Abstract
Since the introduction of biologic agents, increasing data have suggested that conventional size-based RECIST criteria are not accurate in the assessment of response to therapy and non-size-based changes in tumor morphology can be a surrogate marker for assessment of chemotherapeutic effect. The morphologic response criteria are recently introduced, non-size-based criteria for patients undergoing chemotherapy for colorectal liver metastases (CLM). These novel criteria predict pathologic response and long-term survival of patients treated with preoperative chemotherapy, with or without bevacizumab, independent of their RECIST response. They have been validated in patients with resectable and unresectable CLM. These criteria are difficult to apply in small metastases and can be used as an adjunct to RECIST in the assessment of response to preoperative chemotherapy.
Keywords: Colorectal liver metastasis, Chemotherapy, Surgery, RECIST, morphologic
Introduction
In the era of effective chemotherapy, valid assessment of chemotherapeutic effect is necessary not only for monitoring disease progression in advanced disease but also for selecting patients who will benefit from surgery after neoadjuvant systemic therapy. Response to chemotherapy was conventionally assessed by changes in tumor size according to the Response Evaluation Criteria in Solid Tumors (RECIST).[1–3] However, since the introduction of molecular targeted agents, it has been reported that the RECIST criteria may underestimate the response, resulting in studies questioning the adequacy of traditional size-based response criteria.[4–7]
New response criteria incorporating changes in tumor density on computed tomography (CT) were first described in gastrointestinal stromal tumors.[8] Similar changes were later confirmed in patients undergoing systemic therapy with bevacizumab for renal cell carcinoma[9, 10] or colorectal liver metastases (CLM).[11] In this manuscript, we review the significance of non-size-based response criteria for assessing response to systemic therapy in CLM.
Conventional indicators of response to preoperative chemotherapy in CLM
Size-based radiographic change (RECIST)
Degree of size change is a widely accepted response evaluation method in solid tumors. RECIST criteria are based on the sum of the maximal transverse diameters of up to 5 target lesions measured before and after treatment. The percentage difference between the two measurements is used to categorize treatment effect as follows: complete response (CR), disappearance of all lesions; partial response (PR), a decrease of at least 30% in the sum of diameters of target lesions; progressive disease (PD), an increase of at least 20% in the sum of diameters of target lesions and an absolute increase in the sum of at least 5 mm; and stable disease (SD), lack of change between PD and PR.[1]
Although RECIST criteria are validated and established in the assessment of tumor response to therapy, recent studies have questioned the current cut-off points to categorize response to chemotherapy.[12, 13] Suzuki et al. reviewed 567 patients with metastastic colorectal cancer enrolled in the multicenter randomized phase III Nordic VI trial[14] and found that a 10% decrease in tumor diameter at the first follow-up CT correlated with disease-free and overall survival, regardless of the RECIST category.[13] Similarly, De Rook et al. reported that in patients with metastatic colorectal cancer treated with cetuximab, those with KRAS wild type tumors with reduction of tumor size >9.66% at 6 weeks had significantly better survival compared with all other patients.[12] These results indicate that the current cut-off values of RECIST may not apply to all patients.
Pathologic response
In contrast to changes in tumor size after chemotherapy, it has been reported that pathologic response to chemotherapy is strongly correlated with long-term outcome in patients undergoing hepatic resection for CLM. Our group previously reported that cumulative 5-year survival rates after resection of pre-treated CLM were 75% in patients with complete pathologic response (no residual cancer cells), 56% in patients with major pathologic response (1% to 49% residual cancer cells), and 33% in patients with minor response (≥50% residual cancer cells).[15] Maru et al. showed that tumor thickness at the tumor-normal interface in resected specimens is significantly associated with both pathologic and radiographic response, as well as recurrence-free survival.[16] A correlation between the pathologic response and patient survival was also reported by Rubbia-Brandt et al., who reported that histological tumor regression of CLM to chemotherapy corresponds to fibrosis overgrowth and not to increased necrosis.[17]
Morphologic response criteria in CLM
Increasing numbers of studies on the treatment of gastrointestinal stromal tumors or renal cell carcinoma with the biologic agents have reported that changes in tumor density on CT and metabolic response by FDG-PET reflect response to therapy, independent of changes in tumor size.[4–6, 8–10] Our group observed that after preoperative chemotherapy regimens including bevacizumab, CLM undergo morphologic changes on CT, with or without changes in tumor size and reported that morphologic response can be an alternative criteria for evaluating response to preoperative therapy in patients with CLM.[11]
Definition
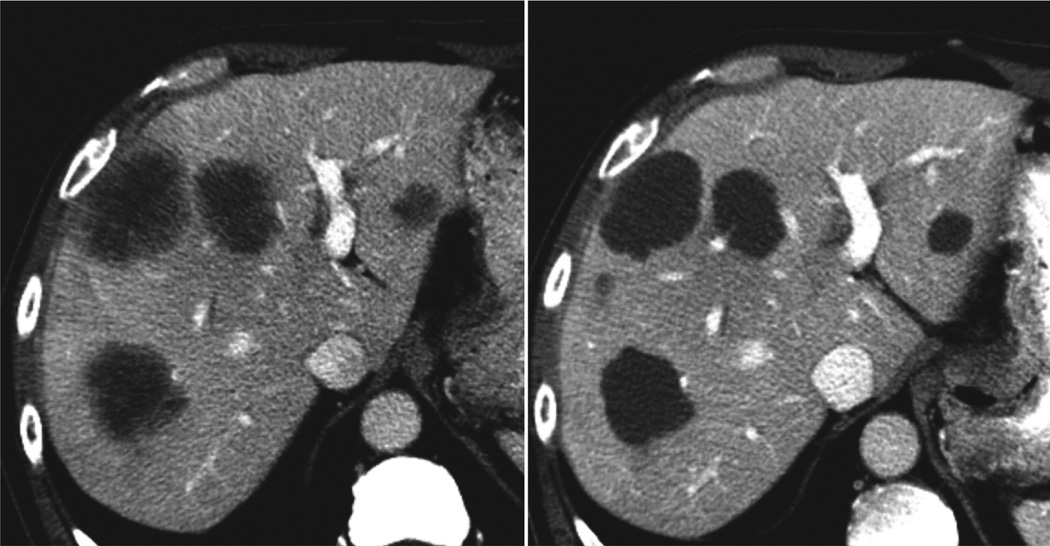
Definitions of morphologic response criteria are summarized in Table 1. A group 1 metastasis is characterized by homogeneous low attenuation with a thin, sharply defined tumor-liver interface. A group 3 metastasis is characterized by heterogeneous attenuation and a thick, poorly defined tumor-liver interface. A group 2 metastasis has morphology that cannot be rated as 1 or 3. Morphologic response criteria are defined as optimal if the metastasis changed from a group 3 or 2 to a 1, incomplete if the group changed from 3 to 2, and none if the group had not changed (Figure 1). In patients with multiple tumors, morphologic response criteria are assigned based on the response seen in the majority of tumors.[11]
Table 1.
Definition of CT Morphologic Groups[11]
| Group | Overall Attenuation | Tumor-Liver Interface |
Peripheral Rim of Enhancement |
|---|---|---|---|
| 3 | Heterogeneous | Ill defined | May be present |
| 2 | Mixed | Variable | If initially present, partially resolved |
| 1 | Homogeneous and hypoattenuating |
Sharp | If initially present, completely resolved |
Optimal response, from Group 3 or 2 to Group 1; Incomplete response, Group 3 to Group 2; No response, no change in Group 2 or 3, or progression
From Chun YS, et al. “Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases.” JAMA 2009, 302:2338-2344, with permission from The American Medical Association.
Figure 1. Optimal morphologic response after systemic chemotherapy.
Group 3 morphology with heterogeneous attenuation and ill defined margin was converted to group 1 morphology with homogeneous attenuation and sharp margin after systemic chemotherapy with bevacizumab.
Correlation between radiographic response criteria and pathologic response
Comparisons of the two radiographic response criteria, the morphologic response criteria and RECIST, have shown that morphologic response was more sensitive in predicting pathologic response, classified by the percentage of residual tumor cells, compared to RECIST. Complete or major pathologic response corresponded to morphologic optimal response in 22 of 29 patients (76%), while minor pathologic response was associated with morphologic incomplete or no response in 17 of 21 patients (81%) (Sensitivity, 0.76; Specificity, 0.81; and Accuracy, 0.78). When correlated with RECIST, complete or major pathologic response corresponded to RECIST PR in 23 of 29 patients (79%), while minor pathologic response was associated with RECIST SD or PD in 10 of 21 patients (48%) (Sensitivity, 0.79; Specificity, 0.48; and Accuracy, 0.66).
Predictors of optimal morphologic response
A recent validation study using a large cohort of patients (n=209) demonstrated that morphologic changes can be observed in patients who receive chemotherapy without bevacizumab. However, the incidence of optimal morphologic response in patients treated without bevacizumab was only 12% (12/102), while an optimal morphologic response was observed in 47% (51/108) of patients treated with bevacizumab. Multivariate analysis confirmed that use of bevacizumab (odds ratio, 6.7) and pretreatment size of tumor ≤ 3 cm (odds ratio, 2.1) were predictive factors for optimal morphologic response to systemic chemotherapy.[18]
Regarding the correlation between the morphologic response and RECIST, optimal morphologic response was observed in 27 out of 69 (39%) patients with RECIST PR and 36 out of 140 (26%) patients with RECIST SD or PD. There was no statistically significant correlation between the morphologic response and RECIST (P =0.06).[18]
Long-term outcomes
Our preliminary report[11] showed that the morphologic optimal response is related to improved survival both in surgical patients and in patients with unresectable metastases, while RECIST PR fails to predict improved survival. Among patients with unresectable metastases, median overall survival was 31 months in patients with optimal response and 19 months with a suboptimal response (P=0.009).[11] Our recent validation study reported that optimal morphologic response is correlated with improved outcome in patients with resectable CLM, both in recurrence-free (21.1 months vs. 11.8 months, p=0.004) and overall survival (114.2 months vs. 49.0 months, p=0.0009). Multivariate analysis confirmed that the optimal morphologic response was a significant prognostic factor for recurrence-free and overall survival and correlated with a 2-fold increase in overall survival. Comparison with currently available indicators for the response to preoperative chemotherapy shows that the morphologic response criteria well stratify the long-term outcome in patients undergoing surgical resection after preoperative systemic therapy (Table 2).
Table 2.
Comparison of prognostic indicators for response to preoperative chemotherapy in patients with colorectal liver metastases
| Indicators | n | median survival (months) |
Overall Survival |
P | ||
|---|---|---|---|---|---|---|
| 1-year | 3-year | 5-year | ||||
| RECIST (MD Anderson unpublished data) | ||||||
| PR | 69 | 88 months | 94% | 78% | 62% | 0.063 |
| SD or PD | 119 | 50 months | 95% | 62% | 45% | |
|
Pathologic response (Blazer et al, J Clin Oncol 2008[15]) |
||||||
| Complete response | 25 | NA | 100% | 100% | 75% | 0.037 0.028 |
| Major response | 97 | 76 months | 95% | 69% | 56% | |
| Minor response | 149 | 42 months | 91% | 58% | 33% | |
|
Morphologic response (Shindoh et al, J Clin Oncol, in press[18]) |
||||||
| Optimal response | 63 | 114 months | 98% | 82% | 74% | 0.0009 |
| Suboptimal response | 146 | 49 months | 93% | 60% | 45% | |
Abbreviations. PR, partial response; SD, stable disease; PD, progressive disease; NA, not available
Limitations and future perspective
Although the morphologic response is a sensitive predictor of postoperative outcomes, there are several limitations for using these criteria. First, determination of the morphologic response relies on high-quality CT scanning parameters allowing for optimal special and contrast resolution and an adequate enhancement protocol to increase the conspicuity of tumor nodules. Therefore, standardization of imaging technique is needed to apply these criteria for the assessment of patients with CLM. Second, morphologic response may be difficult to assess when a tumor is very small (usually less than 1–1.5 cm) due to partial volume effect.[18] Although conventional RECIST criteria are less sensitive than the morphologic response criteria both in prediction of pathologic response and survival, integration of morphologic criteria and RECIST may improve the accuracy of current radiologic response evaluation.
Conclusions
The morphologic response criteria to preoperative chemotherapy, with or without bevacizumab, are significantly correlated with recurrence-free and overall survival after resection of CLM. They are more sensitive than RECIST in prediction of pathologic response and patient survival. These novel response criteria can be a surrogate therapeutic endpoint for patients with CLM.
Acknowledgements
This research was supported in part by the National Institutes of Health through M.D. Anderson’s Cancer Center Support Grant, CA016672.
Footnotes
Disclosure
Junichi Shindoh declares that he has no conflict of interest.
Yun Shin Chun declares that she has no conflict of interest.
Evelyne M. Loyer has received compensation from Roche for lectures including service on speakers bureaus.
Jean-Nicolas Vauthey is supported by a fellowship grant from Roche, and he has also received compensation from Roche for lectures including service on speakers bureaus.
References
Papers of particular interest, published recently, have been highlighted as:
* Of major importance
** Of major importance
- 1.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol. 2006;24:3245–3251. doi: 10.1200/JCO.2006.06.5599. [DOI] [PubMed] [Google Scholar]
- 3.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Antoch G, Kanja J, Bauer S, et al. Comparison of PET, CT and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357–365. [PubMed] [Google Scholar]
- 5.Choi H, Charnsangavej C, de Castro Faria S, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004;183:1619–1628. doi: 10.2214/ajr.183.6.01831619. [DOI] [PubMed] [Google Scholar]
- 6.Stroobants S, Goeminne J, Seegers M, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur J Cancer. 2003;39:2012–2020. doi: 10.1016/s0959-8049(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 7.Thiam R, Fournier LS, Trinquart L, et al. Optimizing the size variation threshold for the CT evaluation of response in metastatic renal cell carcinoma treated with sunitinib. Ann Oncol. 2010;21:936–941. doi: 10.1093/annonc/mdp466. [DOI] [PubMed] [Google Scholar]
- 8.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 9.Smith AD, Lieber ML, Shah SN. Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR Am J Roentgenol. 2010;194:157–165. doi: 10.2214/AJR.09.2941. [DOI] [PubMed] [Google Scholar]
- 10.Smith AD, Shah SN, Rini BI, et al. Morphology, Attenuation, Size, and Structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol. 2010;194:1470–1478. doi: 10.2214/AJR.09.3456. [DOI] [PubMed] [Google Scholar]
- 11. Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. Along with reference 18, this paper first indicated the clinical relevance and predictive value of morphologic response criteria in patients undergoing chemotherapy for colorectal liver metastases. These studies have shown that optimal morphologic response is associated with better survival regardless of the used chemotherapy regimens or surgical treatment.
- 12.De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki C, Blomqvist L, Sundin A, et al. The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Ann Oncol. 2012;23:948–954. doi: 10.1093/annonc/mdr350. This paper has shown the limitation of current version of RECIST in predicting patient outcomes after modern chemotherapy and it also indicates a possibility to improve diagnostic value of RECIST with modifying current cut-off values.
- 14.Glimelius B, Sorbye H, Balteskard L, et al. A randomized phase III multicenter trial comparing irinotecan in combination with the Nordic bolus 5-FU and folinic acid schedule or the bolus/infused de Gramont schedule (Lv5FU2) in patients with metastatic colorectal cancer. Ann Oncol. 2008;19:909–914. doi: 10.1093/annonc/mdm588. [DOI] [PubMed] [Google Scholar]
- 15.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 16.Maru DM, Kopetz S, Boonsirikamchai P, et al. Tumor thickness at the tumor-normal interface: a novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am J Surg Pathol. 2010;34:1287–1294. doi: 10.1097/PAS.0b013e3181eb2f7b. [DOI] [PubMed] [Google Scholar]
- 17.Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 18. Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. doi: 10.1200/JCO.2012.45.2854. In press. Along with reference 11, this paper first indicated the clinical relevance and predictive value of morphologic response criteria in patients undergoing chemotherapy for colorectal liver metastases. These studies have shown that optimal morphologic response is associated with better survival regardless of the used chemotherapy regimens or surgical treatment.