Abstract
Background
Membrane protrusions play important roles in biological processes such as cell adhesion, wound healing, migration, and sensing of the external environment. Cell protrusions are subtype of membrane microdomains composed of cholesterol and sphingolipids, and can be disrupted by cholesterol depletion. Prominins are pentaspan membrane proteins that bind cholesterol and localize to plasma membrane (PM) protrusions. Prominin-1 is of great interest as a marker for stem and cancer cells, while Prominin-2 (Prom2) is reportedly restricted to epithelial cells.
Aim
To characterize the effects of Prom-2 expression on PM microdomain organization.
Methods
Prom2-fluorescent protein was transfected in human skin fibroblasts (HSF) and Chinese hamster ovary (CHO) cells for PM raft and endocytic studies. Caveolae at PM were visualized using transmission electron microscopy. Cdc42 activation was measured and caveolin-1 knockdown was performed using siRNAs.
Results
Prom2 expression in HSF and CHO cells caused extensive Prom2-positive protrusions that co-localized with lipid raft markers. Prom2 expression significantly decreased caveolae at the PM, reduced caveolar endocytosis and increased caveolin-1 phosphorylation. Prom2 expression also inhibited Cdc42-dependent fluid phase endocytosis via decreased Cdc42 activation. Effects on endocytosis were reversed by addition of cholesterol. Knockdown of caveolin-1 by siRNA restored Cdc42 dependent fluid phase endocytosis in Prom2-expressing cells.
Conclusion
Prom2 protrusions primarily localize to lipid rafts and recruit cholesterol into protrusions and away from caveolae, leading to increased phosphorylation of caveolin-1, which inhibits Cdc42-dependent endocytosis. This study provides a new insight for the role for prominins in the regulation of PM lipid organization.
Keywords: lipid rafts, filopodia, sphingolipids, Rho proteins
INTRODUCTION
Membrane protrusions play important roles in biological processes such as cell adhesion, wound healing, migration, and sensing of the external environment [1,2]. Several types of plasma membrane (PM) protrusions exist, such as lamellipodia, sheet-like extensions of the cell supported by branched actin filaments, and filopodia, finger-like projections supported by parallel actin bundles [3,4]. Cell protrusions have been proposed to be a type of membrane microdomain, as they possess elevated levels of cholesterol and glycosphingolipids (GSLs), relative to other regions of the cell membrane [5,6,7] and protrusion structure can be disrupted by cholesterol depletion [8,9,10]
Prominin proteins (Prom1 and Prom2) are pentaspan transmembrane proteins that are enriched at PM protrusions in some cell types [5,11,12]. Both prominins have been shown to directly bind cholesterol and associate with membrane microdomains in living cells [13,14,15]. Prom1 (CD133) has been widely studied as a marker for certain stem cells and cancer stem cells [5,12,16], whereas Prom2 has been shown to be present in some epithelial cells but is otherwise little studied [11,17,18]. The prominins have been proposed to be involved in the organization of membrane protrusions but the specific function of these proteins is presently unknown [5,11].
Our laboratory has been interested in the function and distribution of microdomains on the PM of living cells, and has used various fluorescent probes such as BODIPY-lactosylceramide (Bodipy-LacCer), polyethylene glycol-coupled cholesterol (PEG-Chol), and cholera toxin B subunit (CtxB) to label such domains [19,20,21]. Since cell protrusions have been reported to be a type of GSL-enriched microdomain [7,22], we over-expressed Prom2 as a marker for protrusions and investigated the colocalization of this protein with Bodipy-LacCer and other lipid raft markers. The fluorescent lipid, along with other lipid raft markers, was found to be highly co-localized with Prom2 in protrusions. Over-expression of Prom2 led to significant changes in PM organization and function, including increased protrusions, decreased caveolae at the PM, and decreased caveolar and fluid phase endocytosis. It also resulted in increased caveolin-1 phosphorylation, which inhibited Cdc42-dependent endocytosis due to Cdc42 inactivation. This study provides new insight into possible roles of prominin proteins in regulating PM organization.
MATERIALS AND METHODS
Cell Culture
Normal human skin fibroblasts (HSFs; GM-5659, Coriell Institute for Medical research, Camden, NJ) and CHO cells (ATCC, Manassas, VA) were grown as described [20].
Constructs and transfection experiments
A DNA construct encoding full length human Prom2 was purchased (Thermo Scientific, Waltham MA) and modified (see Suppl). Transfection of DNA constructs was performed using a Nucleofector II apparatus (Lonza).
Lipids, Fluorescent Probes and Miscellaneous Reagents
Bodipy-D-e-LacCer was complexed to defatted BSA as described [23]. Fluorescent AF488-Tfn, AF488-dextran (10kD), and secondary antibodies were from Invitrogen (Eugene, OR). Rhodamine-wheat germ agglutinin (Rh-WGA) was from Vector Laboratories (Burlingame, CA). PEG-Chol (a kind gift from Toshihide Kobayashi, Riken) was labeled with AF488 carboxylic acid (Invitrogen). Caveolin-1 (Cav1) and pY14-Cav1 antibodies were from BD Biosciences (San Jose, CA). A Cdc42 activation kit and HRP-conjugated secondary antibodies were from Millipore (Billerica, MA). All other reagents were from Sigma-Aldrich.
PM labeling with fluorescent probes
HSFs were transfected with Prom2-GFP or -mKate for 48 h. Transfected HSFs were then washed with ice cold HMEM and incubated with 5 μg/ml Rh-WGA, 2.5μM Bodipy-LacCer, 2 μg/ml AF488-CtxB or AF594-StxB, or AF488-PEG-Chol for 30 min at 10°C to label the PM. Samples were then washed, and images were acquired at appropriate wavelengths for the different fluorophores.
Endocytosis assays
For endocytosis assays, HSFs were transfected for 48 h with Prom2 - mKate or –GFP and endocytosis assays were performed as described [20]. In some experiments 5mM methyl ß-cyclodextrin/cholesterol (MßCD/Chol) complex (Sigma) was added to cells for 30 min at 37°C prior to endocytosis assays.
Electron microscopy
Electron microscopy studies were carried out in the Mayo Clinic Electron Microscopy Core Facility (see Suppl.) using FEI Tecnai T12 transmission electron microscope and Hitachi S-4700 cold field-emission electron microscope for transmission and scanning electron microscopy, respectively.
Fluorescence Microscopy and Analysis
Olympus IX70 fluorescence microscope equipped with 60× 1.4NA or 100× 1.35 NA oil immersion objectives was used for fluorescence microscopy. Images were acquired using a Quant EM:512SC CCD camera (Photometrics, Tucson AZ). For quantitation assays, all photomicrographs in a given experiment were exposed and processed identically for a given fluorophore and were analyzed using MetaMorph image processing program (version 7.3.2; Universal imaging Corp.). Quantitative results are expressed as mean ± SE. Images were prepared for individual figures using Photoshop CS (Adobe Systems Inc., San Jose CA). No deconvolution, 3D reconstructions, surface or volume rendering, or gamma adjustments were performed on images.
Miscellaneous procedures
Cells were lysed in RIPA buffer (pH 8.0) with Complete protease inhibitor cocktail (Roche, Indianapolis IN) and Halt phosphatase inhibitor cocktail (Thermo Scientific) for SDS-PAGE and immunoblotting. HSFs were transfected with 100 nM siRNA against human Cav1 or negative siRNA using DharmaFECT 2 (all from Thermo Scientific). Immunofluorescence of formaldehyde-fixed cells was performed as described previously [20,24].
RESULTS
Characterization of membrane protrusions induced by ectopic expression of Prominin-2
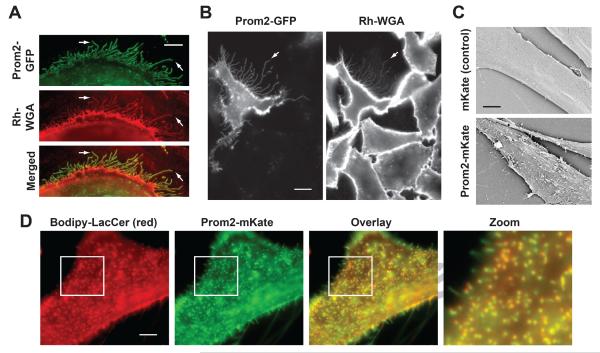
HSFs were transfected with fluorescent Prom2, a marker for protrusions [11,13]. Ectopic expression of Prom2-fluorescent protein induced an extensive network of filopodia-like protrusions projecting from the cell surface (Fig. 1A). A similar induction of membrane protrusions was found when Prom2 was expressed in CHO cells (Fig. 1B). Prom2-positive protrusions were also stained by Rh-WGA (Fig. 1A,B). In contrast, few Rh-WGA-positive protrusions were observed on non-transfected cells (Fig. 1B). To confirm that expression of Prom2 fluorescent protein causes a change in morphology of the cell surface, we performed scanning electron microscopy (SEM) of transfected cells. An extensive network of protrusions was observed in Prom2-transfected cells, which was absent in mKate control transfected cells (Fig. 1C).
Fig. 1. Prom2 expression induced extensive protrusions that colocalize with a lipid raft marker.
HSFs (A) or CHO cells (B) were transfected with Prom2 GFP (48 hr) and stained with Rh-WGA to label cell surface carbohydrates. Note the extensive protrusions labeled with both Prom2-GFP and Rh-WGA (e.g., at arrows) Bar, 10 μm. (C) HSFs were transfected with Prom2-mKate or mKate only and SEM was performed on fixed cells. Transfected cells showed extensive protrusions on their surface whereas control cells had very few protrusions. Bar, 5 μm. (D) Cells transfected with Prom2-mKate, were incubated with Bodipy-LacCer and fluorescence images of living cells were acquired for Prom-2-mKate and Bodipy-LacCer and merged. Note the extensive co-localization of Prom2 with Bodipy-LacCer. Imaging at low temperature inhibited endocytosis.
We next characterized the Prom2-labeled protrusions by co-localization studies using different microdomain/lipid raft markers [19,20,21,25]. HSFs transfected with Prom2-mKate or - GFP (as indicated) were incubated with indicated markers for 30 min at 10°C, and live cell images were acquired at 10°C to inhibit endocytosis. We found an extensive overlap of protrusions with Bodipy-LacCer (Fig. 1D), AF647 labeled PEG-chol and CtxB (binds endogenous GM1 ganglioside) (Suppl. Fig. 1), suggesting that Prom2 protrusions constitute a type of membrane domain. Fluorescent Shiga toxin B subunit (StxB; binds endogenous globoside)-enriched regions showed little overlap with Prom2 protrusions (Suppl. Fig. 1), demonstrating the presence of lipid domains that are distinct from Prom2 protrusions.
Prom2 expression induced a reduction of caveolar endocytosis and caveolae at PM
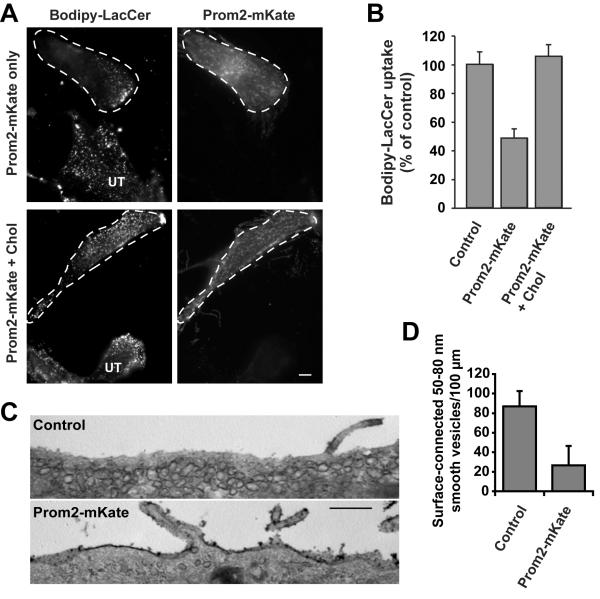
We previously found that Bodipy-LacCer is internalized primarily via caveolae in multiple cell types [20,26,27,28]. Since we demonstrated here that Bodipy-LacCer is extensively localized to Prom2-positive protrusions, we investigated whether Prom2 expression had any effect on endocytosis of Bodipy-LacCer. Uptake of Bodipy-LacCer was significantly (~50%) inhibited in Prom2-expressing cells (Fig. 2A,B). Since Prom2 has been shown to bind cholesterol [13] that is needed for the assembly and function of caveolae [29,30,31], we speculated that the formation of extensive Prom2-positive protrusions might inhibit endocytosis via caveolae by drawing cholesterol into protrusions and away from caveolae. Thus, we added exogenous cholesterol to Prom2-expressing cells and assayed Bodipy-LacCer uptake. We found that treatment of Prom2-positive cells with cholesterol restored Bodipy-LacCer endocytosis (similar to control cells) (Fig. 2B).
Fig. 2. Expression of Prom2 reduced caveolar endocytosis and the numbers of cell-surface attached caveolae.
(A) HSFs transfected ± Prom2-mKate (48 h) were assayed for Bodipy-LacCer endocytosis as described [27]. Transfected cells are outlined with dashed lines (UT-untransfected cells). Bar, 10 μm. (B) HSFs transfected ± Prom2-mKate were quantified for Bodipy-LacCer endocytosis by image analysis. One set of Prom2-expressing cells were pre-treated with 5 mM MßCD/Chol for 30 min at 37°C before Bodipy-LacCer endocytosis. Values are expressed as percentage of control (non-transfected cells in the same field) and are mean ± SE (n>30 cells from three independent experiments). (C) HSFs, transfected with Prom2-mKate or mKate alone (controls), were cultured on gridded glass cover slips for 48 h. Transfected cells (identified by fluorescence microscopy), were stained and processed by TEM to identify PM connected caveolae. Bar, 500 nm. (D) Ruthenium-red positive smooth vesicles (50–80 nm diameters) in TEM images were counted blindly. Values are expressed as means ± SE number/100 μm of length (n>20 cells/condition from two different experiments).
To further elucidate the mechanism by which Prom2 expression inhibits Bodipy-LacCer endocytosis, we examined the effect of Prom2 expression on PM caveolae using transmission electron microscopy (TEM). For these studies, HSFs were transfected with mKate (control) or Prom2-mKate and plated on gridded glass cover slips. Transfected cells were identified and located on grid using fluorescence microscopy. Cells were then fixed, processed using ruthenium red to identify cell surface-connected caveolae [32]. The number of caveolae (ruthenium red positive, uncoated surface vesicles; 50–80 nm in diameter), were counted in a blinded manner. HSFs transfected with mKate only (controls) showed numerous caveolae attached to the PM, whereas HSFs transfected with Prom2-mKate had significantly reduced PM caveolae (Fig. 2C). Quantitation of TEM images demonstrated a ~75% reduction in surface-connected caveolae in Prom2-expressing cells vs. controls (Fig. 2D).
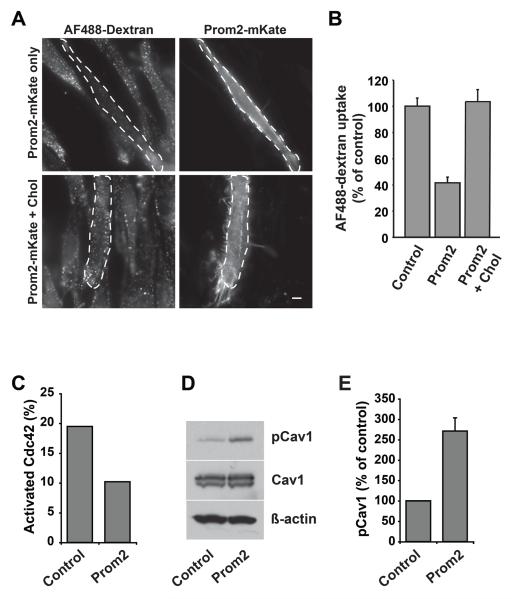
Prom2 expression reduced Cdc42-dependent fluid phase endocytosis, Cdc42 activation and stimulated Cav1 phosphorylation
We next studied the effect of Prom2 over-expression on clathrin-independent Cdc42-dependent fluid phase endocytosis [26,33]. Surprisingly, fluid phase uptake of dextran was inhibited (>60%) in Prom2-positive cells (Fig. 3A, C), with no effect on the clathrin-mediated uptake of transferrin (Suppl. Fig. 2). Since cholesterol addition restored Bodipy-LacCer internalization (Fig. 2A), we next tested the possibility that addition of cholesterol might restore fluid phase endocytosis in Prom2-expressing cells. We found that dextran uptake in Prom2-expressing cells was restored to control levels upon cholesterol addition (Fig. 3A,B). We previously reported that fluid phase endocytosis, a Cdc42-dependent process [26,33], is inhibited under conditions where levels of Y14-phosphorylated-Cav1 (Y14-pCav1) are increased [34]. We proposed that this inhibition is due to the ability of Cav1 to bind to Cdc42 in its inactive, GDP-bound state [35], an interaction which is enhanced by Y14-pCav1 [34]. Thus, we tried to determine if the inhibition of Cdc42-dependent endocytosis occurs by a similar mechanism in Prom2-expressing cells. We found that active Cdc42-GTP was decreased (~45%) in Prom2-GFP-expressing cells compared to GFP-expressing control cells (Fig. 3C). We also found that Y14-pCav1 levels were significantly higher (~2.5-fold) in Prom2-mKate-expressing cells compared to mKate (control)-transfected cells with no effect on total Cav1 and actin levels (Fig. 3D,E). These findings support the concept that Prom2 expression increases Cav1 phosphorylation, thus inhibiting Cdc42-dependent endocytosis.
Fig. 3. Prom2 expression decreased fluid-phase endocytosis, decreased Cdc42 activation and stimulated Y14-phosphorylation of caveolin-1.
HSFs transfected ± Prom2-mKate (48 h) were assayed for dextran (fluid phase uptake) endocytosis. In some cases transfected cells were pretreated with 5mM MßCD/Chol for 30 min at 37°C before endocytosis. (A) Fluorescence images of endocytosis of dextran. Transfected cells are outlined by dashed lines. Bars, 10 μm. Dextran endocytosis was quantified (B) by image analysis as in Fig. 2B. Values are means ± SE (n >30 cells in three independent experiments). Note the inhibition of dextran uptake in Prom2-transfected cells and restoration of dextran endocytosis in Prom2-transfected cells by cholesterol. (C) Cdc42 activation assay was performed in cell lysates from transfected and non-transfected cells. Activated Cdc42 (Cdc42-GTP) and total Cdc42 were immunoblotted and levels of activated Cdc42 were normalized to total Cdc42. Note the decreased activation of Cdc42 in Prom2-transfected cells. (D) Cell lysates (as above) were immunoblotted for Y14-pCav1, Cav1 and actin. Note the increased Cav1 phosphorylation in Prom2-transfected cells compared to mkate alone transfected cells. (E) Quantitation of Y14-pCav1 immunoblots. Values are means ± SE (control 100%) for three different experiments.
Knockdown of Cav1 restores Cdc42 dependent fluid phase endocytosis
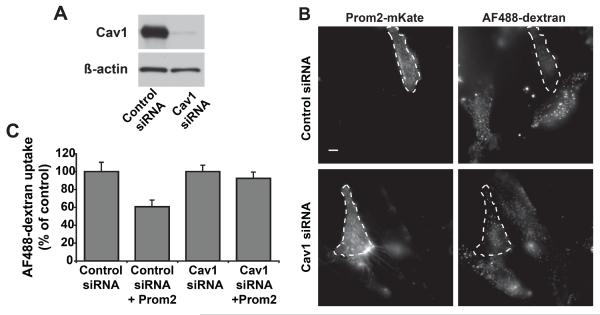
To confirm that the effects of Prom2 on fluid phase endocytosis are due to alterations in Cav1, we used siRNA approach to deplete Cav1 by ~95% relative to control levels (Fig. 4A), and then examined the effects of Prom2-mKate expression on dextran uptake. In cells depleted of Cav1, dextran uptake was unaffected by Prom2 expression, whereas in control cells Prom2 inhibited dextran uptake (Fig. 4B,C), similar to results seen in Fig. 3.
Fig. 4. Knockdown of caveolin-1 restored fluid phase endocytosis in Prom2 expressing cells.
HSFs were treated with Cav1 or negative siRNA for 5 days. (A) Immunoblot showed knockdown of Cav1 in cells treated with Cav1 siRNAs. Equal amounts of protein were loaded (actin immunoblot). (B) Cells treated with Cav1 or negative siRNA were transfected with Prom2-mKate for 48 h and then AF488-dextran uptake was studied as in Fig3. Prom2-transfected cells are outlined. Bar, 10 μm. (C) Quantitation of dextran uptake in Cav1 vs. negative siRNA-treated cells ± Prom2-mKate expression as in Fig3A. Prom2-transfected cells are outlined with dashed lines. Note the reduced level of dextran uptake in negative siRNA/Prom2-expressing cells compared to untransfected cells, but the lack of effect of Prom2 expression on dextran uptake in Cav1 siRNA-treated cells.
DISCUSSION
In the present study, we found that ectopic expression of Prom2 induced an extensive network of membrane protrusions that could be visualized by fluorescence microscopy as well as by SEM. Prom2 expression also decreased the caveolar endocytosis and surface-connected caveolae. A significant inhibition of dextran uptake by the fluid phase pathway was also observed in Prom2-expressing cells, which we propose is a result of increased Cav1 phosphorylation that inhibits Cdc42 activation. Endocytosis via caveolae and dextran uptake could be restored to normal levels by MßCD-cholesterol treatment, a finding which suggests that the effects of Prom2 expression are a result of the redistribution of limiting amounts of cholesterol away from certain domains and into protrusions. Together, these results suggest that Prom2 expression causes a redistribution of PM cholesterol to protrusions, leading to reduced cholesterol at caveolae and inhibiting fluid phase endocytosis as a result of increased Y14-pCav1/Cdc42 interaction. Our findings suggest a novel role for prominins in the organization of PM lipid microdomains and may have significance for the functions of these proteins in epithelia, cancer cells and stem cells.
Expression of Prom2 caused a loss of caveolae from the PM due to recruitment of cholesterol from PM to protrusions as determined by TEM, similar to two different reports in 3T3-L1 adipocytes, where cholesterol depletion caused the loss/flattening of caveolae [31,36]. We hypothesized that Prom expression results in a redistribution of cholesterol away from caveolae membranes, leading to the flattening/loss of PM caveolae. This premise is supported by experiments showing that the reduction of Bodipy-LacCer endocytosis by Prom expression can be reversed by incubation of cells with cholesterol (Fig. 2B). In addition, cholesterol depletion is reported to result in increased Y14-pCav1 [30]. Similarly, we found that Prom2 expression resulted in an increase in Y14-pCav1 (Fig. 3), a result consistent with the idea that the effects of Prom2 expression on Cav1 may be mediated by cholesterol redistribution.
The finding that Prom2 expression reduced PM caveolae and the endocytosis of dextran led us to consider whether these two observations are linked. Cav1, the key protein in caveolae biogenesis, was reported to act as a guanine nucleotide dissociation inhibitor of Cdc42, thus inhibiting Cdc42-dependent secretion [35]. We previously reported that fluid phase endocytosis was inhibited by Cav1 over-expression and stimulated by Cav1 depletion by siRNA, and provided evidence that this is a result of the ability of Y14-pCav1 to inhibit Cdc42 activation [34]. Here, we found that Prom2 expression caused a reduction in Cdc42 activation and dramatic increase in pCav1 levels (Fig. 3). These finding led to the hypothesis that increased pCav1 is responsible for inhibition of fluid phase uptake in Prom2 over-expressing cells. The lack of effect of Prom2 expression on fluid phase uptake in cells depleted of Cav1 (Fig. 4B,C) supports this hypothesis. Our results are consistent with a model in which Prom expression causes local losses of cholesterol in caveolar membranes resulting in increased Y14-pCav1. Higher levels of pCav1 interact with Cdc42, leading to retention of Cdc42 in its inactive state and decreasing fluid phase endocytosis.
Based on the localization of prominin proteins to cellular protrusions, they have been hypothesized to play a role in the membrane organization [5,13,16]. Further, given the ability of Prom1 and Prom2 to interact with cholesterol, it has been proposed that prominins function to regulate lipid composition in protrusions [5,13]. Our study shows that prominin protein expression has the potential to reorganize PM domains such as those associated with caveolae, in addition to promoting the formation of cell protrusions. This membrane reorganizing function may be one mechanism by which prominins affect cell differentiation and migration. Y14-pCav1 has been reported to be important for the regulation of migration [37,38,39]. Our studies suggest that prominin expression in stem cells and cancer cells might influence migration by altering the phosphorylation of Cav1. In conclusion, our findings suggest a role for prominin proteins in regulating PM lipid rafts, a concept that may aid in our understanding of the significance of prominin expression in different cell types.
Supplementary Material
Highlights
Prominin-2 expression induced protrusions that co-localized with lipid raft markers
Prominin-2 expression decreased caveolae, caveolar endocytosis and increased pCav1
Prominin-2 expression inhibited fluid phase endocytosis by inactivation of Cdc42
These endocytic effects can be reversed by adding exogenous cholesterol
Caveolin1 knockdown restored fluid phase endocytosis in Prominin2 expressing cells
ACKNOWLEDGEMENTS
This research was supported by grant R01GM022942 from the National Institute Of General Medical Sciences. This work is dedicated to the memory of Richard E. Pagano.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- [2].Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- [3].Ladwein M, Rottner K. On the Rho'd: the regulation of membrane protrusions by Rho-GTPases. FEBS Lett. 2008;582:2066–2074. doi: 10.1016/j.febslet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- [4].Nambiar R, McConnell RE, Tyska MJ. Myosin motor function: the ins and outs of actin-based membrane protrusions. Cell Mol Life Sci. 2010;67:1239–1254. doi: 10.1007/s00018-009-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Corbeil D, Roper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- [6].Heijnen HF, Van Lier M, Waaijenborg S, Ohno-Iwashita Y, Waheed AA, Inomata M, Gorter G, Mobius W, Akkerman JW, Slot JW. Concentration of rafts in platelet filopodia correlates with recruitment of c-Src and CD63 to these domains. J Thromb Haemost. 2003;1:1161–1173. doi: 10.1046/j.1538-7836.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- [7].Janich P, Corbeil D. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett. 2007;581:1783–1787. doi: 10.1016/j.febslet.2007.03.065. [DOI] [PubMed] [Google Scholar]
- [8].Jeon JH, Kim SK, Kim HJ, Chang J, Ahn CM, Chang YS. Lipid raft modulation inhibits NSCLC cell migration through delocalization of the focal adhesion complex. Lung Cancer. 2010;69:165–171. doi: 10.1016/j.lungcan.2009.10.014. [DOI] [PubMed] [Google Scholar]
- [9].Petrovic N, Schacke W, Gahagan JR, O'Conor CA, Winnicka B, Conway RE, Mina-Osorio P, Shapiro LH. CD13/APN regulates endothelial invasion and filopodia formation. Blood. 2007;110:142–150. doi: 10.1182/blood-2006-02-002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Poole K, Meder D, Simons K, Muller D. The effect of raft lipid depletion on microvilli formation in MDCK cells, visualized by atomic force microscopy. FEBS Lett. 2004;565:53–58. doi: 10.1016/j.febslet.2004.03.095. [DOI] [PubMed] [Google Scholar]
- [11].Fargeas CA, Florek M, Huttner WB, Corbeil D. Characterization of prominin-2, a new member of the prominin family of pentaspan membrane glycoproteins. J Biol Chem. 2003;278:8586–8596. doi: 10.1074/jbc.M210640200. [DOI] [PubMed] [Google Scholar]
- [12].Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Florek M, Bauer N, Janich P, Wilsch-Braeuninger M, Fargeas CA, Marzesco AM, Ehninger G, Thiele C, Huttner WB, Corbeil D. Prominin-2 is a cholesterol-binding protein associated with apical and basolateral plasmalemmal protrusions in polarized epithelial cells and released into urine. Cell Tissue Res. 2007;328:31–47. doi: 10.1007/s00441-006-0324-z. [DOI] [PubMed] [Google Scholar]
- [14].Marzesco AM, Wilsch-Brauninger M, Dubreuil V, Janich P, Langenfeld K, Thiele C, Huttner WB, Corbeil D. Release of extracellular membrane vesicles from microvilli of epithelial cells is enhanced by depleting membrane cholesterol. FEBS Lett. 2009;583:897–902. doi: 10.1016/j.febslet.2009.01.048. [DOI] [PubMed] [Google Scholar]
- [15].Roper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2:582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- [16].Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- [17].Jaszai J, Fargeas CA, Haase M, Farkas LM, Huttner WB, Corbeil D. Robust expression of Prominin-2 all along the adult male reproductive system and urinary bladder. Histochem Cell Biol. 2008;130:749–759. doi: 10.1007/s00418-008-0445-4. [DOI] [PubMed] [Google Scholar]
- [18].Jaszai J, Farkas LM, Fargeas CA, Janich P, Haase M, Huttner WB, Corbeil D. Prominin-2 is a novel marker of distal tubules and collecting ducts of the human and murine kidney. Histochem Cell Biol. 2010;133:527–539. doi: 10.1007/s00418-010-0690-1. [DOI] [PubMed] [Google Scholar]
- [19].Marks DL, Bittman R, Pagano RE. Use of Bodipy-labeled sphingolipid and cholesterol analogs to examine membrane microdomains in cells. Histochem Cell Biol. 2008;130:819–832. doi: 10.1007/s00418-008-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Singh RD, Holicky EL, Cheng Z, Kim SY, Wheatley CL, Marks DL, Bittman R, Pagano RE. Inhibition of caveolar uptake, SV40 infection, and ®1-integrin signaling by a non-natural glycosphingolipid stereoisomer. J Cell Biol. 2007;176:895–901. doi: 10.1083/jcb.200609149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Singh RD, Marks DL, Holicky EL, Wheatley CL, Kaptzan T, Sato SB, Kobayashi T, Ling K, Pagano RE. Gangliosides and beta1-integrin are required for caveolae and membrane domains. Traffic. 2010;11:348–360. doi: 10.1111/j.1600-0854.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen Y, Qin J, Chen ZW. Fluorescence-topographic NSOM directly visualizes peak-valley polarities of GM1/GM3 rafts in cell membrane fluctuations. J Lipid Res. 2008;49:2268–2275. doi: 10.1194/jlr.D800031-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Singh RD, Liu Y, Wheatley CL, Holicky EL, Makino A, Marks DL, Kobayashi T, Subramaniam G, Bittman R, Pagano RE. Caveolar endocytosis and microdomain association of a glycosphingolipid analog is dependent on its sphingosine stereochemistry. J Biol Chem. 2006;281:30660–30668. doi: 10.1074/jbc.M606194200. [DOI] [PubMed] [Google Scholar]
- [24].Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, Enrich C, Parton RG. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol Biol Cell. 2005;16:2091–2105. doi: 10.1091/mbc.E04-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kovbasnjuk O, Edidin M, Donowitz M. Role of lipid rafts in Shiga toxin 1 interaction with the apical surface of Caco-2 cells. J Cell Sci. 2001;114:4025–4031. doi: 10.1242/jcs.114.22.4025. [DOI] [PubMed] [Google Scholar]
- [26].Cheng ZJ, Singh RD, Sharma DK, Holicky EL, Hanada K, Marks DL, Pagano RE. Distinct mechanisms of clathrin-independent endocytosis have unique sphingolipid requirements. Mol Biol Cell. 2006;17:3197–3210. doi: 10.1091/mbc.E05-12-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Puri V, Watanabe R, Singh RD, Dominguez M, Brown JC, Wheatley CL, Marks DL, Pagano RE. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J Cell Biol. 2001;154:535–547. doi: 10.1083/jcb.200102084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Singh RD, Puri V, Valiyaveettil JT, Marks DL, Bittman R, Pagano RE. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol Biol Cell. 2003;14:3254–3265. doi: 10.1091/mbc.E02-12-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chang WJ, Rothberg KG, Kamen BA, Anderson RG. Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J Cell Biol. 1992;118:63–69. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Park EK, Park MJ, Lee SH, Li YC, Kim J, Lee JS, Lee JW, Ye SK, Park JW, Kim CW, Park BK, Kim YN. Cholesterol depletion induces anoikis-like apoptosis via FAK down-regulation and caveolae internalization. J Pathol. 2009;218:337–349. doi: 10.1002/path.2531. [DOI] [PubMed] [Google Scholar]
- [31].Parpal S, Karlsson M, Thorn H, Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J Biol Chem. 2001;276:9670–9678. doi: 10.1074/jbc.M007454200. [DOI] [PubMed] [Google Scholar]
- [32].Parton RG, Molero JC, Floetenmeyer M, Green KM, James DE. Characterization of a distinct plasma membrane macrodomain in differentiated adipocytes. J Biol Chem. 2002;277:46769–46778. doi: 10.1074/jbc.M205683200. [DOI] [PubMed] [Google Scholar]
- [33].Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct Cdc42-regulated, clathrin-independent pinocytic pathway. Develop Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- [34].Cheng ZJ, Singh RD, Holicky EL, Wheatley CL, Marks DL, Pagano RE. Co-regulation of caveolar and Cdc42-dependent fluid phase endocytosis by phosphocaveolin-1. J Biol Chem. 2010;285:15119–15125. doi: 10.1074/jbc.M109.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nevins AK, Thurmond DC. Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. J Biol Chem. 2006;281:18961–18972. doi: 10.1074/jbc.M603604200. [DOI] [PubMed] [Google Scholar]
- [36].Gustavsson J, Parpal S, Karlsson M, Ramsing C, Thorn H, Borg M, Lindroth M, Peterson KH, Magnusson KE, Stralfors P. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB Journal. 1999;13:1961–1971. [PubMed] [Google Scholar]
- [37].Joshi B, Strugnell SS, Goetz JG, Kojic LD, Cox ME, Griffith OL, Chan SK, Jones SJ, Leung SP, Masoudi H, Leung S, Wiseman SM, Nabi IR. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68:8210–8220. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- [38].Park JH, Han HJ. Caveolin-1 plays important role in EGF-induced migration and proliferation of mouse embryonic stem cells: involvement of PI3K/Akt and ERK. Am J Physiol Cell Physiol. 2009;297:C935–944. doi: 10.1152/ajpcell.00121.2009. [DOI] [PubMed] [Google Scholar]
- [39].Podar K, Shringarpure R, Tai YT, Simoncini M, Sattler M, Ishitsuka K, Richardson PG, Hideshima T, Chauhan D, Anderson KC. Caveolin-1 is required for vascular endothelial growth factor-triggered multiple myeloma cell migration and is targeted by bortezomib. Cancer Res. 2004;64:7500–7506. doi: 10.1158/0008-5472.CAN-04-0124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.