Abstract
The mammalian intestinal tract is colonized by trillions of beneficial commensal bacteria that are anatomically restricted to specific niches. However, the mechanisms that regulate anatomical containment remain unclear. Here we identify that interleukin (IL)-22-producing innate lymphoid cells (ILCs) are present in intestinal tissues of healthy mammals. Depletion of ILCs resulted in peripheral dissemination of commensal bacteria and systemic inflammation, which was prevented by administration of IL-22. Disseminating bacteria were identified as Alcaligenes species originating from host lymphoid tissues. Alcaligenes was sufficient to promote systemic inflammation following ILC-depletion in mice, and Alcaligenes-specific systemic immune responses were associated with Crohn's disease and progressive HCV infection in patients. Collectively, these data indicate that ILCs regulate selective containment of lymphoid-resident bacteria to prevent systemic inflammation associated with chronic diseases.
Colonization of the mammalian gastrointestinal tract by commensal bacteria is essential for promoting normal intestinal physiology (1-3). In healthy mammals, commensal bacteria are anatomically restricted to either the intestinal lumen, the epithelial surface or within the underlying gut-associated lymphoid tissues (GALT) (1-5). Anatomical containment is essential to limit inflammation and maintain normal systemic immune cell homeostasis (1, 2). Loss of containment and subsequent dissemination of commensal bacteria to peripheral organs promotes inflammation and is a hallmark of multiple chronic human infectious and inflammatory diseases including progressive human immunodeficiency virus (HIV) infection, hepatitis virus infection and inflammatory bowel disease (IBD) (6-10). Therefore, understanding the pathways that promote anatomical containment of commensal bacteria required to prevent systemic inflammation may provide novel targets for treatment and prevention of chronic human diseases.
Studies in murine models identified a critical role for the cytokine IL-22 in regulating intestinal immunity, inflammation and tissue repair (11, 12). CD4+ T cells and innate lymphoid cells (ILCs) are sources of IL-22 (11-14), however, whether T cell- or ILC-derived IL-22 contributes to the anatomical containment of commensal bacteria and prevention of systemic inflammation in the steady state has not been investigated. To address this issue, we sought to identify the IL-23-responsive cell populations that express IL-22 in intestinal tissues and GALT of healthy human donors. Following ex vivo stimulation with recombinant (r) IL-23, a population of IL-22+ cells was found in intestinal samples from healthy human donors that lacked expression of lineage markers CD20, CD56 and CD3 (Fig. 1A), and was CD127+, CD45-intermediate (CD45INT) and RORγt+ (Fig. 1B), a phenotype consistent with ILCs in humans (11, 14). IL-22+ cells in the mesenteric lymph node (mLN) of healthy human donors also exhibited an ILC phenotype (Fig. S1A, B). Examination of tissues from healthy non-human primates revealed an analogous population of IL-22+ cells that exhibited an ILC phenotype in rectal tissues (Fig. 1C, D) and inguinal LN (Fig. S1C, D). A population of IL-22+ cells was also constitutively present in intestinal tissues or mLN from naïve mice that lacked expression of lineage markers CD3 or NK1.1 (Fig. 1E and S1E), but were CD127+, CD45INT, RORγt+ and CD90.2 (Thy1)+ (Fig. 1F and S1F), indicating they were ILCs (11, 14). The presence of IL-22-producing ILCs in mice was independent of commensal bacteria, as their frequencies were similar in conventional versus germ-free mice (Fig. S2A, B). Collectively, these data identify that ILCs are a dominant IL-23-responsive, IL-22-producing cell population constituently present in the intestine and GALT of healthy mammals.
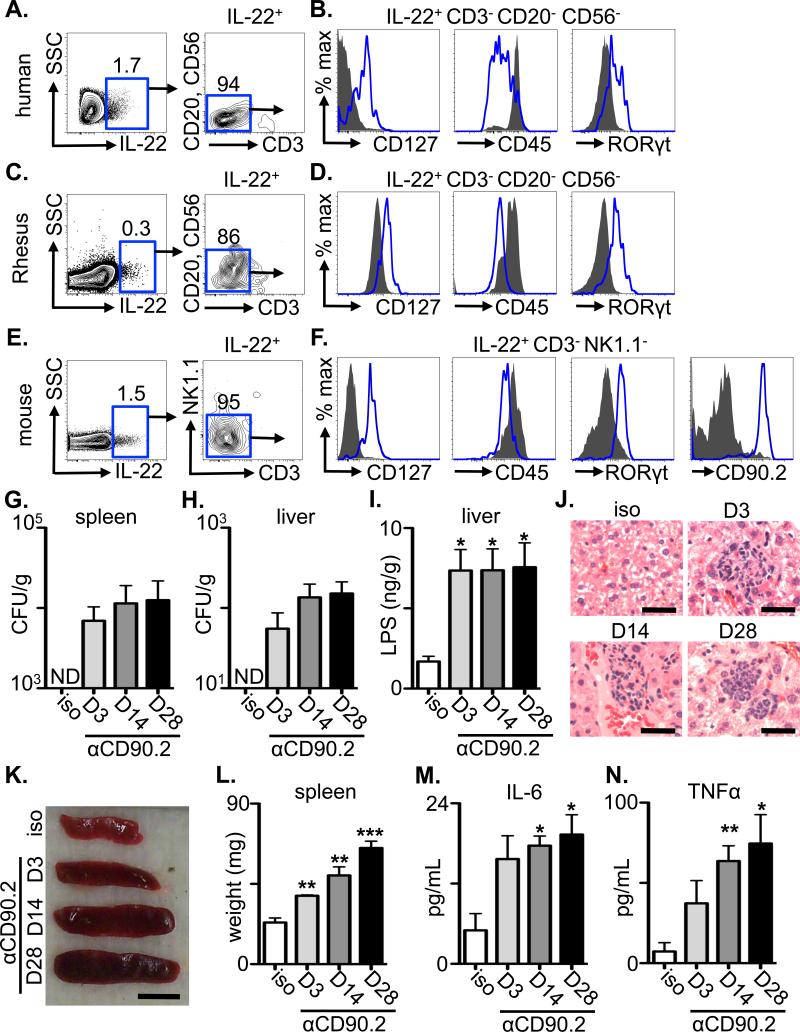
Figure 1. Innate lymphoid cells are resident in intestinal tissues of healthy mammals and limit bacterial dissemination and systemic immune activation in naïve mice.
(A, C and E) The frequency of live IL-22+ cells was examined by flow cytometry from ex vivo IL-23-stimulated cells from (A) intestinal tissues from healthy humans, (C) the rectum of healthy rhesus macaques (E) or the small intestine lamina propria of naïve C57BL/6 mice. (B, D and F) The gated live IL-22+ populations from (B) humans, (D) rhesus macaques or (F) mice were stained with the indicated markers (open blue histogram) and compared to negative population controls (human and rhesus macaques: CD20+ CD56+, solid grey histograms; mice: CD19+ B cell populations, solid grey histograms). (G-N) Naïve C57BL/6 Rag1-/- mice were administered an isotype control or anti-CD90.2 mAb starting on day 0 and sacrificed on day 3, 14 or 28. Colony forming units (CFU) present in homogenates from the (G) spleen and (H) liver of antibody treated mice. (I) LPS concentrations in homogenates from the liver of antibody treated mice. (J) H&E stained histological sections of the liver of antibody treated mice. Bar, 5 μm. Spleen (K) size and (L) weight from antibody treated mice. Bar, 5 mm. Serum concentrations of (M) IL-6 and (N) TNFα from antibody treated mice. All data are representative of 3 independent experiments of 3 individual mice per experiment, 5 total individual human donors or 2 total individual Rhesus macaques. Data shown are the mean ± SEM. Statistics compare days post depletion versus isotype using the Student's t test. * p < 0.05, ** p < 0.01, *** p < 0.001. ND, none detected.
To test whether ILCs contribute to the anatomical containment of commensal bacteria in the steady state, control or anti-CD90.2 monoclonal antibody (mAb) was administered to naïve Rag1-/- mice to deplete ILC populations. Prior to depletion, Rag1-/- mice exhibited a population of IL-22-producing CD90.2+ ILCs in the intestine and mLN (Fig. S2C, D). Strikingly, while peripheral tissues from isotype-treated or anti-NK1.1 mAb-treated Rag1-/- mice were sterile, spleen and liver from anti-CD90.2 mAb-treated Rag1-/- mice contained culturable bacteria and significantly increased levels of LPS in the liver at days 3, 14 and 28 post-depletion (Fig. 1G-I and S3A-D). Collectively, these data indicate a requirement for ILCs in the anatomical containment of commensal bacteria under steady-state conditions.
We sought to test whether depletion of ILCs and subsequent bacterial dissemination elicited systemic immune activation in the steady state. In comparison to isotype mAb-treated Rag1-/- mice, examination of peripheral organs from anti-CD90.2 mAb-treated Rag1-/- mice revealed hepatic inflammation characterized by foci of neutrophils, increased spleen size and weight and elevated serum levels of IL-6 and tumor necrosis factor α (TNFα) at days 3, 14 and 28 post depletion (Fig. 1J-N). Further, anti-CD90.2 mAb-treated Rag1-/- mice that were administered oral antibiotics to deplete intestinal commensal bacteria (15) did not exhibit peripheral dissemination of culturable bacteria or systemic inflammation (Fig. S4A-H), collectively implicating a critical role for ILC-mediated containment of commensal bacteria to prevent systemic inflammation in lymphocyte-deficient mice.
To test whether ILC-mediated anatomical containment of commensal bacteria was dependent on IL-22-IL-22R interactions, naïve Rag1-/- mice were treated with isotype or anti-IL-22 mAb. Anti-IL-22 mAb-treated mice but not isotype mAb-treated mice exhibited culturable bacteria in the spleen and liver (Fig. 2A and B), and significantly increased levels of hepatic LPS (Fig. 2C). Anti-IL-22 mAb-treated mice also exhibited signs of systemic inflammation (Fig. 2D and E), indicating that neutralization of IL-22 in Rag1-/- mice is sufficient to promote bacterial dissemination and systemic inflammation.
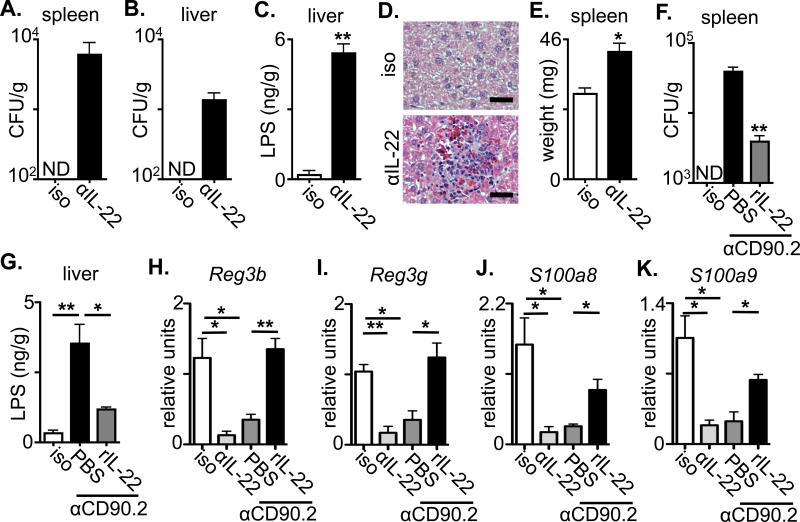
Figure 2. ILCs regulate anatomical containment of commensal bacteria through IL-22-dependent induction of anti-microbial peptides.
(A-E) Naïve C57BL/6 Rag1-/- mice were administered an isotype control or anti-IL-22 mAb starting on day 0 and sacrificed on day 14. Colony forming units (CFU) present in homogenates from the (A) spleen and (B) liver of antibody treated mice. (C) LPS concentrations in homogenates from the liver of antibody treated mice. (D) H&E stained histological sections of the liver of antibody treated mice. Bar, 5 μm. (E) Spleen weight from antibody treated mice. (F-K) Naïve C57BL/6 Rag1-/- mice were administered an isotype control or anti-CD90.2 mAb with PBS control or rL-22 starting on day 0 and sacrificed on day 14. (F) Colony forming units (CFU) present in homogenates from the spleen of antibody treated mice. (G) LPS concentrations in homogenates from the liver of antibody treated mice. Relative fold change of (H) Reg3b, (I) Reg3g, (J) S100a8 and (K) S100a9 transcript in terminal ileum epithelial RNA from treated mice. All data are representative of 2 or more independent experiments with a minimum of 3-4 mice per group. Data shown are the mean ± SEM. Statistics compare treatment versus isotype unless otherwise noted using the Student's t test. * p < 0.05 ** p < 0.01. ND, none detected.
To determine whether therapeutic delivery of exogenous IL-22 could restore anatomical containment of commensal bacteria in ILC-depleted mice, anti-CD90.2 mAb-treated Rag1-/- mice were treated with either PBS control or rIL-22. ILC-depleted mice that received rIL-22 exhibited decreased amounts of culturable bacteria in the spleen (Fig. 2F) and significantly decreased levels of hepatic LPS (Fig. 2G) as compared to control anti-CD90.2 mAb-treated mice. Examination of intestinal epithelial cells from anti-IL-22 mAb or anti-CD90.2 mAb treated Rag1-/- mice demonstrated a significant reduction in expression of the IL-22 regulated anti-microbial proteins Reg3b, Reg3g, S100a8 and S100a9 (11-13, 16, 17), which could be restored with delivery of rIL-22 to ILC-depleted mice (Fig. 2H-K). Collectively, these data indicate that ILCs are critical in promoting IL-22-dependent pathways that limit pl dissemination of commensal bacteria and systemic inflammation.
Peripheral dissemination of intestinal commensal bacteria is commonly associated with impaired intestinal epithelial barrier integrity, resulting in the translocation of commensal bacteria from the intestinal lumen (1, 2, 5, 6, 18-20). However, both isotype and anti-CD90.2 mAb-treated Rag1-/- mice exhibited no significant differences in levels of serum FITC following oral administration of FITC-dextran (21), fecal albumin or intestinal expression of the tight-junction proteins claudin-1 or claudin-2 (Fig. S5A-D), and also did not exhibit histological signs of intestinal inflammation (Fig. S5E). Further, the mLN of control and anti-CD90.2 mAb-treated mice contained equivalent frequencies of macrophages and dendritic cells, while anti-CD90.2 mAb-treated mice contained significantly higher frequencies of neutrophils in the mLN (Fig. S5F-H), suggesting that depletion of ILCs does not result in a global impairment of intestinal epithelial barrier integrity and that disseminating bacteria may not originate from the intestinal lumen.
Metabolic profiling (22) of bacterial colonies from the liver or spleen of anti-CD90.2 mAb-treated Rag1-/- mice identified that the disseminating bacteria were Alcaligenes spp. (Fig. S6A), a genus of gram-negative bacteria that reside within the Peyer's patches (PPs) and mLN of healthy humans, non-human primates and mice (4, 23). 16S-directed PCR confirmed the presence of Alcaligenes spp. in liver and spleen from anti-CD90.2 mAb-treated, but not isotype-treated Rag1-/- mice (Fig. S6B) and pyrosequencing of 16S rDNA tags demonstrated that samples from the intestinal lumen of untreated Rag1-/- mice contained multiple phylogenetic groups of commensal bacteria, whereas cultures from the liver and spleen of ILC-depleted Rag1-/- mice exhibited a homogenous population of Alcaligenaceae (Fig. 3A). Analysis of these sequences identified the species as Alcaligenes xylosoxidians (also referred to as Achromobacter xyloxoxidans). To interrogate the origins of the Alcaligenes spp., tissues from naïve mice were analyzed by fluorescent in situ hybridization (FISH) using Alcaligenes-specific probes. Consistent with a previous report (4), Alcaligenes spp. were found in the interior of PPs and mLN of healthy mice (Fig. 3B, C and S7). Collectively, these results indicate that loss of ILCs results in selective dissemination of lymphoid-resident Alcaligenes spp. to peripheral tissues.
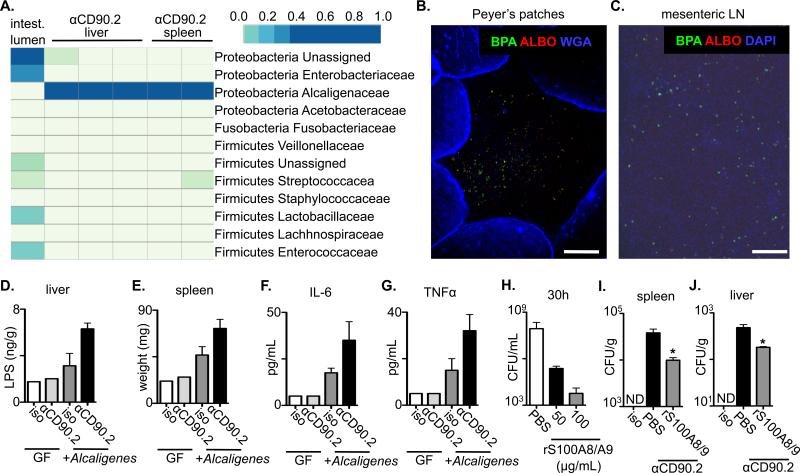
Figure 3. Innate lymphoid cells regulate selective anatomical containment of Alcaligenes species to limit systemic inflammation.
(A-C) Naïve C57BL/6 Rag1-/- mice were administered an isotype control or anti-CD90.2 mAb starting on day 0 and sacrificed on day 14. Tissues from the liver and spleen were homogenized and cultured in LB broth. (A) Pyrosequencing of contents from the intestinal lumen or tissue cultures from anti-CD90.2 mAb treated Rag1-/- mice. Top right bar represents sequence frequency. (B) Peyer's patches and (C) mesenteric lymph node (LN) were analyzed by FISH using probes to identify Alcaligenes spp. (BPA and ALBO) and epithelial cells (WGA) or DNA (DAPI). Bar, 100 μm. (D-G) Naïve germ-free (GF) or Alcaligenes monoassociated Rag1-/- mice were administered an isotype control or anti-CD90.2 mAb starting on day 0 and sacrificed on day 5. (D) LPS concentrations in homogenates from the liver of antibody treated mice. (E) Spleen weight from antibody treated mice. Serum concentrations of (F) IL-6 and (G) TNFα from antibody treated mice. (H) Alcaligenes was cultured in the presence of PBS or recombinant (r) S100A8/S100A9 and colony forming units (CFU) were measured. Naïve Rag1-/- mice were administered an isotype control or anti-CD90.2 mAb with PBS control or rS100A8/S100A9 on day 0 and sacrificed on day 5. Colony forming units (CFU) present in homogenates from the (I) spleen and (J) liver of antibody treated mice. All data are representative of 2 independent experiments with a minimum of 2-3 mice per group. Data shown are the mean ± SEM. Statistics compare PBS versus rS100S8/A9 treatments using Student's t test * p < 0.05. ND, none detected.
To determine whether Alcaligenes spp. were sufficient to promote inflammation, Alcaligenes was administered systemically to Rag1-/- mice. In comparison to isotype-treated mice, both anti-CD90.2 mAb treated Rag1-/- mice and Rag1-/- mice that received systemic Alcaligenes spp. exhibited significantly increased hepatic LPS and systemic inflammation (Fig. S8A-F). Furthermore, whereas germ-free Rag1-/- mice exhibited no increases in hepatic LPS or systemic inflammation after administration of anti-CD90.2 mAb, germ-free Rag1-/- mice that were mono-associated with Alcaligenes and treated with anti-CD90.2 mAb exhibited increased hepatic LPS, increased spleen weight and elevated levels of serum IL-6 and TNFα as compared to isotype-treated monoassociated mice (Fig. 3D-G).
To examine whether decreased expression of IL-22-regulated anti-microbial peptides (Fig 2H-K) impact Alcaligenes, rS100A8/S100A9 (calprotectin) (24) was added to cultures and found to inhibit the growth of Alcaligenes and limit colony formation in a dose-dependent manner (Fig. 3H and S9). Furthermore, delivery of rS100A8/S100A9 in vivo significantly reduced burdens of Alcaligenes in the spleen and liver of anti-CD90.2 mAb treated Rag1-/- mice (Fig. 3I and J). Collectively, these results suggest that in healthy mice, ILCs promote anatomical containment of Alcaligenes spp. in part through promoting expression of calprotectin to limit disruption of systemic immune homeostasis.
To test whether ILCs contribute to prevention of dissemination of Alcaligenes in lymphocyte-replete mice, CD90-disparate Rag1-/- chimeric mice were generated that permit the selective depletion of CD90.2+ ILCs without depleting CD90.1+ lymphocytes (Fig. S10A) (13). Administration of anti-CD90.2 mAb to CD90-disparate Rag1-/- chimeric mice resulted in peripheral dissemination of Alcaligenes to the spleen and liver at day 3 post depletion (Fig. 4A, B and Fig. S10B). Chimeric mice exhibited elevated levels of hepatic LPS and inflammation, increased spleen size, elevated levels of serum IL-6 and TNFα at days 3, 14 and 28 post-depletion (Fig. 4C-G), and significantly higher frequencies of splenic Ki-67+ CD4+ T cells, Ki-67+ CD8+ T cells and Ki-67+ CD19+ B cells (Fig. S10C-E). Splenocyte cultures were restimulated with Alcaligenes-derived antigens and significantly higher frequencies of IL-6+ CD4+ T cells and TNFα+ CD4+ T cells were observed in ILC-depleted chimeric mice (Fig. S10F). Anti-CD90.2 mAb-treated chimeric mice also exhibited significantly elevated serum IgG responses specific for Alcaligenes-derived antigens, but not luminal-resident Escherichia coli-derived antigens (Fig. S10G) or opportunistic viruses (Table S1). The inability to culture Alcaligenes at days 14 and 28 was associated with the development of systemic IgG specific for Alcaligenes spp. (Fig. 4H), indicating that despite persistent systemic inflammation, the adaptive immune system can limit the presence of live bacteria in the periphery. Collectively, these data suggest that ILCs are essential to promote anatomical containment of Alcaligenes to lymphoid-tissues and limit the induction of systemic inflammation in lymphocyte-replete hosts.
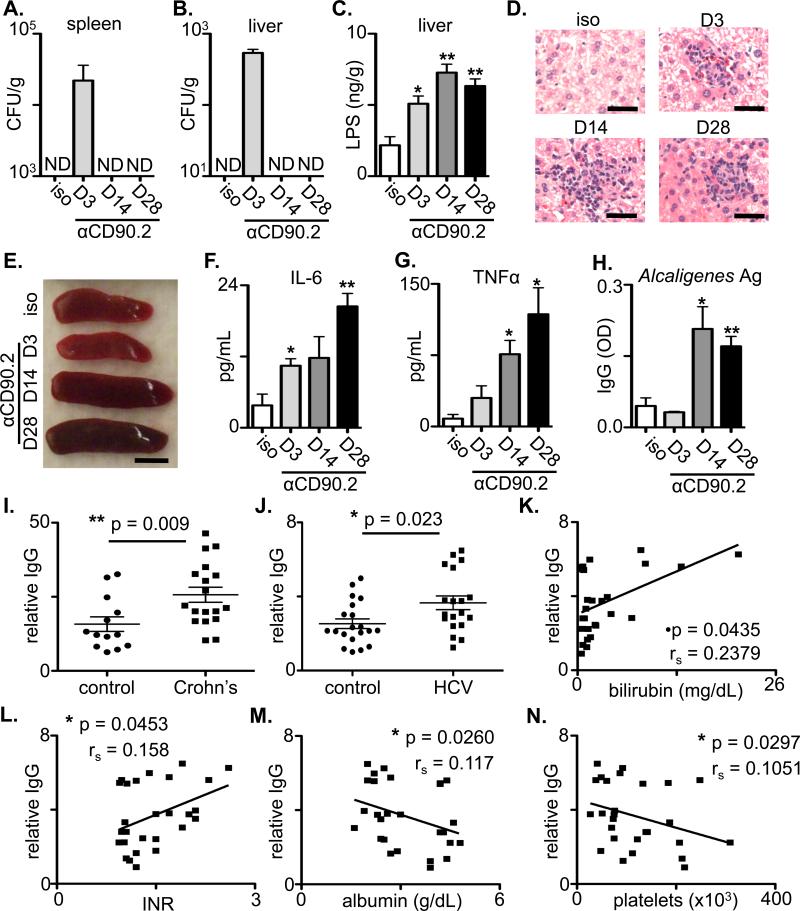
Figure 4. ILCs regulate anatomical containment of Alcaligenes in lymphocyte-replete hosts and Alcaligenes-specific responses are associated with chronic human disease.
(A-H) Naïve CD90-disparate chimeric mice were administered an isotype control or anti-CD90.2 mAb starting on day 0 and sacrificed on day 3, 14 or 28. Colony forming units (CFU) present in homogenates from the (A) spleen and (B) liver of antibody treated chimeric mice. (C) LPS concentrations in homogenates from the liver of antibody treated mice. (D) H&E stained histological sections of the liver of antibody treated chimeric mice. Bar, 5 μm. (E) Spleen size from antibody treated chimeric mice. Bar, 5 mm. Serum concentrations of (F) IL-6 and (G) TNFα from antibody treated chimeric mice. (H) Relative optical density (OD) values of serum IgG specific to Alcaligenes crude antigens in treated chimeric mice. All data are representative of 3 independent experiments with a minimum of 3-5 mice per group. Statistics compare days post depletion versus isotype using the Student's t test. (I-N) Relative serum IgG (OD values per g/mL total serum IgG) specific to Alcaligenes crude antigens in (I) control (n=13) versus pediatric Crohn's disease patients (n=18) or (J) control (n=20) versus cirrhotic HCV-infected patients awaiting orthotopic liver transplantation (n=19). Statistics compare disease status using the Mann-Whitney test. Relative serum IgG specific for Alcaligenes crude antigen in chronically HCV-infected individuals (n=27) were correlated with levels of serum (K) bilirubin, (L) international normalized ratio (INR) of prothrombin time, (M) albumin and (N) platelets. The association between Alcaligenes-specific IgG levels and clinical parameters were compared by non-parametric Spearman's rank correlation coefficient.
Loss of containment of commensal bacteria and chronic systemic inflammation is associated with several chronic human diseases (6-8). To determine whether these diseases were also associated with a loss of containment of Alcaligenes spp., serum samples from cohorts of pediatric Crohn's disease patients or chronically HCV-infected adults were analyzed for the presence of Alcaligenes-specific IgG. In comparison to age-matched controls, serum from pediatric Crohn's disease patients and plasma from cirrhotic HCV-infected individuals awaiting liver transplantation exhibited significantly elevated levels of relative IgG specific for Alcaligenes spp. (Fig. 4I and J). Although further analysis of HCV-infected individuals with and without cirrhosis demonstrated no correlations between Alcaligenes-specific IgG levels and patient age or serum alanine transaminase (sALT) (Fig. S11A, B), there were significant correlations between plasma levels of Alcaligenes-specific IgG and laboratory measures of liver disease including increased serum bilirubin and international normalized ratio (INR) of prothrombin time as well as decreased serum albumin and platelets (Fig. 4K-N).
Mammals have evolved multiple immunologic and physiologic mechanisms to promote the anatomical containment of commensal bacteria to intestinal sites including promoting physical barriers (via epithelial cell tight junctions), biochemical barrier (via production of mucus layers and anti-microbial peptides) and immunologic barriers (via IgA-mediated immune exclusion, intra-epithelial lymphocytes and innate pathways involving phagocytosis, TLR-mediated sensing and oxidative bursts) (1, 2, 18, 19, 25). The demonstration that depletion of ILCs results in the selective dissemination and survival of Alcaligenes spp. in peripheral tissues of mice indicates that in addition to established pathways that non-selectively maintain intestinal barrier function, more discriminatory processes may have evolved to promote the selective anatomical containment of phylogenetically-defined communities of lymphoid-resident commensal bacteria (Fig. S12). It is remarkable that Acaligenes spp. has recently been identified to be a dominant lymphoid-resident commensal species colonizing the PPs and mLN of mammals (4). Moreover, peripheral dissemination of Alcaligenes spp. have been reported in patients with HIV infection, cancer and cystic fibrosis (26-29). The identification of a pathway through which IL-22-producing ILCs can prevent dissemination of lymphoid-resident Alcaligenes spp. and limit systemic inflammation highlights the selectivity of immune-mediated containment of defined commensal bacterial species and could offer novel therapeutic strategies to limit inflammation associated with multiple debilitating chronic human diseases.
Supplementary Material
Acknowledgements
We thank members of the Artis laboratory for discussions and critical reading of the manuscript. We also thank S. Olland, R. Zollner, K. Lam and A. Root at Pfizer for the preparation of IL-22 cytokine and antibodies. The research is supported by the National Institutes of Health (AI061570, AI087990, AI074878, AI083480, AI095466 and AI095608 to D.A., T32-AI007532 to G.F.S and L.A.M., and T32-RR007063, K08-DK093784 to T.A., AI47619 to K.M.C.), the NIH funded Penn Center for AIDS Research (P30 AI 045008 to G.F.S. and D.A.), the Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease Award (to D.A.), the Philadelphia VA Medical Research and Merit Review and American Gastroenterological Association (to K.M.C.), the Ministry of Education, Culture, Sports, Science and Technology of Japan (to J.K., N.S. and H.K) and the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (to J.K.). We also thank the Matthew J. Ryan Veterinary Hospital Pathology Lab, the National Institute of Diabetes and Digestive and Kidney Disease Center for the Molecular Studies in Digestive and Liver Disease Molecular Pathology and Imaging Core (P30DK50306), the Penn Microarray Facility and the Abramson Cancer Center Flow Cytometry and Cell Sorting Resource Laboratory (partially supported by NCI Comprehensive Cancer Center Support Grant (#2-P30 CA016520)) for technical advice and support. Several human tissue samples were provided by the Cooperative Human Tissue Network which is funded by the National Cancer Institute. The data presented in the paper are tabulated in the main paper and in the supplementary materials.
Abbreviations used
- CFU
colony forming unit
- GALT
gut associated lymphoid tissue
- HIV
human immunodeficiency virus
- IBD
inflammatory bowel disease
- IL
interleukin
- ILC
innate lymphoid cell
- ILF
isolated lymphoid follicle
- INR
international normalized ratio
- LPL
lamina propria lymphocytes
- mLN
mesenteric lymph node
- PPs
Peyer's patches
- r
recombinant
- SI
small intestine
- spp.
species
- Th
T helper
- TLR
toll-like receptor
References and Notes
- 1.Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010 Mar;28:623. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010 Mar;10:159. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 3.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006 Feb 24;124:837. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Obata T, et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci U S A. 2010 Apr 20;107:7419. doi: 10.1073/pnas.1001061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004 Mar 12;303:1662. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 6.Brenchley JM, Douek DC. Microbial Translocation Across the GI Tract (*). Annu Rev Immunol. 2012 Apr 23;30:149. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandler NG, et al. Host Response to Translocated Microbial Products Predicts Outcomes of Patients with HBV or HCV infection. Gastroenterology. 2011 Jul 1; doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009 Jan;15:100. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- 9.Lescut D, et al. Bacterial translocation in colorectal cancers. Gastroenterol Clin Biol. 1990;14:811. [PubMed] [Google Scholar]
- 10.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000 May;32:742. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011 May;12:383. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008 Apr;28:454. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011 Jan 28;34:122. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012 Apr 23;30:647. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 15.Hill DA, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18:538. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008 Mar;14:282. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 17.Aujla SJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008 Mar;14:275. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaishnava S, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011 Oct 14;334:255. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slack E, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009 Jul 31;325:617. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004 Jun;4:478. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 21.Bergstrom KS, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010 May;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funke G, Monnet D, deBernardis C, von Graevenitz A, Freney J. Evaluation of the VITEK 2 system for rapid identification of medically relevant gram-negative rods. J Clin Microbiol. 1998 Jul;36:1948. doi: 10.1128/jcm.36.7.1948-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busse HJ, Stolz A. In: Prokaryotes. Dworkin M, editor. Springer; New York: 2006. pp. 675–700. e. a. [Google Scholar]
- 24.Ryckman C, et al. HIV-1 transcription and virus production are both accentuated by the proinflammatory myeloid-related proteins in human CD4+ T lymphocytes. J Immunol. 2002 Sep 15;169:3307. doi: 10.4049/jimmunol.169.6.3307. [DOI] [PubMed] [Google Scholar]
- 25.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008 Oct;6:776. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aisenberg G, Rolston KV, Safdar A. Bacteremia caused by Achromobacter and Alcaligenes species in 46 patients with cancer (1989-2003). Cancer. 2004 Nov 1;101:2134. doi: 10.1002/cncr.20604. [DOI] [PubMed] [Google Scholar]
- 27.Espinoza-Gomez F, Newton-Sanchez OA, Melnikov V, Virgen-Gonzalez O, Unrau J. Meningitis caused by Alcaligenes xylosoxidans in a patient with HIV/AIDS. Braz J Infect Dis. 2007 Dec;11:603. doi: 10.1590/s1413-86702007000600015. [DOI] [PubMed] [Google Scholar]
- 28.Duggan JM, Goldstein SJ, Chenoweth CE, Kauffman CA, Bradley SF. Achromobacter xylosoxidans bacteremia: report of four cases and review of the literature. Clin Infect Dis. 1996 Sep;23:569. doi: 10.1093/clinids/23.3.569. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, et al. Ribosomal DNA-directed PCR for identification of Achromobacter (Alcaligenes) xylosoxidans recovered from sputum samples from cystic fibrosis patients. J Clin Microbiol. 2002 Apr;40:1210. doi: 10.1128/JCM.40.4.1210-1213.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.