Abstract
Germinal centers (GC) are large aggregates of proliferating B lymphocytes within follicles of lymphoid tissue that form during adaptive immune responses. GCs are the source of long-lived B cells that form the basis for pathogen-specific lifelong B cell immunity. The complex architecture of these structures includes subdomains that differ significantly in their stromal cell and T lymphocyte subset composition. In part due to their structural complexity and potential to generate some lymphomas, much interest and many theories about GC dynamics have emerged. Here we review recent research employing in vivo imaging that has begun to untangle some of the mysteries.
Keywords: germinal center, lymphocyte, migration, follicular dendritic cell, two-photon laser scanning microscopy
Introduction
The progressive increase in the affinity of antibodies following immunization, known as ”affinity maturation”, is a hallmark of adaptive immune responses. This process critically depends on the formation of organized aggregates of proliferating B and T cells known as germinal centers (GC) that emerge within B cell follicles during immune responses. Within this dynamic environment, GC B cells with higher affinities for foreign antigens are selectively expanded and instructed to differentiate into one of two lineages essential to persistent immunity; long-lived high-affinity antibody-forming cells (AFCs) and memory B cells. These events occur within complex microenvironments where less fit B cells may succumb to apoptotic cell death. In this way, GCs shape, magnify and add permanence to the most effective B cells of the immune response.
These intriguing features of GCs have fueled decades of research and spawned much controversy. Until recently, however, insight into GC B cell dynamics had been largely shaped by notions of cell contacts perceived from histology images. In combination with cellular and molecular in vitro studies, a tremendous amount of information has been garnered. However, knowledge of temporal processes has been inherently limited by the static nature of these approaches and could only be investigated in dynamic mathematical models. In this regard, recent in vivo imaging studies of germinal centers are of particular interest. A combination of technical advances and novel analytic approaches to time-resolved imaging in vivo have empowered research into GC T and B cell movement within lymphoid tissue. Multiple studies have examined GC T and B cell behavior via two-photon laser scanning microscopy, a technique that allows the movement of fluorescently labeled cells to be followed through time and space within either intact excised tissue or living anesthetized mice. The visualization of GC B cells in vivo has shed light on some of the dynamic processes that had long been the subject of speculation and challenged some aspects of classical thinking. Although these reports generated important insights as the very first of their kind, they have raised many questions and spurred new interest in the unresolved elements of GC function.
GC architecture and historical background
GCs are large clusters of antigen specific T and B cells that emerge within B cell follicles during productive immune responses. As the GC expands, non-responding B cells with a naïve phenotype are peripherally displaced to form a crest around the GC referred to as the follicular mantle (Figure 1). The highly structured architecture of GCs is primarily comprised of two subdomains, the light zone and dark zone, a historical nomenclature based on their relative appearance in haematoxylin/eosin-stained tissue sections [21, 85, 91]. Within these environs, B cells responding to foreign proteins clonally expand while undergoing somatic hypermutation (SHM) of the immunoglobulin (Ig) gene segments that encode for antigen specific B cell receptors (BCR) [59, 60]. The cellular “products” of germinal center reactions, long-lived memory B cells and plasma cells, express BCR that are typically isotype switched and of high affinity for the eliciting antigen [39, 80, 81, 140].
Figure 1. Germinal center orientation and zones.
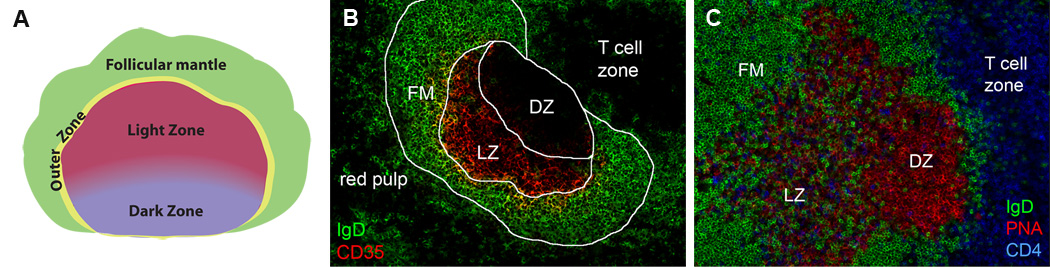
(A) Cartoon of GC architecture within a B cell follicle; DZ (purple), LZ (red), OZ (yellow), FM (green). (B) Murine spleen obtained 12 days after ip immunization. An Ig H chain gene knockin strain that encodes for an anti-hapten specificity in ~5% of B cells was immunized with NP-CGG prepared in alum. Staining for anti-IgD-AL488 (green) and anti-CD35-bi/SA-AL568 (red). (C) Lymph node section obtained 9 days after footpad immunization in complete Freunds adjuvant stained for anti-IgD-AL488 (green), PNA-bi/SA-AL568 (red) and anti-CD4-AL647 (blue).
The complex architecture of established GCs is reproducibly oriented within B cell follicles (Figure 1).The light zone (LZ) is more proximal to the subcapsular sinus of lymph nodes or the marginal sinus enveloping the white pulp of spleens. The dark zone (DZ) is positioned between the GC LZ and the base of the follicle bordering with the T cell zone. Many of the proliferating B cells of a GC are found within the DZ which is comprised of activated B cells that are dividing at a very rapid rate, among the fastest of any known cell type [3, 51, 55, 155]. Activation induced cytidine deaminase (AID) drives a unique process of SHM of V regions of Ig genes (CDR) that can introduce amino acid substitutions in the antibodies produced [103]. SHM was thought to occur in the DZ because SHM is introduced during DNA replication which is more evident in this compartment in tonsils [104, 120]. DZ B cells, also referred to as centroblasts, typically express lower levels of a variety of surface markers, including BCRs, giving this domain a more homogeneous appearance.
The Light Zone (LZ) is distinguished by the presence of follicular dendritic cells (FDCs), the fine reticular processes of which form an extended mesh of dendrites that entirely comprise the stromal cell matrix in this region [86]. FDCs are not hematopoietically derived [57, 151] but are phenotypically and functionally distinct from other stromal cells within B cell follicles. In addition to elevated levels of the adhesion molecules VCAM-1 and ICAM-1 [11, 73], FDCs also express high levels of complement receptors [23, 89] and the Fc receptor FcgRIIB [116]. Because FDCs retain antigen-antibody complexes on the cell surface and could serve as long-term depots of undegraded Ag and activated complement components [23, 70, 92, 137], they have classically been considered an important site of antigen presentation to B cells.
Based in part on studies of human tonsils, a widely accepted view emerged of GC dynamics promoting affinity maturation [91]. Because the most evident deposits of antigen are present on the surface of FDCs in the form of C3d tagged Ag in immune complexes, it has been largely presumed that antigen encounters occur within the LZ. On the other hand, that SHM is concomitant with DNA replication engendered the idea that mutation of BCRs must occur in the DZ where the bulk of proliferation is found in tonsilar GCs. This vision of GCs created a disconcerting functional split between zones that were physically separated. To reconcile the need for these sequential events to occur in separate zones, a migration model emerged termed “Cyclic Re-entry” in which GC B cells move from one zone to the other in order to achieve multiple rounds of proliferation and selection [64, 90, 96, 107]. In this model, GC B cells that had recently mutated and divided in the DZ migrate to the LZ where they compete for the acquisition of antigen and/or selective survival signals provided by direct contact with other cell types such as FDCs or a specialized T cell subset. B cells that have successfully survived this test were predicted to return to the DZ for another round of division and mutation.
A subset of CD4+ T helper cells known as T follicular helpers (Tfh) that supply key cytokines to GC B cells are primarily found within the LZ where they can often be present in great numbers, but can also be found distributed throughout the follicle mantle (Figure 1) [7, 69, 91]. Tfh can be distinguished from other cell subsets by a high level of expression of the molecules PD-1, ICOS, and the chemokine receptor CXCR5 [19, 67, 124, 153], which is thought to mediate Tfh attraction to the LZ FDCs that are a known source of the chemokine CXCL13 [47]. Within the DZ, Tfh cells can also be observed at the periphery of the GC that borders with the follicular mantle, also known as the Outer Zone (OZ), but are rarely present in the DZ interior. A subset of Tfh have been shown previously to promote differentiation to AFCs, as well as cell proliferation and class-switch recombination (reviewed in [145]). Thus, Tfh are not strictly confined to the LZ within GCs and may serve distinct purposes in different locations, a notion that is discussed more at length below.
The sites of GC B cell proliferation and AFC promoting factors have historically been seen as indicators of potential function of each zone. In this regard, it is interesting to note that Ki67+ B cells in cell cycle are present in the LZ of both tonsils and murine LNs, indicating that proliferation can occur in both GC compartments [3, 21, 54, 55, 148]. In contrast to the DZ however, smaller cells that are not actively cycling are more likely to be found in the LZ [54]. LZ B cells, often referred to as centrocytes, express higher surface levels of BCRs and elevated levels of a survival promoting factor, B cell lymphoma x (Bcl-x), than DZ B cells [75, 144]. Moreover, transcription factors associated with the development of antibody secreting cells, including IRF4, XBP and BLIMP, have been observed at elevated levels in the nucleus of some LZ B cells in GCs of tonsils and murine spleen [5, 9, 26, 28, 71]. These observations coupled with the presence of rare B cells with acentric nuclei has led to the idea that differentiation toward long-term plasma cells occurs within the LZ [87].
In addition to the prominent proliferation of GC B cells, apoptotic cell death is also quite evident, putatively resulting from a form of negative selection. It is thought that GC B cell death results from a failure to compete for limiting resources perhaps due to the expression of inadequent BCRs [50, 88]. Apoptotic cell bodies and their debris are primarily found within the phagosomes of tingible body macrophages [131, 135, 137]. This observation has led to the idea that dying GC B cells are rapidly engulfed by roving macrophages. Apoptotic cell bodies are predominantly found at the interface between the LZ and DZ, a region of finer FDC dendrites and where tingible body macrophages are most often located [54, 84]. However, apoptotic cells can be seen throughout GCs, in both the LZ and DZ [54]. It is unclear whether cell death is a passive process that results from neglect or an actively induced process. Reports of poor affinity maturation in CD95 (Fas) deficient mice are consistent with an active induction of B cell death [53, 139], however not all studies are in agreement [50, 132].
GC evolution and zonal segregation
The early GC developmental stages are still a bit of a mystery, in part due to the inscrutible nature of events prior to the detection of surface markers characteristic of GC B cells. Commonly accepted markers of GC B cells include a high level of expression of CD95 (Fas) on follicular B cells [93, 132], a loss of IgD and a gain in the ability to bind peanut agglutinin (PNA). Interestingly, expression patterns of the ecto-enzyme CD38 expression substantially differ in mice and man – whereas human naïve B cells downregulate CD38 as they become GC B cells, the exact opposite is true with murine B cells [106]. During the early stages of GC development in T cell dependent immune responses, prominent DZ formation precedes significant LZ expansion; large clusters of IgD lo PNA hi B cells beneath the FDC zone are readily apparent when PNA hi cells are still sparse within the FDC network [21, 148]. Activation of FDCs occurs around this time, which then display elevated levels of ICAM-1 and VCAM-1 [37]. As antigen specific B cells more fully populate the FDC network, GCs take on a bi-lobed shape, an appearance that becomes less apparent at later stages of GC development.
The chemokine receptors CXCR5 and CXCR4 play an important role in the creation and maintenance of GC zones. LZ and DZ B cells express distinct chemokine receptors which are required for proper zonal segregation [1]. DZ B cells have a higher level of CXCR4 expression than B cells found within the LZ. Moreover, CXCR4−/− GC B cells fail to populate the DZ of GCs in chimeric mice. Likewise, CXCR5−/− B cells were found to only reside in the DZ of GCs of chimeras, indicating that chemotaxis to the chemokines CXCL12 and CXCL13 is a critical component of GC zonal formation.
That one zone appears before the other suggests that GC compartments may form independently, or with different kinetics. This idea is further supported by the nature of GCs present during T cell-independent immune responses to antigens that are not proteinaceous. Such GCs emerge more rapidly, fail to expand PNA+ cells within the FDC network and quickly abort at a point in time when the FDCs would mature and the LZ would typically expand [35, 42, 78, 143].
The existence of distinct GC compartments effectively creates micro-climates that may foster unique forms of growth and differentiation. Resident cell types and the cytokines and chemokines they secrete are strong influences on lymphocyte activation, proliferation and differentiation [1]. The segregation of GC cells into compartments with distinct cellular composition ensures that lymphocyte activation in one zone will occur in a molecular context that is different from the other. One could imagine that a restricted path of differentiation may be promoted when GC lymphocytes remain within one zone during sequential activation events, as lymphocytes would then continue to be exposed to the same molecular milieu.
The nature of selection
High affinity antigen specific B cells are nurtured and expanded within GCs, while B cells of lower affinity fail to thrive [17, 34, 50, 61, 127]. SHM results in the introduction of mutations that could either enhance, reduce or even obliterate BCR binding to its cognate antigen ligand. Computer modeling studies have indicated that selection of B cells on the basis of their affinity for antigen must occur frequently and reiteratively to obtain the high replacement/silent substitution ratios in the complementarity-determining regions of antibody. [72, 128, 129]. Events that favor GC B cells with higher affinity BCR variants are predicted to occur on the order of every division in order to achieve the rates of affinity maturation that are observed.
Much has been learned from studies of transgenic mice that express BCR of defined affinities for model antigens. Such studies have revealed that B cells with low affinity are not a priori excluded from GCs if B cells of higher affinities are not present, but are rapidly supplanted by higher affinity clones in competitive circumstances. The selective enrichment of higher affinity B cell clones is evident even at the earliest stages of GC development; clonal diversity is rapidity reduced in young GCs that are not yet mature [34, 61, 127]. Murine GCs induced by immunization with model antigens are typically oligoclonal by the peak of the GC reaction [60, 76].
The enrichment of higher affinity GC B cells could theoretically be achieved by either a positive selection of meritable cells or the culling of unfit cells by an active elimination process. There is evidence to suggest that both forms of selection may be operating within GCs, and reasons why this would be desirable. A study of CD95 deficient mice suggest that the elimination of unwanted cells plays a role in shaping the GC repertoire via this death receptor family member, presumably mediated via CD95L expressed on GC T cells [139]. However, in the absence of CD95, clonal selection may be less efficient, but not eliminated, suggesting that the relative expansion of desirable cells can still occur even in the absence of negative selection [50, 132]. Due to the risk of generating autoimmune specificities as a result of SHM, the timely elimination of self-reactive B cell clones would seem to be a prudent mechanism to have in place [36, 50, 142]. Similarly, an impaired affinity maturation was observed in transgenic mice that constitutively express Bcl-xl, a protein that inhibit cell death and promotes survival [93, 138]. Thus, under conditions where all GC B cells survive irrespective of BCR ‘fitness”, the degree of affinity maturation decreases but is not abolished, suggesting that prescribed cell death plays a key role in determining which B cell clones will prevail.
A number of mechanisms have been proposed to result in the enrichment of higher affinity variants, all of them reliant on acquisition of cognate antigen by GC B cells: 1) assessment of the extent of BCR cross-linking or the resulting signal strength, an indicator of BCR affinity when antigen is limiting [4, 14, 77]. 2) presentation of antigen-derived peptide in MHC II to Tfh cells that provide pro-survival factors, an indication of a B cell’s ability to acquire proteins that are complexed to their nominal antigen [2, 49, 98]. 3) the elimination of B cells that fail to present antigen-derived peptide to T cells expressing CD95L (FasL) [53, 98] 4) competition for the limited space available on (follicular) DC cell bodies in order to derive survival signals dependent on sustained contact mediated by antigen recognition on its cell surface [66, 74, 79, 141].
Although some of these potential modes of B cell selection are not mutually exclusive, they have inspired many migration theories and mathematical modeling [4, 38, 64–66, 90, 97, 98, 100, 101, 107]. A heightened interest in the mechanism of GC B cell selection provided the impetus to visualize directly the cellular contacts within the GC microenvironments that might lead to differentiation, death or instead incite further proliferation. Through in vivo imaging, some of the concepts discussed above have now been put to the test and several unexpected features of GCs been revealed in the process.
Technical limitations of imaging by two-photon laser scanning microscopy
After decades of inferring lymphocyte migratory behavior from static histology images, two-photon laser scanning microscopy arrived on the stage as a new champion, capable of visualizing the events we were once trying to guess at (reviewed in [20, 44, 133]. Two photon lasers differ from conventional lasers in several ways; they are tunable within a broad range of wavelengths (typically 750–1000 nm) and are pulsed lasers that emit photon packets that are short in duration (femtoseconds). Because of the inherently lower energy of long wavelength photons, fluorophores must absorb two (or more) photons for excitation. Moreover, it is only at the plane of focus that the density of photons is sufficient to allow for two photons to be absorbed simultaneously. As a result, all of the fluorescence that is emitted during two-photon laser scanning can be attributed to excitation at the plane of focus, unlike traditional confocal microscopy that elicits fluorescence above the plane of focus as well, reducing phototoxicity and improving resolution.
With the power to make movies of live B and T cells interacting and moving in the wild, so to speak, it would seem that answering questions about complex lymphocyte behavior should be no more difficult than playing the movies back to see what the cells do. However, as is often the case with new technologies, this is actually a much more difficult task than it would appear to those not directly involved in performing the experiments themselves. In truth, with current technology and for the foreseeable future, it is more like trying to reconstruct lymphocyte behavior from short video clippings, often taken from poor angles with much of the action taking place off screen.
To date, most published two-photon studies of leukocyte behavior have been based on observations of an hour or less. The reasons for this are mostly technical; longer imaging can result in tissue damage if the operator is not careful, and limitations in software and computing power can prevent the collection and analysis of larger data sets. However, many of the behaviors one might hope to elucidate take longer than an hour from beginning to end, complicating interpretation [15, 38]. Movies of limited duration can only observe a relatively small part of this process at a time. Similarly, there are limits to how much of the tissue can be imaged at one time. Two-photon microscopy generates three-dimensional movies by stacking multiple two-dimensional images taken at incremental depths. Each stack represents a “frame” in the movie. The X and Y dimensions of the frame are determined by a combination of the scanning parameters set at the beginning of the experiment and the objective on the microscope (and therefore differ between experiments and facilities). The Z-dimension is even more limiting. While a reasonably good two-photon microscope can image to depths of 200 µm or more into a lymph node, it is not possible to generate a movie that makes use of this full range while still permitting tracking of individual cells. This is because the time interval between frames must be short enough to be able to accurately follow a moving cell from one to the next. In our experience, 20 seconds between frames permits accurate cell tracking. Therefore, the depth of the volume is limited by the number of images that can be captured in within this time period. Because of this, the imaged depth in the z dimension can not include an entire mature germinal center and the tissue volume to be imaged for a particular experiment must be chosen with a specific question in mind. While larger volumes could be imaged, this would be balanced by a loss in temporal resolution and the potential for inaccurate cell tracking.
The result of these limits on two-photon imaging is that many events are observed in pieces, rather than as a whole. While the ideal situation would be to follow individual cells right from the beginning to the end of a particular process, we instead must rely on disjointed observations of larger numbers of cells at different stages of the event to reconstruct the whole. Theoretical models of the event can be used to make predictions about what sort of behavior should be observed by certain numbers of cells at a particular time and place. However, there are several major issues to consider when interpreting cell tracking data. First, if the observation is too short or the imaging volume too small, much of the context in which the behavior occurs (ex. if it follows cell division or interactions with another cell type) is lost. Second, basing conclusions on the behavior of a relatively large number of cells can mask rare, but extremely important events. This is a particularly important point to keep in mind when considering rare selection events during affinity maturation, for example. Finally, only fluorescent cells or structures can be observed, while the rest of the surrounding tissue remains dark and uninformative. Therefore, while two-photon microscopy is, in many ways, a very large step forward compared to static histology for the study of lymphocyte activation, differentiation and migration in lymphatic tissues, much like histology it still can only produce snapshots of the whole process and creativity must be used to identify the important pieces to reassemble the whole. A recent review discussing considerations in the analysis of cell tracking data can be found in reference [16].
In vivo imaging insights into GC biology
The zonal structure of GCs makes them very suitable subjects for analysis by two-photon microscopy. It is therefore not a surprise that quite a number of papers have been published in the past 3 years that use this technique to address migration patterns of GC B and T cells in order to bring to light how this highly organized tissue structure supports the generation and selection of high affinity BCR mutants. The initiation was made by 3 contemporaneous papers, including one from our own group, which used slightly different experimental approaches but provided unique insights and perspectives on otherwise similar observations, as discussed below in detail [3, 55, 125]. As the very first of their kind, these papers generated a lot of excitement and provided a fertile ground for the work of others. While these papers investigated the migration of B cells within GCs, recent publications have focused on the immigration of naïve B cells into GCs [43, 58, 111, 126], deposition of antigen on FDCs [22, 113, 121], B cell acquisition of antigen from FDCs [134], apoptosis [154], GC initiation [33], and the role of Tfh cells [115].
Pleomorphic GC B cells project cytosolic processes
In vivo imaging studies revealed an unexpected dynamism of GC B cells that are exceptionally variable in shape. Some cells displayed a highly polarized shape typical of cells moving under a strong chemotactic gradient, with a defined leading edge and long, narrow trailing uropod (Figure 2). Most motile cells did not adapt this morphology however. Both mobile and stationary GC B cells appeared to continuously probe their environment with dendrite-like structures, an appearance that may be more striking because of the abundant cytosol of GC B cells, which are very large (10–15 µm). The active extension and retraction of long membrane projections of GC B cells was observed in both the LZ and DZ. In contrast, naïve B cells typically had a more rounded appearance with little membrane ruffling. In this regard, it is interesting to note that B cells of T cell dependent GCs transcribe genes that are also known to regulate axon growth [152].
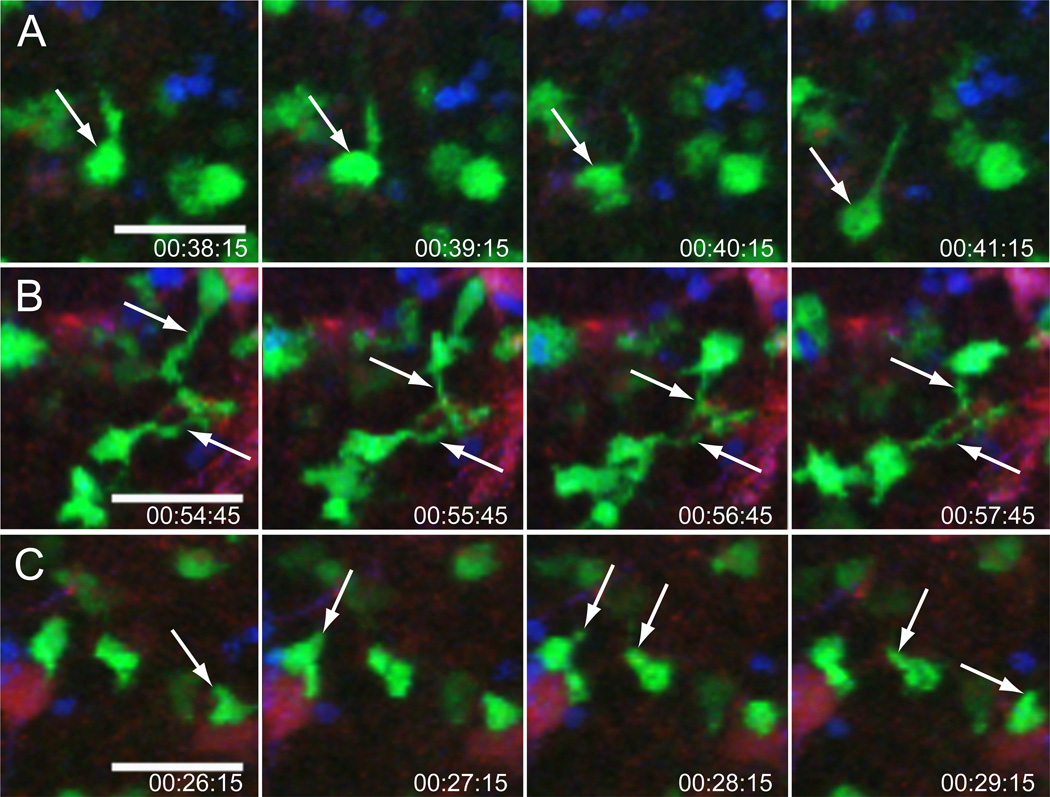
Figure 2. Morphology of GC and examples of GC B cells as imaged by intravital microscopy.
The images shown here are representative of those obtained by intravital time resolved imaging of a popliteal lymph node 9 days after immunization. GC B cells (green), naïve B cells (blue) and FDC cell bodies and dendrites (red). (A) An example of a polarized GC B cell moving directionally (white arrow). (B) Tethered cells with long, anchored cytoplasmic extensions in close association with FDCs. (C) Irregularly shaped GC B cells probing their environment with filopodia. Hapten specific B cells expressing GFP under the direction of the chicken-b-actin promoter were transferred into GFP tolerant recipients. The next day, recipients were immunized s.c. with NP-CGG. Two days before imaging, recipients were injected in the footpad with anti-CD35 Fab fragments conjugated to AL633 to highlight FDCs. They also received wild type naïve B cells that were labeled with Hoechst 33342. Scale bar, 30 µm. Originally published in Hauser et al. Immunity 26:655–667 (2007) and reprinted with permission from Elsevier.
GCs with permeable borders accept novel B cells
The remarkable paucity of naive B cells in GCs seen in tissue sections might seem to suggest that a physical barrier is present that enforces the segregation of responding and non-responding cell types. To the contrary, in vivo imaging studies indicate that GC boundaries are permeable and allow B cells that were not previously participating in the GC reaction to enter, particularly within the LZ [3, 55, 125]. Naïve B cells can be seen to move into the GCs with great fluidity and seemingly without constraint, although the GC did not appear to be their preferred environment since they did not typically remain. In this way, “open” GCs allow access to all follicular B cells, but likewise permit cells that do not find the environment desirable to leave just as readily. In some cases, B cells that were adoptively transferred into recipients with ongoing GC reactions further proliferated within pre-existing GCs. This has been demonstrated using B cells with antigen specificities that could react with the original immunogen, as well as non-specific B cells that were stimulated with lipopolysaccharide (LPS) just prior to adoptive transfer [58, 125].
The idea that novel B cells freshly emerging from the bone marrow can re-seed ineffective GCs has teleologic appeal. Mice with immune responses committed to a small number of B cell clones or to low affinity BCRs antigen are ill-fated. A limited BCR repertoire of long-term persisting AFCs and memory cells is less likely to protect against the pathogen that elicited the response and even less likely to quell variants of that pathogen. Therefore a diverse yet efficacious collection of B cells is ideal and any mechanisms that allow for an influx of B cells with higher affinity is prudent. Of course, the reverse is not desirable – new clones with poor affinities should not disproportionately populate or diminish the number of higher affinity clones. It is well known that higher affinity B cells will out-compete lower affinity clones that are present at the time of immunization, quickly prevailing in even young GCs. However, the dependence of GC initiation on what was perceived to be transient T cell help promoted the vision of GCs as functionally “closed” cages in which rival B cell clones compete.
Recent research by Nussenzweig and colleagues demonstrates that higher affinity B cell clones that ingress GCs already well established can nevertheless preferentially expand and extensively populate both the DZ and LZ [126]. However, in situ expansion of newly arriving B cells only occurred when the transferred cells were of higher affinity than the pre-existing GC population. The requirement for a superior affinity indicates that the ability of a newly emerging specificity to dominate is not merely due to a delayed influx of fresh cells. Much like the introduction of new BCR variants created by somatic hypermutation, selective pressures must be in place to ensure enrichment of the most fit cells. Therefore, clonal dominance is not mediated by the physical exclusion of other clones, but is instead the result of a still incompletely defined process that preserves and expands B cell clones that are better fit. It is tempting to speculate that progeny of highly successful GCs could emigrate and enter GCs that had not yet succeeded in producing high affinity B cells. However, there is no evidence to date demonstrating that B cells emigrating from one GC have the capacity to enter another GC in a separate follicle.
Freshly transferred B cells of a different specificity can participate in established GCs, but the kinetics are substantially delayed under such conditions [126]. This is because adoptively transferred B cells do not directly enter established GCs, but instead first collect at the follicle border with the adjacent T cell zone, a pattern that is typical of B cells during the earliest stages of primary activation. This result suggests that naïve B cells must still be licensed by Th cells at the periphery of follicles in order to later participate in the GCs within the follicular interior, even when abundant numbers of Tfh are present within the GC environment itself. Two recent studies have shown that downregulation of the orphan G-protein coupled receptor EBI2, a receptor mediating chemotaxis to an unknown ligand, is essential for movement of activated B cells from the outer follicle periphery into the follicle interior [43, 111]. B cells that overexpress EBI2 are unable to move back into the follicle interior to form GCs, despite successfully having undergone the appropriate activation steps at the T zone border [111]. Although EBI2 deficient B cells transferred into mice with established GCs of WT B cells migrate into the GC environment, they are restricted to the GC border with the follicular mantle, the GC outer zone [43].
Although naïve B cells were more often observed to enter GCs through the LZ, adoptively transferred LPS stimulated B cells exhibit a preference for the DZ of established GCs [58]. Although these non-specifically activated B cells were shown to divide in situ, they were largely confined to the DZ and could go on to dominate the population in that zone. LPS treated B cells appeared to be attracted to each other, often pausing during homotypic contacts that, while brief (~4 minutes), lasted longer than those observed in co-transferred, untreated B cells [40][8]. That most adoptively transferred LPS blasts within the DZ are short lived is compatible with the idea that LPS blasts have acquired a phenotype that confers a preference for the DZ environment, but are only enabled to divide a limited number of times. This is reminiscent of T cell independent GCs that form with fast kinetics and have a “DZ only” appearance that lacks substantial expansion within the FDC network. T cell independent GCs are typically elicited with immunogens that support strong BCR crosslinking, lack protein antigens and contain TLR agonists. Such immunogens do not generate antigen specific T cell help and elicit short-lived GCs that often involute within a few days [35, 42, 78, 143], despite being comprised of B cells that proliferate substantially in situ initially. It should be noted that although Tfh cells are likely to be present within the LZ of the previously established GCs of the experimental system described above, these T cells are unlikely to have a TCR specificity that would allow them to make meaningful contacts with the transferred LPS blasts [35, 42, 78, 143].
Contacts with Follicular Dendritic Cells
Due to the retention of immune complexes and activated complement components on FDC processes, it was long thought that FDC served as a depot for Ag that could be presented on the cell surface in a less degraded format. FDCs are able to retain antigen for long periods of time [70, 92] in the form of C3d tagged Ag in immune complexes via their expression of Fc receptors [116] and the complement receptors CD21/35 [23, 89]. It is envisioned that the surface presentation of retained protein antigen would improve access to the epitopes that rely on secondary structure. Internalized protein antigens would instead be proteolytically degraded, destroying key epitopes that could provide enhanced protection against native viral and bacterial proteins. It has been suggested that surface of FDCs may serve as a rigid platform of a multimeric array of antigens, thereby promoting stronger BCR ligation derived signals than might be obtained with soluble antigen [13, 14]. In vivo imaging has demonstrated that antigen retained by FDCs can also be acquired by B cells and transported elsewhere [134].
FDC expression of the adhesion molecules ICAM and VCAM [37, 73] and factors that promote survival, such as sonic hedgehog [123], further supported the view that BCR recognition of antigen on FDC cell surface could serve to positively select higher affinity B cells via competition for access to retained antigen. That FDCs have been shown to reduce the spontaneously occurring apoptotic cell death of purified GC B cells in vitro lends support to this idea [74, 79, 110]. In this regard, it is interesting to note a recent study by Vinuesa and colleagues of Dock8 mutant mice that fail to form mature GCs and long-term B cell immunity although the short-term antibody response remained unaffected. This strain exhibits a defect in ICAM recruitment to BCR immunological synapses which were smaller than those of WT B cells, suggesting that sustained BCR synapses may be required for LZ expansion and the differentiation of memory B cells [117].
However, there is evidence that suggests that the retention of immune complexes by FDCs is not required for normal GC formation, calling into question the role of antibody complexed antigen (reviewed in [48]). However, whether the production of memory B cells and long-term plasma cells is compromised when antigen is not retained via FcR or complement receptors remains controversial [12, 29, 52]. Such studies fostered intrigue concerning the nature of GC B cell interactions with FDCs.
The tracking of GC B cell movement via in vivo imaging provided an assessment of contacts with FDCs for the first time. GC B cell contacts with FDC dendrites are transient; LZ B cells could be seen to fluidly glide along dendrites, occasionally pausing, seemingly scanning the surface [3, 125]. The brevity of these interactions might suggest that brief contacts with FDCs are sufficient to transfer retained antigen to GC B cells, potentially promoting BCR ligation and conferring enhanced survival. A study published by our own group, Hauser et al., observed clusters of GC B cells that appeared to be firmly attached to FDC cell bodies although sustained attachments to dendrites were not apparent. These tethered B cells seemed to strain and pull against prolonged connections to FDC cell bodies in contacts that typically lasted longer than the one hour imaging time period (Figure 2). Persistent B cell interactions with FDCs have also been observed in primary follicles shortly after immunization and prior to GC formation [134]. Which molecules might mediate such long-term cell contacts remains to be determined, as does the exact biological significance of them.
Stationary GC B cells that are not adjacent to fluorescently tagged cells were observed in one in vivo imaging report [55]. In Hauser et al., examples of GC B cells dwelling in one location on the order of one hour are not uncommon, a time period is similar to the contact times reported for antigen specific engagement of T cells with myeloid dendritic cells (DC) [94, 99]. In most cases stationary cells continued to actively extend cytoplasmic extensions and were therefore alive. Non-motile cells were observed throughout the GC interior but were also prominently located at the peripheral margins of the GC (OZ). Stationary cells were also observed in the distal portion of the DZ nearest the T cell zone. Although it cannot be determined from this study whether stationary tracks represent B cells receiving positive or negative signals, the T-B border and OZ are likely arenas of extended interaction of GC B cells with other cell types. Due to technical limitations in the number of different fluorophores that can be detected at one time, simultaneous imaging of Tfh, GC B cells, FDCs and the defining follicular mantle has not yet been reported. Nevertheless, the presence of animated stationary B cells that are not adjacent to FDCs may suggest that other cell types other than FDCs and Tfh may contact and influence GC B cell dynamics. Additional accessory cell types such as tingible body macrophages and a hematopoietically derived GC DC subset are also present within GCs and could theoretically regulate GC B cell behavior [46].
Tfh cell contacts with GC B cells
It has been proposed that the primary role of GC T cells is to promote differentiation in the LZ [30]. Great numbers of Tfh cells can found in the LZ of GCs, a subset of which has been shown previously to promote differentiation to AFCs, as well as cell proliferation and class-switch recombination in vitro (reviewed in [145]). Tfh secrete the cytokines IL-4 and IL-21 and express CD40L, molecules that are required for the full development and maintenance of mature GCs [24, 31, 119]. In mice that lack IL-4 or IL-21 or their receptors, GCs are either reduced or inapparent and the amount of serum IgG levels greatly reduced [105, 108, 146].
However, reports of Tfh secretion profiles are not all in agreement, perhaps in part because the definition of a “Tfh” varied between investigations, sometimes defined solely on the basis of CXCR5 expression by T cells (Reviewed in [145]). A subset of human tonsillar Tfh cells found along the OZ can be distinguished from other apical LZ Tfh by an absence of expression of CD57. Although both CD57+ and CD57− Tfh subsets express CD40L by microarray analysis [68], by one report only the CD57− subset were found to harbor preformed cytoplasmic stores of CD40L that can be rapidly externalized from preformed intracellular stores after TCR mediated stimulation [24]. It is intriguing that although the CD57− subset often comprises a significant fraction of Tfh in tonsilllar GCs, it is less capable of promoting Ab secretion in B cell co-cultures in vitro, despite having a greater capacity to secrete IL-4 [62, 68]. Moreover, a recent study that employed strains of mice that fluorescently reported IL-4 or γIFN expression indicates that T cells within the LZ are more likely to produce IL-4 in contrast to GC T cells at the base of the DZ that primarily produced γIFN [119]. This is a clear indication that not all Tfh are alike and perhaps should not be viewed as a single uniform subset, particularly irrespective of location [67].
It has also been suggested that a primary role of Tfh is to promote the selection of higher affinity BCR variants within the LZ, presumably by providing factors that encourage GC B cell survival [3, 24, 25, 98, 147] Here it is envisioned that B cells with higher affinity BCRs acquire more antigen and present greater amounts of proteolytically degraded protein antigen in the context of MHC II, enabling them to more effectively engage Tfh specific for the cognate antigen [136]. In this scenario, B cells that fail to present antigen derived peptides recognizable by TCRs of Tfh cells might then be ignored and fail to thrive due to neglect. Although one role of Tfh is thought to be promotion of plasma cell differentiation, the rapid incorporation of the thymidine nucleotide BrdU by some LZ B cells raises the possibility that interactions with Tfh cells may promote proliferation instead, or in addition. In vitro studies demonstrating that CD40 +IL-4 prevented GC B cell death that occurs with BCR crosslinking, suggests that T cell derived factors in the GC milieu could impart survival to B cells otherwise prone to apoptosis [41, 83]. Moreover, sustained CD40 signaling in B cells inhibits AFC formation in vitro, suggesting that CD40 stimulation in the absence of AFC promoting cytokines may instead elicit continued proliferation [118]. The ease with which GCs are disrupted with anti-CD40(L) reagents is also consistent with CD40-CD40L interactions during T-B contacts playing a critical role in the maintenance of a high proliferative rate [39, 49] although other mechanisms of GC disruption have not been ruled out [45].
A clue to the potential functions of Tfh cells comes from the in vivo imaging study of Allen et al., who reported rapid movement of specific T cells throughout the LZ which slow down upon contact with GC B cells [3]. Despite the high density of T cells within the LZ, only a small percentage of GC B cells were observed in prolonged stable contact with T cells. Most of T-B contacts were very brief (<4 minutes), a length of time similar to non-specific interactions seen with non-cognate T-DC contacts. Fragments of B cells presumably resulting from normal apoptosis were found to be attached to T cells, suggesting to the authors that cell debris resulting from naturally occurring apoptosis would sterically hinder most B cells and promote competition for T cell interactions. These observations were interpreted as being consistent with the notion that positive selection of high-affinity GC B cells results from competition for Tfh cell surface contact within the LZ, even under conditions where the density of Tfh cells is great.
A recent imaging study by Germain and colleagues of mice deficient in SAP have greatly enhanced our understanding of the factors that promote GC retention of Tfh [115]. SAP is an intracellular adaptor protein that modulates TCR signaling and in its absence, GC development is profoundly compromised [102]. The defect in GC promotion is particularly intriguing given that the early activation steps of SAP−/− T cells and engagement with antigen presenting DCs appear unaffected in vivo. SAP−/− T cells could enter into B cell follicles and acquire a Tfh phenotype comparable to SAP+/+ T cells. However, SAP−/− T cells displayed a reduced capacity to form long-lasting contacts with antigen specific B cells during early stages of the immune response. Although SAP−/− T cells formed T-B conjugates of shorter duration that were nevertheless longer than would be expected of non-specific interactions, B contacts with SAP−/− T cells were insufficient to produce GC B cells. Intravital imaging experiments of mice that had received an adoptive transfer of both SAP sufficient and deficient T cells revealed significant differences in their migration patterns. Consistent with previous observations that GCs do not physically exclude non-participating cells from entering, both SAP+/+ and SAP−/− T cells could enter GCs. However, SAP−/− T cells tended to turn away from GC boundaries and remain outside the GC while SAP+/+ T cells demonstrated a preference to remain within GCs. Elegant bone marrow chimera experiments demonstrated that the retention of T cells within the GC boundaries was dependent on the capacity of GC B cells to present cognate antigen. These results indicate that cognate T-B interactions are required to retain T cells within the LZ, and by extension, that failure to do so prevents the maintenance of fully mature GCs. Moreover, it suggests that productive encounters with GC B cells programs Tfh cells to alter their migratory propensities, presumably the result of as yet unappreciated phenotypic changes. Finally, this study further suggests the environment containing LZ GC B cells is perceived to be different from the surrounding follicle mantle by such Tfh, despite the fact that FDC dendrites typically extend beyond LZ GC borders.
T cells within GCs have also been previously proposed to deliver negative signals via CD95/CD95L interactions that induce apoptosis [56, 139]. GC B cell apoptotic cell death is clearly evident in tissue sections and cellular debris is apparent in some in vivo movies, supporting the view that GC B cell death plays a prominent role in shaping the B cell repertoire [2, 50, 88]. The spontaneous apoptosis of GC B cells in vitro has been shown to involve the death receptor signaling pathway [56]. Immunized CD95 deficient mice have increased numbers of B cells with lower affinity and a reduced affinity maturation of serum antibodies [53, 139]. The high levels of CD95 expression by GC B cells and CD95L by Tfh cells makes this molecular interaction feasible, although the uniform expression by all B cells should call into question how selectivity could be imposed. It has been suggested that T cells that interact with multiple B cells at the same time might polarize toward the B cell with the highest density of pMHC presentation, selectively killing the others via a FasL mediated signal [98]. The extent to which CD95 mediated negative selection against B cells with poor BCR affinity could function as the sole means to affinity maturation is controversial [50, 132].
However, CD95L mediated B cell death remains the only identified mechanism to date for the active elimination of newly emerging self-reactive clones. It seems unlikely that the predominantly brief interactions between Tfh and GC B cells would result in cell death, since the high density of T cells within the LZ could result in frequent CD95 ligation. It is unclear whether apoptosis requires a lengthy CD95 crosslinking, which could be achieved during the more sustained T cell contacts occasionally observed in vivo. In this regard, it is interesting to note that lytic death of a B cell tumor in vivo incited by cytotoxic CD8+ T cells required only an average of 17 minutes of contact time and was preceded by a stage of active conjugate motility with only brief arrest prior to overt signs of death [95].
Migration patterns of GC B cells
Three contemporaneous imaging studies, including one from our own group, examined the migration patterns of GC B cells in vivo [3, 55, 125]. Each of these studies queried the rate of GC B cell movement between zones, a migration pattern predicted by the cyclic reentry migration model [64, 90, 107]. All three studies employed the adoptive transfer of antigen specific B cells that express GFP as well as fluorescently tagged naïve B cells to highlight the boundaries of B cell follicles. In order to define B cell migration patterns and the timing of cellular contacts within distinguishable GC zones, the FDC networks were highlighted by the injection of either fluorophore conjugated antibodies against FDC-M1 [125], Fabs against CD35 [55], or phycoerythrin-containing immune complexes [3]. Hauser et al. and Schwickert et al. used intravital microscopy in the popliteal and inguinal lymph node. Most of the time-lapse movies in the publication by Allen et al. were done in explanted lymph nodes.
In the classical version of the cyclic reentry migration model discussed above, it is envisioned that DZ B cells that have recently undergone SHM and DNA replication, move to the LZ in order to acquire antigen from FDC membrane bound deposits and selective survival signals. This vision, coupled with a perceived need for selection of higher affinity B cells between every round of division [72, 129], predicted that GC B cell movement between zones would occur frequently. However, a relatively low percentage of cell tracks were found to cross the LZ/DZ interface in each of these reports, which ranged from 5–8% per hour to 13% per hour. Different conclusions were drawn about whether this rate of exchange between zones was sufficient to remain consistent with the cyclic reentry model. Two of these reports concluded that the observed frequency and bidirectional nature of the zonal interchange was compatible with the cyclic reentry model [3, 125]. By contrast, Hauser et al. concluded that the rate of inter-zonal crossing was insufficient to allow for movement between zones and back again between every B cell division, as prescribed by the classical cyclic reentry model However, neither the Allen et al. or Schwickert et al. analyses stipulated that positive selection within the light zone must occur between each division in the dark zone, which might account for the differing interpretation of otherwise comparable results. The observed transzone migration rates in all of the studies are consistent with a cyclic re-entry model in which movement between zones after every division is not strictly imposed
The frequency of interzonal cell migration proscribed by the cyclic reentry model is a function of the rate of GC B cell division, the estimates of which differ substantially. Although a variety of technical approaches have been used to determine cell division rates, a common approach assesses the ability of cells to take up the thymidine analog, BrdU. Cells which are in S phase of the cell cycle incorporate BrdU into the newly synthesized DNA strand as long as the nucleotide analog is available in the extracellular environment. A brief exposure to BrdU in vivo is ideal in order to deliver in a tight “pulse” of BrdU to the cells. By staining cells with DAPI or Hoechst to quantify total DNA content by Flow cytometry, the rate at which cells progress through the cell cycle can be determined at the population level. Using this approach, the estimations of the division rate of GC B cells range from every 6 to 12 hours [3, 55]. Based on this range of cell cycle times, a computational simulation of the classical cyclic re-entry model estimated the fraction of cells expected to cross the dark-zone–light-zone interface to be significantly greater than the amount observed with in vivo imaging. The infrequent movement of B cells between GC zones could remain consistent with a modified version of the cyclic reentry model in which multiple rounds of division without selection within the DZ are coupled with less frequent visits to LZ selection. However, this model is less favored because it would be more difficult to account for the efficiency of affinity maturation and R-mutation enrichment achieved by the GC B cells if the requirement for selection at every division were relaxed [32, 122, 149]
The migration patterns of GC B cells reported by Hauser et al. appear to instead reflect predominantly intrazonal movement. GC B cells were observed turning back to their respective zone. Moreover, the cell tracks at the DZ-LZ interface moved mainly parallel to, and not perpendicular to this interface which otherwise would have been predicted if migration between zones was strictly required. The proposed ‘intra-zonal circulation” model does not exclude occasional movement of B cells between functionally independent zones, but does not rely on it for successive rounds of mutation and selection within a zone. The observation reported by Kehrl and colleagues that adoptively transferred B cells stimulated with LPS preferentially remain within the DZ of established GCs is consistent with the idea that motile GC B cells may display a preference to remain within one zone [58].
Support for GC zonal independence
Several lines of evidence suggest that at least some functional aspects of GC compartments can be segregated. A study of a strain of mice with a CD19 signaling defect concluded that proliferation within the LZ and DZ are initiated and regulated by different mechanisms [148]. Likewise, poor LZ formation of T cell independent GCs in WT mice despite the continued proliferation of DZ B cells over the course of several days indicates that migration to the LZ is not necessary for every round of division within the DZ [35, 42, 78, 143]. In addition to dynamic in vivo data, the early expansion of the DZ relative to the LZ compartment during T cell dependent responses implies that the progression of one is not wholly contingent on the other [21, 148]. Finally, the relative size of the two zones is rarely comparable within mature GCs. Neither of these compartments is invariably larger than the other, a feature of GC development that is consistent with at least some elements of intra-zonal dynamics being independent of one another.
B cells within the LZ are thought to be undergoing either some form of competitive selection for higher affinity variants or programmatic differentiation. As we discuss further below, all the cell types that have been proposed to control the next round of division, such as T cells and GC DC subsets as well as FDCs, exist within the basal LZ, apical region or OZ. In murine GCs, B cells that have incorporated BrdU during very brief exposures to injected antigen can be found throughout the LZ [3, 55]. In the LZ of tonsils, Ki67+ B cells are also more likely to be found in the OZ [54], a region that harbors a phenotypically distinct Tfh population and AID expressing B cells [27]. It remains unknown whether the proliferation observed at the OZ is linked to higher rates of SHM, but if it were, all the same potential modes of selection would also be present in this region. Ongoing proliferation within the LZ of LNs does not conflict with the idea that this region may also guide differentiation toward long-term AFCs as several rounds of division may be required for plasma cell formation [6].
All the previously proposed mechanisms for selection also remain available to DZ B cells and could occur between each division. The IgD+ follicular mantle around murine GCs is often very thin, and at times even non-existent, at the DZ base adjacent to the T zone, a site well known for the initiation of T dependent B cell responses. This border with the T cell zone is a location rich in activated T cells and dendritic cells during T-dependent responses and could serve as an alternative site for proliferative signals (Figure 3). Even at later stages of immune responses when GCs are fully mature, cytokine producing CD4+ T cells can be found at this interface [119]. The directed movement of DZ B cells to and from the base of the DZ abutting the T zone border is consistent with this region playing a role in sustaining DZ homeostasis [55]. DZ B cells could be observed to form contacts lasting longer than 10 minutes with unknown cell types that were not fluorescently labeled at the DZ/T zone border. Thus, interactions with other cell types proposed to promote B cell proliferation and differentiation are available within the DZ arena.
Figure 3. Close association of the DZ with T zone border.
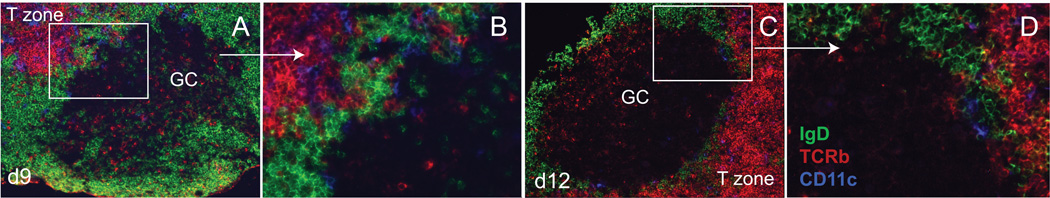
B cells at the base of the DZ have the potential to have intimate contact with T and DC cells near the T zone. The base of the DZ is typically long and flattened against the T cell zone. T cells and DCs (CD11c+) are present at the periphery of the DZ of mature GCs. Murine LNs were obtained 9 or 12 days after footpad immunization. Mouse immunization details are as described in Figure 1. Staining for anti-IgD-AL488 (green), anti-TCRb-bi/SA-AL568 (red) and anti-CD11c-AL647 (blue).
Although FDCs do not exist within the DZ, myeloid DCs at the DZ periphery as well as the T zone border are theoretically capable of providing similar proliferative and survival signals. DCs have previously been shown to promote the proliferation and sustained survival of B cells [10, 82]. During intravital imaging, DCs adjacent to paracortical sinuses have been shown to retain undegraded protein antigen non-specifically and incite Ca++ flux within specific B cells upon contact, presumably the result of BCR cross-linking [114]. Likewise, antigen complexed with specific Ig can be taken up by DCs in a non-degradative pathway via FcgRIIB and subsequently re-expressed at the DC surface. Such a mode of Ag acquisition and presentation to B cells has been shown to be sufficient to promote B cell proliferation in vitro [18, 150]. Naturally occurring components of viral and bacterial pathogens constitute highly multivalent antigenic arrays that, like anti-IgM stimulation in vitro, could allow for activation via BCR crosslinking. Intravital imaging studies have shown that larger particulate antigens, such as inert beads, intact viruses or immune complexes, are initially excluded from follicles during the earliest stages of antigen exposure, but can be shuttled across the subcapsular sinus of LNs by macrophages that line this sinus [22, 63, 112]. However, particulate antigens primarily filter through larger sinuses that pass through interfollicular regions to paracortical high endothelial venules. Although these reports illustrate how antigen is shepherded during the very earliest antigen exposures, uptake and transport to other locations by accessory cells such as myeloid DCs does occur in some circumstances [10]. These reports, coupled with the presence of CD11c+ DCs at the DZ base, suggests a potential mechanism for the presentation of intact protein antigens on the surface of an accessory cell.
However, it is important to keep in mind that recognition of antigen in the context of an accessory cell is not strictly required for B cell activation. As discussed above, competition for T cell help is one of the proposed mechanisms that may selectively encourage the proliferation or survival of higher affinity B cells. DZ B cells that have acquired soluble antigen, chronically released by FDCs or shed or secreted by pathogens, could process internalized antigen and present peptide determinants in the context of MHC II to stimulatory T cells. In vivo imaging studies have demonstrated that small molecular complexes can permeate and very rapidly disseminate throughout the follicle interior via thin conduits that extend directly from sinuses or possibly via small gaps between cells lining sinuses that transport afferent lymph [109, 121]. These studies and others clearly demonstrated that B cell access to such soluble antigens and internalization can occur immediately upon antigen arrival in follicles, and by extension could do so with soluble antigens disseminated by other means at later stages in the immune response.
It is possible that retention within a zone may be mediated by unique features in the extra-cellular and stromal cell matrix, an idea that is supported by some GC features. The stromal cell subset within the DZ is phenotypically distinct, expressing less VCAM-1 and ICAM-1. Although adhesion via integrins is not essential for locomotion through lymphoid tissue [40], this difference in adhesion molecule expression might suggest that DZ and LZ stromal cells differ in other molecules influencing migratory dispositions, analogous to the retention of resting T cells within the T cell zone [8]. Predispositions to respond to zonal differences in chemokines could also promote the retention of GC B cells within a zone. LZ and DZ B cells differ in the expression of CXCR5 and CXCR4, chemokine receptors that have been shown to be critically required for the proper organization of GC B cells into distinct zones [1].
It is tempting to speculate that different types of B cells might emerge from each zone, but there is insufficient evidence to support such an idea. Due to the presence of AFC promoting transcription factors within the LZ of tonsillar GCs, it seems natural to suggest that development of long-term AFCs occurs within that compartment. It is conceivable that events within the LZ may be directly responsible for producing both cell types. Some features of DZ B cells lend credence to the idea that this compartment could produce memory B cells. The higher density of actively proliferating B cells is suggestive of a greater potential for self-renewal in situ, perhaps an environment that might foster the development of memory B cells that retain an ability to self-renew when pathogens are encountered later in life. The observation of B cells exiting follicles via a cortical sinus close to the base of the follicle seems compatible with the notion of a more proximal DZ exporting a cellular “product” [130]. However, the behavior of B cells of either zone immediately after division is yet to be determined. Similarly, nothing is known about the exact route of B cell exit and the zonal origins of B cells that egress.
Conclusions
The utilization of two-photon microscopy in order to explore GCs in vivo has undoubtedly led to a boost of research in this field. Many unexpected features have been revealed that have contributed significantly to our understanding of the GC reaction through the application of this technology. Despite these advances in the field, there are still many important open questions. Although some elements of the cellular interactions between cell types within GCs have now been visualized, it is yet to be determined which events definitively control the positive selection and enrichment of GC B cells with higher affinity BCR. Further insights into the cellular and molecular interactions that regulate GC B cell behavior are much needed in order to deepen our understanding of lymphomagenesis and autoimmunity as well.
The intravital imaging studies described above used adoptively transferred cells that express fluorescent proteins throughout the cytosol, an approach that permits the visualization a cell in its entirety and tracking of its movements through an otherwise dark tissue volume. More mechanistic experimental approaches will be aided by the generation of murine strains of mice that express fluorescent fusion proteins. This would greatly enhance our ability to observe events on a sub-cellular level and more effectively query key molecular interactions. The ability to image over extended time periods in combination with the generation of an increasing variety of fluorescent reporter mice will likewise empower future studies to determine functional consequences and potential differences of location-specific or cytokine-specific interactions. Beyond data acquisition, the development of more standardized, automated approaches for analysis will help to extract the important pieces from the complexity of information generated by time-resolved intravital imaging. Altogether, this will further refine our knowledge on GCs and provide greater insight into the dynamics and mechanisms that form the basis of these fascinating structures.
Acknowledgements
We thank Mark Shlomchik for critical reading of the manuscript and thoughtful discussion. This work was supported in part by grants from NIH grant 1R01AI080850-01A1 (AMH and SMK) and Deutsche Forschungsgemeinschaft (DFG) grant HA5354/1-4 (AEH).
References
- 1.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 2.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 4.Anderson SM, Khalil A, Uduman M, Hershberg U, Louzoun Y, Haberman AM, Kleinstein SH, Shlomchik MJ. Taking Advantage: High-Affinity B Cells in the Germinal Center Have Lower Death Rates, but Similar Rates of Division, Compared to Low-Affinity Cells. J Immunol. 2009;183:7314–7325. doi: 10.4049/jimmunol.0902452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol. 2000;165:5462–5471. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- 6.Arce S, Luger E, Muehlinghaus G, Cassese G, Hauser A, Horst A, Lehnert K, Odendahl M, Honemann D, Heller KD, Kleinschmidt H, Berek C, Dorner T, Krenn V, Hiepe F, Bargou R, Radbruch A, Manz RA. CD38 low IgG-secreting cells are precursors of various CD38 high-expressing plasma cell populations. J Leukoc Biol. 2004;75:1022–1028. doi: 10.1189/jlb.0603279. [DOI] [PubMed] [Google Scholar]
- 7.Arnold CN, Campbell DJ, Lipp M, Butcher EC. The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur J Immunol. 2007;37:100–109. doi: 10.1002/eji.200636486. [DOI] [PubMed] [Google Scholar]
- 8.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balague O, Mozos A, Martinez D, Hernandez L, Colomo L, Mate JL, Teruya-Feldstein J, Lin O, Campo E, Lopez-Guillermo A, Martinez A. Activation of the endoplasmic reticulum stress-associated transcription factor x box-binding protein-1 occurs in a subset of normal germinal-center B cells and in aggressive B-cell lymphomas with prognostic implications. Am J Pathol. 2009;174:2337–2346. doi: 10.2353/ajpath.2009.080848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 11.Balogh P, Aydar Y, Tew JG, Szakal AK. Appearance and phenotype of murine follicular dendritic cells expressing VCAM-1. Anat Rec. 2002;268:160–168. doi: 10.1002/ar.10148. [DOI] [PubMed] [Google Scholar]
- 12.Barrington RA, Pozdnyakova O, Zafari MR, Benjamin CD, Carroll MC. B lymphocyte memory: role of stromal cell complement and FcgammaRIIB receptors. J Exp Med. 2002;196:1189–1199. doi: 10.1084/jem.20021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8:751–759. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 14.Batista FD, Neuberger MS. B cells extract and present immobilized antigen: implications for affinity discrimination. Embo J. 2000;19:513–520. doi: 10.1093/emboj/19.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beltman JB, Maree AF, de Boer RJ. Spatial modelling of brief and long interactions between T cells and dendritic cells. Immunol Cell Biol. 2007;85:306–314. doi: 10.1038/sj.icb.7100054. [DOI] [PubMed] [Google Scholar]
- 16.Beltman JB, Maree AF, de Boer RJ. Analysing immune cell migration. Nat Rev Immunol. 2009;9:789–798. doi: 10.1038/nri2638. [DOI] [PubMed] [Google Scholar]
- 17.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 18.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cahalan MD, Parker I, Wei SH, Miller MJ. Real-time imaging of lymphocytes in vivo. Curr Opin Immunol. 2003;15:372–377. doi: 10.1016/s0952-7915(03)00079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camacho SA, Kosco-Vilbois MH, Berek C. The dynamic structure of the germinal center. Immunol Today. 1998;19:511–514. doi: 10.1016/s0167-5699(98)01327-9. [DOI] [PubMed] [Google Scholar]
- 22.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 24.Casamayor-Palleja M, Khan M, MacLennan IC. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J Exp Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casamayor-Palleja M, Gulbranson-Judge A, MacLennan IC. T cells in the selection of germinal center B cells. Chem Immunol. 1997;67:27–44. doi: 10.1159/000058676. [DOI] [PubMed] [Google Scholar]
- 26.Cattoretti G, Angelin-Duclos C, Shaknovich R, Zhou H, Wang D, Alobeid B. PRDM1/Blimp-1 is expressed in human B-lymphocytes committed to the plasma cell lineage. J Pathol. 2005;206:76–86. doi: 10.1002/path.1752. [DOI] [PubMed] [Google Scholar]
- 27.Cattoretti G, Buttner M, Shaknovich R, Kremmer E, Alobeid B, Niedobitek G. Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells. Blood. 2006;107:3967–3975. doi: 10.1182/blood-2005-10-4170. [DOI] [PubMed] [Google Scholar]
- 28.Cattoretti G, Shaknovich R, Smith PM, Jack HM, Murty VV, Alobeid B. Stages of germinal center transit are defined by B cell transcription factor coexpression and relative abundance. J Immunol. 2006;177:6930–6939. doi: 10.4049/jimmunol.177.10.6930. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Koralov SB, Gendelman M, Carroll MC, Kelsoe G. Humoral immune responses in Cr2−/− mice: enhanced affinity maturation but impaired antibody persistence. J Immunol. 2000;164:4522–4532. doi: 10.4049/jimmunol.164.9.4522. [DOI] [PubMed] [Google Scholar]
- 30.Choe J, Li L, Zhang X, Gregory CD, Choi YS. Distinct role of follicular dendritic cells and T cells in the proliferation, differentiation, and apoptosis of a centroblast cell line, L3055. J Immunol. 2000;164:56–63. doi: 10.4049/jimmunol.164.1.56. [DOI] [PubMed] [Google Scholar]
- 31.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 32.Clarke SH, Huppi K, Ruezinsky D, Staudt L, Gerhard W, Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985;161:687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffey F, Alabyev B, Manser T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity. 2009;30:599–609. doi: 10.1016/j.immuni.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J Immunol. 1998;161:5373–5381. [PubMed] [Google Scholar]
- 35.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond B, Scharff MD. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984;81:5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Shikh ME, El Sayed R, Szakal AK, Tew JG. Follicular dendritic cell (FDC)-FcgammaRIIB engagement via immune complexes induces the activated FDC phenotype associated with secondary follicle development. Eur J Immunol. 2006;36:2715–2724. doi: 10.1002/eji.200636122. [DOI] [PubMed] [Google Scholar]
- 38.Figge MT, Garin A, Gunzer M, Kosco-Vilbois M, Toellner KM, Meyer-Hermann M. Deriving a germinal center lymphocyte migration model from two-photon data. J Exp Med. 2008;205:3019–3029. doi: 10.1084/jem.20081160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claassen E, Noelle RJ. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 41.Galibert L, Burdin N, Barthelemy C, Meffre G, Durand I, Garcia E, Garrone P, Rousset F, Banchereau J, Liu YJ. Negative selection of human germinal center B cells by prolonged BCR cross-linking. J Exp Med. 1996;183:2075–2085. doi: 10.1084/jem.183.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaspal FM, McConnell FM, Kim MY, Gray D, Kosco-Vilbois MH, Raykundalia CR, Botto M, Lane PJ. The generation of thymus-independent germinal centers depends on CD40 but not on CD154, the T cell-derived CD40-ligand. Eur J Immunol. 2006;36:1665–1673. doi: 10.1002/eji.200535339. [DOI] [PubMed] [Google Scholar]
- 43.Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009;31:259–269. doi: 10.1016/j.immuni.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Germain RN, Miller MJ, Dustin ML, Nussenzweig MC. Dynamic imaging of the immune system: progress, pitfalls and promise. Nat Rev Immunol. 2006;6:497–507. doi: 10.1038/nri1884. [DOI] [PubMed] [Google Scholar]
- 45.Gray D, Siepmann K, Wohlleben G. CD40 ligation in B cell activation, isotype switching and memory development. Semin Immunol. 1994;6:303–310. doi: 10.1006/smim.1994.1039. [DOI] [PubMed] [Google Scholar]
- 46.Grouard G, Durand I, Filgueira L, Banchereau J, Liu YJ. Dendritic cells capable of stimulating T cells in germinal centres. Nature. 1996;384:364–367. doi: 10.1038/384364a0. [DOI] [PubMed] [Google Scholar]
- 47.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 48.Haberman AM, Shlomchik MJ. Reassessing the function of immune-complex retention by follicular dendritic cells. Nat Rev Immunol. 2003;3:757–764. doi: 10.1038/nri1178. [DOI] [PubMed] [Google Scholar]
- 49.Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- 50.Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J Exp Med. 1995;182:1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanna MG., Jr An Autoradiographic Study of the Germinal Center in Spleen White Pulp during Early Intervals of the Immune Response. Lab Invest. 1964;13:95–104. [PubMed] [Google Scholar]
- 52.Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hao Z, Duncan GS, Seagal J, Su YW, Hong C, Haight J, Chen NJ, Elia A, Wakeham A, Li WY, Liepa J, Wood GA, Casola S, Rajewsky K, Mak TW. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29:615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardie DL, Johnson GD, Khan M, MacLennan IC. Quantitative analysis of molecules which distinguish functional compartments within germinal centers. Eur J Immunol. 1993;23:997–1004. doi: 10.1002/eji.1830230502. [DOI] [PubMed] [Google Scholar]
- 55.Hauser AE, Junt T, Mempel TR, Sneddon MW, Kleinstein SH, Henrickson SE, von Andrian UH, Shlomchik MJ, Haberman AM. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007;26:655–667. doi: 10.1016/j.immuni.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Hennino A, Berard M, Krammer PH, Defrance T. FLICE-inhibitory protein is a key regulator of germinal center B cell apoptosis. J Exp Med. 2001;193:447–458. doi: 10.1084/jem.193.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Humphrey JH, Grennan D, Sundaram V. The origin of follicular dendritic cells in the mouse and the mechanism of trapping of immune complexes on them. Eur J Immunol. 1984;14:859–864. doi: 10.1002/eji.1830140916. [DOI] [PubMed] [Google Scholar]
- 58.Hwang IY, Park C, Harrison K, Kehrl JH. TLR4 signaling augments B lymphocyte migration and overcomes the restriction that limits access to germinal center dark zones. J Exp Med. 2009;206:2641–2657. doi: 10.1084/jem.20091982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 61.Jacob J, Przylepa J, Miller C, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johansson-Lindbom B, Ingvarsson S, Borrebaeck CA. Germinal centers regulate human Th2 development. J Immunol. 2003;171:1657–1666. doi: 10.4049/jimmunol.171.4.1657. [DOI] [PubMed] [Google Scholar]
- 63.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 64.Kepler TB, Perelson AS. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol Today. 1993;14:412–415. doi: 10.1016/0167-5699(93)90145-B. [DOI] [PubMed] [Google Scholar]
- 65.Kesmir C, De Boer RJ. A mathematical model on germinal center kinetics and termination. J Immunol. 1999;163:2463–2469. [PubMed] [Google Scholar]
- 66.Kesmir C, De Boer RJ. A spatial model of germinal center reactions: cellular adhesion based sorting of B cells results in efficient affinity maturation. J Theor Biol. 2003;222:9–22. doi: 10.1016/s0022-5193(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 67.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim JR, Lim HW, Kang SG, Hillsamer P, Kim CH. Human CD57+ germinal center-T cells are the major helpers for GC-B cells and induce class switch recombination. BMC Immunol. 2005;6:3. doi: 10.1186/1471-2172-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 70.Klaus GG, Humphrey JH, Kunkl A, Dongworth DW. The follicular dendritic cell: its role in antigen presentation in the generation of immunological memory. Immunol Rev. 1980;53:3–28. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 71.Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, Ludwig T, Rajewsky K, Dalla-Favera R. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 72.Kleinstein SH, Singh JP. Toward quantitative simulation of germinal center dynamics: biological and modeling insights from experimental validation. J Theor Biol. 2001;211:253–275. doi: 10.1006/jtbi.2001.2344. [DOI] [PubMed] [Google Scholar]
- 73.Koopman G, Parmentier HK, Schuurman HJ, Newman W, Meijer CJ, Pals ST. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J Exp Med. 1991;173:1297–1304. doi: 10.1084/jem.173.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koopman G, Keehnen RM, Pals ST. Interaction through the LFA-1/ICAM-1 pathway prevents programmed cell death of germinal center B cells. Adv Exp Med Biol. 1993;329:387–392. doi: 10.1007/978-1-4615-2930-9_65. [DOI] [PubMed] [Google Scholar]
- 75.Krajewski S, Krajewska M, Shabaik A, Wang HG, Irie S, Fong L, Reed JC. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 1994;54:5501–5507. [PubMed] [Google Scholar]
- 76.Kroese FG, Wubbena AS, Seijen HG, Nieuwenhuis P. Germinal centers develop oligoclonally. Eur J Immunol. 1987;17:1069–1072. doi: 10.1002/eji.1830170726. [DOI] [PubMed] [Google Scholar]
- 77.Leanderson T, Kallberg E, Gray D. Expansion, selection and mutation of antigen-specific B cells in germinal centers. Immunol Rev. 1992;126:47–61. doi: 10.1111/j.1600-065x.1992.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 78.Lentz VM, Manser T. Cutting edge: Germinal centers can be induced in the absence of T cells. Journal of Immunology. 2001;167:15–20. doi: 10.4049/jimmunol.167.1.15. [DOI] [PubMed] [Google Scholar]
- 79.Lindhout E, Mevissen ML, Kwekkeboom J, Tager JM, de Groot C. Direct evidence that human follicular dendritic cells (FDC) rescue germinal centre B cells from death by apoptosis. Clin Exp Immunol. 1993;91:330–336. doi: 10.1111/j.1365-2249.1993.tb05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Linton PJ, Lo D, Lai L, Thorbecke GJ, Klinman NR. Among naive precursor cell subpopulations only progenitors of memory B cells originate germinal centers. Eur J Immunol. 1992;22:1293–1297. doi: 10.1002/eji.1830220526. [DOI] [PubMed] [Google Scholar]
- 81.Linton PL, Decker DJ, Klinman NR. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 1989;59:1049–1059. doi: 10.1016/0092-8674(89)90761-7. [DOI] [PubMed] [Google Scholar]
- 82.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 84.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 85.Liu YJ, Johnson GD, Gordon J, MacLennan IC. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992;13:17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 86.Liu YJ, Grouard G, de Bouteiller O, Banchereau J. Follicular dendritic cells and germinal centers. Int Rev Cytol. 1996;166:139–179. doi: 10.1016/s0074-7696(08)62508-5. [DOI] [PubMed] [Google Scholar]
- 87.Liu YJ, Banchereau J. Regulation of B-cell commitment to plasma cells or to memory B cells. Semin Immunol. 1997;9:235–240. doi: 10.1006/smim.1997.0080. [DOI] [PubMed] [Google Scholar]
- 88.Liu YJ, de Bouteiller O, Fugier-Vivier I. Mechanisms of selection and differentiation in germinal centers. Curr Opin Immunol. 1997;9:256–262. doi: 10.1016/s0952-7915(97)80145-8. [DOI] [PubMed] [Google Scholar]
- 89.Liu YJ, Xu J, de Bouteiller O, Parham CL, Grouard G, Djossou O, de Saint-Vis B, Lebecque S, Banchereau J, Moore KW. Follicular dendritic cells specifically express the long CR2/CD21 isoform. J Exp Med. 1997;185:165–170. doi: 10.1084/jem.185.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.MacLennan IC, Johnson GD, Liu YJ, Gordon J. The heterogeneity of follicular reactions. Res Immunol. 1991;142:253–257. doi: 10.1016/0923-2494(91)90070-y. [DOI] [PubMed] [Google Scholar]
- 91.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 92.Mandel TE, Phipps RP, Abbot A, Tew JG. The follicular dendritic cell: long term antigen retention during immunity. Immunol Rev. 1980;53:29–59. doi: 10.1111/j.1600-065x.1980.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 93.Martinez-Valdez H, Guret C, de Bouteiller O, Fugier I, Banchereau J, Liu YJ. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J Exp Med. 1996;183:971–977. doi: 10.1084/jem.183.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 95.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 96.Meyer-Hermann M. A mathematical model for the germinal center morphology and affinity maturation. J Theor Biol. 2002;216:273–300. doi: 10.1006/jtbi.2002.2550. [DOI] [PubMed] [Google Scholar]
- 97.Meyer-Hermann M, Figge MT, Toellner KM. Germinal centres seen through the mathematical eye: B-cell models on the catwalk. Trends Immunol. 2009;30:157–164. doi: 10.1016/j.it.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 98.Meyer-Hermann ME, Maini PK, Iber D. An analysis of B cell selection mechanisms in germinal centers. Math Med Biol. 2006;23:255–277. doi: 10.1093/imammb/dql012. [DOI] [PubMed] [Google Scholar]
- 99.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moreira JS, Faro J. Modelling two possible mechanisms for the regulation of the germinal center dynamics. J Immunol. 2006;177:3705–3710. doi: 10.4049/jimmunol.177.6.3705. [DOI] [PubMed] [Google Scholar]
- 101.Moreira JS, Faro J. Re-evaluating the recycling hypothesis in the germinal centre. Immunol Cell Biol. 2006;84:404–410. doi: 10.1111/j.1440-1711.2006.01443.x. [DOI] [PubMed] [Google Scholar]
- 102.Morra M, Howie D, Grande MS, Sayos J, Wang N, Wu C, Engel P, Terhorst C. X-linked lymphoproliferative disease: a progressive immunodeficiency. Annu Rev Immunol. 2001;19:657–682. doi: 10.1146/annurev.immunol.19.1.657. [DOI] [PubMed] [Google Scholar]
- 103.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 104.Neuberger MS, Di Noia JM, Beale RC, Williams GT, Yang Z, Rada C. Somatic hypermutation at A.T pairs: polymerase error versus dUTP incorporation. Nat Rev Immunol. 2005;5:171–178. doi: 10.1038/nri1553. [DOI] [PubMed] [Google Scholar]
- 105.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oliver AM, Martin F, Kearney JF. Mouse CD38 is down-regulated on germinal center B cells and mature plasma cells. J Immunol. 1997;158:1108–1115. [PubMed] [Google Scholar]
- 107.Oprea M, van Nimwegen E, Perelson AS. Dynamics of one-pass germinal center models: implications for affinity maturation. Bull Math Biol. 2000;62:121–153. doi: 10.1006/bulm.1999.0144. [DOI] [PubMed] [Google Scholar]
- 108.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 109.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 110.Park CS, Yoon SO, Armitage RJ, Choi YS. Follicular dendritic cells produce IL-15 that enhances germinal center B cell proliferation in membrane-bound form. J Immunol. 2004;173:6676–6683. doi: 10.4049/jimmunol.173.11.6676. [DOI] [PubMed] [Google Scholar]
- 111.Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460:1122–1126. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007 doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 113.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10:786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 115.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qin D, Wu J, Vora KA, Ravetch JV, Szakal AK, Manser T, Tew JG. Fc gamma receptor IIB on follicular dendritic cells regulates the B cell recall response. J Immunol. 2000;164:6268–6275. doi: 10.4049/jimmunol.164.12.6268. [DOI] [PubMed] [Google Scholar]
- 117.Randall KL, Lambe T, Johnson A, Treanor B, Kucharska E, Domaschenz H, Whittle B, Tze LE, Enders A, Crockford TL, Bouriez-Jones T, Alston D, Cyster JG, Lenardo MJ, Mackay F, Deenick EK, Tangye SG, Chan TD, Camidge T, Brink R, Vinuesa CG, Batista FD, Cornall RJ, Goodnow CC. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10:1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Randall TD, Heath AW, Santos-Argumedo L, Howard MC, Weissman IL, Lund FE. Arrest of B lymphocyte terminal differentiation by CD40 signaling: mechanism for lack of antibody-secreting cells in germinal centers. Immunity. 1998;8:733–742. doi: 10.1016/s1074-7613(00)80578-6. [DOI] [PubMed] [Google Scholar]
- 119.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature Immunology. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rogerson B, Hackett J, Jr, Peters A, Haasch D, Storb U. Mutation pattern of immunoglobulin transgenes is compatible with a model of somatic hypermutation in which targeting of the mutator is linked to the direction of DNA replication. Embo J. 1991;10:4331–4341. doi: 10.1002/j.1460-2075.1991.tb05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, von Andrian UH, Carroll MC. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sablitzky F, Wildner G, Rajewsky K. Somatic mutation and clonal expansion of B cells in an antigen-driven immune response. Embo J. 1985;4:345–350. doi: 10.1002/j.1460-2075.1985.tb03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sacedon R, Diez B, Nunez V, Hernandez-Lopez C, Gutierrez-Frias C, Cejalvo T, Outram SV, Crompton T, Zapata AG, Vicente A, Varas A. Sonic hedgehog is produced by follicular dendritic cells and protects germinal center B cells from apoptosis. J Immunol. 2005;174:1456–1461. doi: 10.4049/jimmunol.174.3.1456. [DOI] [PubMed] [Google Scholar]
- 124.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 126.Schwickert TA, Alabyev B, Manser T, Nussenzweig MC. Germinal center reutilization by newly activated B cells. J Exp Med. 2009 doi: 10.1084/jem.20091225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shih TA, Meffre E, Roederer M, Nussenzweig MC. Role of BCR affinity in T cell dependent antibody responses in vivo. Nat Immunol. 2002;3:570–575. doi: 10.1038/ni803. [DOI] [PubMed] [Google Scholar]
- 128.Shlomchik MJ, Litwin S, Weigert M. The influence of somatic mutation on clonal expansion. Prog Immunol Proc 7th Int Cong Immunol. 1990;7:415–423. [Google Scholar]
- 129.Shlomchik MJ, Watts P, Weigert MG, Litwin S. Clone: a Monte-Carlo computer simulation of B cell clonal expansion, somatic mutation, and antigen-driven selection. Curr Top Microbiol Immunol. 1998;229:173–197. doi: 10.1007/978-3-642-71984-4_13. [DOI] [PubMed] [Google Scholar]
- 130.Sinha RK, Park C, Hwang IY, Davis MD, Kehrl JH. B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity. 2009;30:434–446. doi: 10.1016/j.immuni.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith JP, Lister AM, Tew JG, Szakal AK. Kinetics of the tingible body macrophage response in mouse germinal center development and its depression with age. Anat Rec. 1991;229:511–520. doi: 10.1002/ar.1092290412. [DOI] [PubMed] [Google Scholar]
- 132.Smith KG, Nossal GJ, Tarlinton DM. FAS is highly expressed in the germinal center but is not required for regulation of the B-cell response to antigen. Proc Natl Acad Sci U S A. 1995;92:11628–11632. doi: 10.1073/pnas.92.25.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sumen C, Mempel TR, Mazo IB, von Andrian UH. Intravital microscopy: visualizing immunity in context. Immunity. 2004;21:315–329. doi: 10.1016/j.immuni.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 134.Suzuki K, Grigorova I, Phan TG, Kelly LM, Cyster JG. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J Exp Med. 2009;206:1485–1493. doi: 10.1084/jem.20090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Swartzendruber DC, Congdon CC. Electron Microscope Observations on Tingible Body Macrophages in Mouse Spleen. J Cell Biol. 1963;19:641–646. doi: 10.1083/jcb.19.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Szakal AK, Kosco MH, Tew JG. A novel in vivo follicular dendritic cell-dependent iccosome-mediated mechanism for delivery of antigen to antigen-processing cells. J Immunol. 1988;140:341–353. [PubMed] [Google Scholar]
- 137.Szakal AK, Kosco MH, Tew JG. Microanatomy of lymphoid tissue during humoral immune responses: structure function relationships. Annu Rev Immunol. 1989;7:91–109. doi: 10.1146/annurev.iy.07.040189.000515. [DOI] [PubMed] [Google Scholar]
- 138.Takahashi Y, Cerasoli DM, Dal Porto JM, Shimoda M, Freund R, Fang W, Telander DG, Malvey EN, Mueller DL, Behrens TW, Kelsoe G. Relaxed negative selection in germinal centers and impaired affinity maturation in bcl-xL transgenic mice. J Exp Med. 1999;190:399–410. doi: 10.1084/jem.190.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14:181–192. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 140.Tarlinton DM, Smith KG. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol Today. 2000;21:436–441. doi: 10.1016/s0167-5699(00)01687-x. [DOI] [PubMed] [Google Scholar]
- 141.Tew JG, Wu J, Fakher M, Szakal AK, Qin D. Follicular dendritic cells: beyond the necessity of T-cell help. Trends Immunol. 2001;22:361–367. doi: 10.1016/s1471-4906(01)01942-1. [DOI] [PubMed] [Google Scholar]
- 142.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Toellner KM, Jenkinson WE, Taylor DR, Khan M, Sze DM, Sansom DM, Vinuesa CG, MacLennan IC. Low-level hypermutation in T cell-independent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J Exp Med. 2002;195:383–389. doi: 10.1084/jem.20011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tuscano JM, Druey KM, Riva A, Pena J, Thompson CB, Kehrl JH. Bcl-x rather than Bcl-2 mediates CD40-dependent centrocyte survival in the germinal center. Blood. 1996;88:1359–1364. [PubMed] [Google Scholar]
- 145.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 146.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 147.Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Lane PJ. Co-stimulation and selection for T-cell help for germinal centres: the role of CD28 and OX40. Immunol Today. 2000;21:333–337. doi: 10.1016/s0167-5699(00)01636-4. [DOI] [PubMed] [Google Scholar]
- 148.Wang Y, Carter RH. CD19 regulates B cell maturation, proliferation, and positive selection in the FDC zone of murine splenic germinal centers. Immunity. 2005;22:749–761. doi: 10.1016/j.immuni.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 149.Weiss U, Rajewsky K. The repertoire of somatic antibody mutants accumulating in the memory compartment after primary immunization is restricted through affinity maturation and mirrors that expressed in the secondary response. J Exp Med. 1990;172:1681–1689. doi: 10.1084/jem.172.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- 151.Yoshida K, Kaji M, Takahashi T, van den Berg TK, Dijkstra CD. Host origin of follicular dendritic cells induced in the spleen of SCID mice after transfer of allogeneic lymphocytes. Immunology. 1995;84:117–126. [PMC free article] [PubMed] [Google Scholar]
- 152.Yu D, Cook MC, Shin DM, Silva DG, Marshall J, Toellner KM, Havran WL, Caroni P, Cooke MP, Morse HC, MacLennan IC, Goodnow CC, Vinuesa CG. Axon growth and guidance genes identify T-dependent germinal centre B cells. Immunol Cell Biol. 2008;86:3–14. doi: 10.1038/sj.icb.7100123. [DOI] [PubMed] [Google Scholar]
- 153.Yu D, Batten M, Mackay CR, King C. Lineage specification and heterogeneity of T follicular helper cells. Curr Opin Immunol. 2009;21:619–625. doi: 10.1016/j.coi.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 154.Zaheen A, Boulianne B, Parsa JY, Ramachandran S, Gommerman JL, Martin A. AID constrains germinal center size by rendering B cells susceptible to apoptosis. Blood. 2009;114:547–554. doi: 10.1182/blood-2009-03-211763. [DOI] [PubMed] [Google Scholar]
- 155.Zhang J, MacLennan IC, Liu YJ, Lane PJ. Is rapid proliferation in B centroblasts linked to somatic mutation in memory B cell clones? Immunol Lett. 1988;18:297–299. doi: 10.1016/0165-2478(88)90178-2. [DOI] [PubMed] [Google Scholar]