Abstract
This paper reports effects of salts on in vitro activity of phosphoenolpyruvate carboxylase and ribulose-1,5-diphosphate carboxylase, isolated from species differing in salt tolerance.
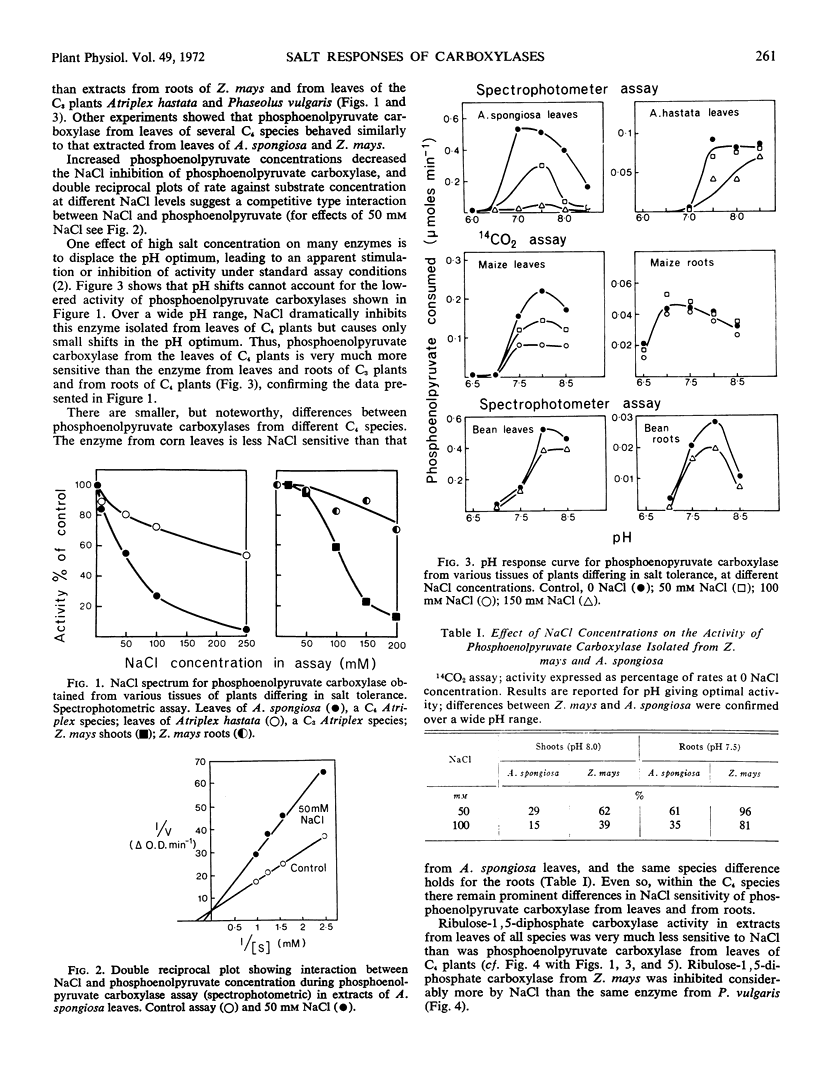
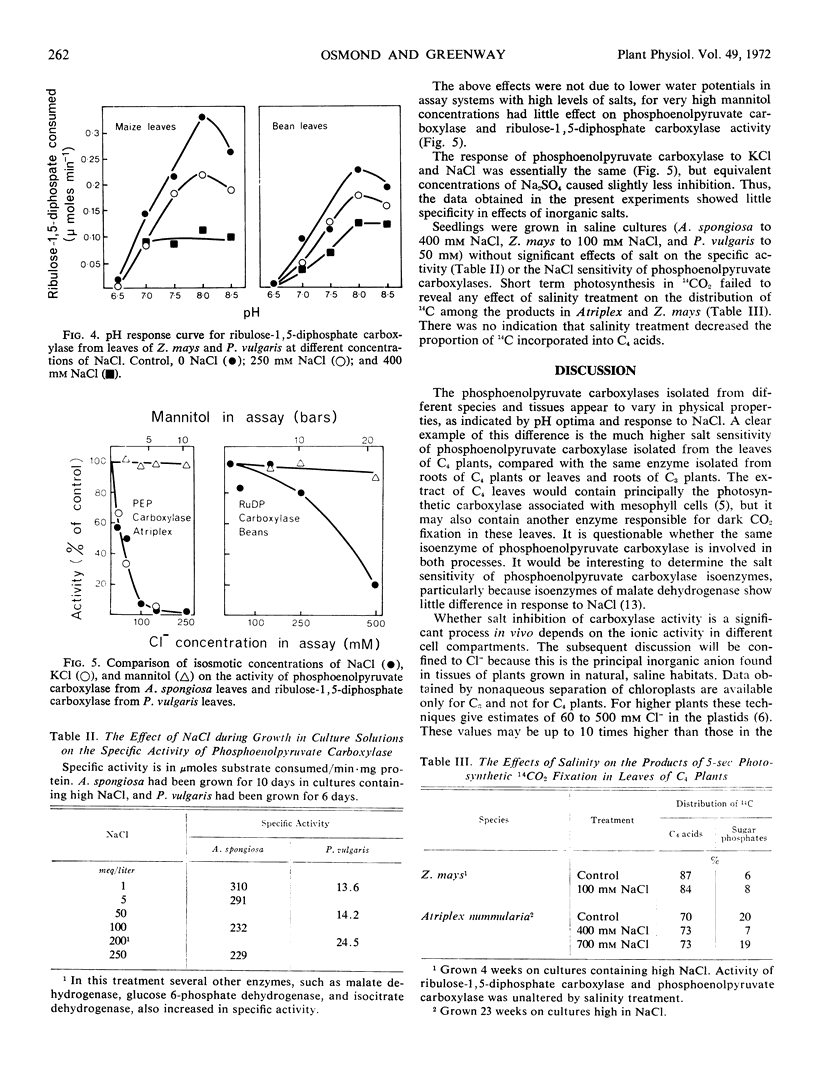
Inhibition of phosphoenolpyruvate carboxylase by the inorganic salts KCl, NaCl, and Na4SO4 depended on the source of the enzyme. Phosphoenolpyruvate carboxylase isolated from leaves of C4 plants was extremely sensitive to inorganic salts, whereas the enzyme extracted from roots of C4 plants or from both shoots and roots of C3 plants was much less sensitive. Ribulose-1,5-diphosphate carboxylase was less salt-sensitive than the phosphoenolpyruvate carboxylases. Differences in salt sensitivity of carboxylases were observed over a wide pH range. The results suggest substantial physical-chemical differences between phosphoenolpyruvate carboxylases functioning in photosynthesis and in CO2 dark fixation.
Among C4 species, phosphoenolpyruvate carboxylase from halophytic species was more salt-sensitive than that from a salt-sensitive species. This anomaly, between in vitro response of enzymes and growth response of the plants, is briefly discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldry C. W., Bucke C., Coombs J. Light-phosphoenolpyruvate dependent carbodioxide fixation by isolated sugar cane chloroplasts. Biochem Biophys Res Commun. 1969 Nov 20;37(5):828–832. doi: 10.1016/0006-291x(69)90966-8. [DOI] [PubMed] [Google Scholar]
- Greenway H. Salt responses of enzymes from species differing in salt tolerance. Plant Physiol. 1972 Feb;49(2):256–259. doi: 10.1104/pp.49.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum A. W., Hill A. E. Ion and water transport in Limonium. V. The ionic status of chloroplasts in the leaf of Limonium vulgare in relation to the activity of the salt glands. Biochim Biophys Acta. 1970 Mar 17;203(1):133–138. doi: 10.1016/0005-2736(70)90043-x. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D., Goodchild D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 Sep;114(3):489–498. doi: 10.1042/bj1140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimberg R. An electrophoretic analysis of the isozymes of malate dehydrogenase in several different plants. Plant Physiol. 1968 Apr;43(4):622–628. doi: 10.1104/pp.43.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimberg R. Effect of sodium chloride on the activity of a soluble malate dehydrogenase from pea seeds. J Biol Chem. 1967 Jun 25;242(12):3000–3006. [PubMed] [Google Scholar]


