selection of cases for small, circumscribed volumes confers a bias to our results that cannot be overcome outside a randomized study.
Independent of the potential radiosensitization effects of bevacizumab detailed above are the propitious effects of administering the drug during the post-irradiation period twice monthly until failure. Malignant gliomas relapse in their original bed in over 90% of patients (39). Maintaining bevacizumab treatment in the post-irradiation patient as done in this study may help to prevent the tumor bed from recovering to the point where it allows tumor regrowth. RT is known to damage the tumor stroma and delays regrowth, in part by the inability of the damaged bed to provide adequate vascularity (40). Revascularization after RT depends upon both sprouting of local vessels and the incorporation of bone marrow-derived VEGFR-2-positive endothelial progenitor cells and perivascular infiltration of VEGFR-1-positive myelomonocytic cells (20, 41). All of these mechanisms are suppressed by anti-VEGF therapies (41–43). Daily endostatin administered after radiation has been shown to block xenograft revascularization in mice, prolonging disease free survival (44). The growth rate of lung cancer xenografts, recurrent after irradiation, is slower in animals treated with DC101 (45).
The higher values of perfusion MRI parameters at baseline in the tumor compared to the contralateral side imply that tumor associated vasculature was leaky (high Ktrans) and vessel density was high (high fBV and rCBV); these findings are consistent with known pathological characteristics of recurrent malignant gliomas, which have high vascularity and poor capillary integrity. Perfusion MRI demonstrated a decrease in capillary permeability and microvessel density after patients received only two doses of bevacizumab, which is similar to studies of other anti-VEGF studies (42, 46). Although its implications are limited by the small number of patients studied, the mismatch observed between perfusion and anatomical imaging findings suggests that in a small subset of patients (those with stable radiographic disease), perfusion imaging may be helpful in determining response to anti-angiogenic therapy when conventional imaging does not. Larger studies are required but such imaging markers have the potential of being useful in dose-seeking studies or as early markers of response and survival for antiangiogenic agents (42, 47). VEGF was previously known as vascular permeability factor (48) and decrease in peritumoral vasogenic edema and decreased corticosteroid dependence is another benefit of anti-VEGF treatment; as demonstrated by the decrease in Ktrans (42).
This novel treatment approach combining bevacizumab with HFSRT was exceptionally well tolerated with promising signs of efficacy in heavily pretreated malignant glioma patients, suggesting that it is reasonable to increase the size of recurrences treated with this regimen and also to export this approach to a newly diagnosed patient population. The safety of RT with bevacizumab in recurrent malignant glioma patients provides important reassurance as coming trials with newly-diagnosed patients move bevacizumab into the RT window at the beginning of treatment.
Acknowledgments
Genentech provided drug and research support. We thank Judy Lampron for her expert editorial support.
Footnotes
This study has been presented in part at the 2007 Annual Meeting of the American Society of Clinical Oncology (Chicago, June 1–5, 2007) at the 49th Annual Meeting of the American Society for Therapeutic Radiology (Los Angeles, October 28 to November 1, 2007) and Oncology, at the 16th Annual Meeting of the International Society for Magnetic Resonance in Medicine (Toronto, May 3–9, 2008), and at the 46th Annual Meeting of the American Society of Neuroradiology (New Orleans, May 31–June 5, 2008).
Conflict of Interest Notification: Drs. Abrey and Gutin received research support and consultation fees from Genentech.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 2.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 3.Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev. 2000;26:397–409. doi: 10.1053/ctrv.2000.0191. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 5.Lamszus K, Ulbricht U, Matschke J, et al. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res. 2003;9:1399–1405. [PubMed] [Google Scholar]
- 6.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 8.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 9.Moeller BJ, Cao Y, Li CY, et al. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 12.Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 13.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 14.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 15.Cloughesy TF, Prados MD, Wen PY, et al. A phase II, randomized, non- comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM) J Clin Oncol. 2008;28 abstr 2010b. [Google Scholar]
- 16.Czito BG, Bendell JC, Willett CG, et al. Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: Phase I trial results. Int J Radiat Oncol Biol Phys. 2007;68:472–478. doi: 10.1016/j.ijrobp.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 18.Lai A, Filka E, McGibbon B, et al. Phase II Pilot Study of Bevacizumab in Combination with Temozolomide and Regional Radiation Therapy for Up-Front Treatment of Patients With Newly Diagnosed Glioblastoma Multiforme: Interim Analysis of Safety and Tolerability. Int J Radiat Oncol Biol Phys. 2008;71:1372–1380. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 19.Narayana A, Golfinos J, Knopp E, et al. Feasibility of using bevacizumab with radiation therapy in high grade gliomas. Int J Radiat Oncol Biol Phys. 2007;69:S51–S51. doi: 10.1016/j.ijrobp.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 20.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozin SV, Boucher Y, Hicklin DJ, et al. Vascular endothelial growth factor receptor-2-blocking antibody potentiates radiation-induced long-term control of human tumor xenografts. Cancer Res. 2001;61:39–44. [PubMed] [Google Scholar]
- 22.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 25.Beitler JJ, Makara D, Silverman P, et al. Definitive, high-dose-per-fraction, conformal, stereotactic external radiation for renal cell carcinoma. Am J Clin Oncol. 2004;27:646–648. doi: 10.1097/01.coc.0000145289.57705.07. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185–190. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848–855. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- 28.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Nyman J, Johansson KA, Hulten U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer--mature results for medically inoperable patients. Lung Cancer. 2006;51:97–103. doi: 10.1016/j.lungcan.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Shiau CY, Sneed PK, Shu HK, et al. Radiosurgery for brain metastases: relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Phys. 1997;37:375–383. doi: 10.1016/s0360-3016(96)00497-x. [DOI] [PubMed] [Google Scholar]
- 31.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 32.Brown JM, Koong AC. High-dose single-fraction radiotherapy: exploiting a new biology? Int J Radiat Oncol Biol Phys. 2008;71:324–325. doi: 10.1016/j.ijrobp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Jubb AM, Oates AJ, Holden S, et al. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006;6:626–635. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 35.Larsson HB, Tofts PS. Measurement of blood-brain barrier permeability using dynamic Gd-DTPA scanning--a comparison of methods. Magn Reson Med. 1992;24:174–176. doi: 10.1002/mrm.1910240119. [DOI] [PubMed] [Google Scholar]
- 36.Zachary I. Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- 37.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 38.Vordermark D, Kolbl O, Ruprecht K, et al. Hypofractionated stereotactic re-irradiation: treatment option in recurrent malignant glioma. BMC Cancer. 2005;5:55. doi: 10.1186/1471-2407-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907–911. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- 40.Milas L, Ito H, Hunter N, et al. Retardation of tumor growth in mice caused by radiation-induced injury of tumor bed stroma: dependency on tumor type. Cancer Res. 1986;46:723–727. [PubMed] [Google Scholar]
- 41.Rafii S, Lyden D, Benezra R, et al. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 42.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itasaka S, Komaki R, Herbst RS, et al. Endostatin improves radioresponse and blocks tumor revascularization after radiation therapy for A431 xenografts in mice. Int J Radiat Oncol Biol Phys. 2007;67:870–878. doi: 10.1016/j.ijrobp.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozin SV, Winkler F, Garkavtsev I, et al. Human tumor xenografts recurring after radiotherapy are more sensitive to anti-vascular endothelial growth factor receptor-2 treatment than treatment-naive tumors. Cancer Res. 2007;67:5076–5082. doi: 10.1158/0008-5472.CAN-06-3664. [DOI] [PubMed] [Google Scholar]
- 46.Gossmann A, Helbich TH, Kuriyama N, et al. Dynamic contrast-enhanced magnetic resonance imaging as a surrogate marker of tumor response to anti-angiogenic therapy in a xenograft model of glioblastoma multiforme. J Magn Reson Imaging. 2002;15:233–240. doi: 10.1002/jmri.10072. [DOI] [PubMed] [Google Scholar]
- 47.Duda DG, Batchelor TT, Willett CG, et al. VEGF-targeted cancer therapy strategies: current progress, hurdles and future prospects. Trends Mol Med. 2007;13:223–230. doi: 10.1016/j.molmed.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]


