Abstract
Difficulties in the ability to successfully inhibit impulsive behaviors have been reported in marijuana (MJ) smokers, yet few studies have made direct comparisons between early (prior to age 16) and late (age 16 or later) onset MJ smokers, specifically during behavioral inhibition tasks. The current study utilized the Multi-Source Interference Task (MSIT) during functional magnetic resonance imaging (fMRI) in chronic, heavy MJ smokers and healthy non-MJ smoking controls which revealed a more focal pattern of anterior cingulate activity in controls relative to smokers. Early onset smokers had more focal activation but tended to make more errors of commission relative to late onset smokers, suggesting a possible neural adaptation despite difficulty with behavioral inhibition. Further investigation is warranted, as early exposure to MJ may result in reorganization of critical brain regions.
Keywords: marijuana, cognitive control, inhibition, age of onset, fMRI
Introduction
Marijuana (MJ) remains the most widely used illicit substance in the United States, with 14.4 million Americans aged 12 and older reporting at least one instance of abuse in the past month [22]. Difficulties in the ability to successfully monitor and inhibit impulsive behaviors have been reported in adult MJ smokers [3,11,17], and neuroimaging studies have reported altered activation of frontal brain regions during inhibitory tasks [8,13,15]. While previous investigations have reported alterations in brain function which are associated with the age of onset of MJ use, few have made direct comparisons between early and late onset MJ smokers, specifically during tasks requiring behavioral inhibition [2,18]. Studies of brain structure have indicated that the frontal cortex matures more slowly than other brain regions, and development of this region parallels the improvements in both cognitive control and behavioral inhibition that emerge during the transition into adulthood [6]. It is not surprising, therefore, that the neuromaturational changes that occur during adolescence which result in cognitive and emotional changes may be adversely affected by early exposure to MJ. Several animal studies have reported that the pubertal through adolescent period is a highly susceptible time frame for exposure to cannabinoids, resulting in more pronounced alteration in behaviors in adulthood than exposure during other developmental periods [19–20].
The current study examined cortical response using functional magnetic resonance imaging (fMRI) in chronic, heavy MJ smokers as compared to healthy, non-MJ smoking control subjects during a task that requires behavioral inhibition, the Multi-Source Interference Task (MSIT). The MSIT reliably and robustly activates cingulo-frontal-parietal circuitry associated with cognitive/attentional pathways [4]. Further, in order to identify the potential impact of age of onset of MJ use on brain function, we directly compared activation patterns of those who began using MJ prior to age 16 (early onset) to those who began smoking MJ after the age of 16 (late onset). While no uniformly accepted definition of early vs late onset exists, several studies have used age 16 as a cutoff [7,11–12]. Based on findings from previous imaging studies of MJ smokers during tasks requiring executive control and inhibition [8,13], we hypothesized that during the interference condition of the task, 1) chronic, heavy MJ smokers would exhibit a different pattern of activation despite similar task performance, and 2) early onset MJ smokers would demonstrate a different pattern of activation and have more difficulty relative to late onset MJ smokers.
Materials and Methods
Participants
Twenty-three well-characterized, adult chronic heavy MJ smokers and sixteen non-MJ smoking healthy control (HC) subjects were recruited from the greater Boston area, with participants from both downtown and suburban locations. Recruitment sites included local colleges and universities, athletic centers, and other public locations. All subjects received the Structured Clinical Interview for DSM-IV, Patient Edition (SCID-P) [9] to ensure that no Axis I pathology was present, including current or previous drug/alcohol abuse or dependence (excluding MJ for the smokers). In addition, subjects were excluded if they reported more than 15 lifetime uses of any category of illicit drugs including sedative-hypnotics, stimulants, cocaine, opioids, hallucinogens, methylenedioxymetamphetamine (MDMA) and marijuana (in the control group), had a positive urine screen for any illicit substance (excluding MJ for the smokers) or routinely had more than 15 drinks per week. Additionally, subjects were excluded if they reported a history of head injury, neurological disorder, or use of psychotropic medications.
To qualify for study entry, MJ smokers had to have smoked MJ a minimum of 2500 times, used MJ at least five of the last seven days, tested positive for urinary cannabinoids, and met DSM-IV criteria for MJ abuse or dependence. MJ smokers were also required to abstain from smoking for at least 12 hours before their scan visit to ensure they were not acutely intoxicated at the time of testing, and were told they would have to give a urine sample upon arrival at the laboratory. To ensure compliance of 12-hour abstinence, subjects were led to believe that this sample would allow us to detect use of MJ within this time frame. Urine samples were tested for the presence of illicit substances using TRIAGE test kits (Triage® Drugs of Abuse Panel: Immediate Response Diagnostics, San Diego, CA). This procedure was required to (1) ensure subjects did not test positive for other drugs, (2) determine whether subjects had used MJ recently enough to have a positive urine screen, and (3) to encourage subjects to abstain from MJ from the previous evening; subjects were repeatedly reminded that they would be tested for MJ. A portion of the sample was sent to an outside laboratory for quantification of urinary cannabinoid concentration via gas chromatography–mass spectrometry (GC–MS). Prior to participation, study procedures were explained, and subjects were required to read and sign an informed consent form approved by the McLean Hospital Institutional Review Board, which described the procedures and voluntary nature of the study.
To assess the potential impact of age of onset of MJ use on inhibitory function, MJ smokers were divided into early onset (regular MJ use prior to age 16; n=9) and late onset (regular MJ use at age 16 or older; n=14) groups. To assess differences in MJ use between early and late onset smokers, frequency (smokes per week), magnitude (grams of MJ used per week), and mode of use were calculated using a time line follow back procedure. Lifetime use was also determined using the SCID-P.
Study Design
Subjects completed the MSIT while undergoing fMRI, described previously [4–5]. Briefly, subjects were presented with sets of three numbers (1, 2, 3, or 0) for 1.75 seconds with a prerelease of 0.5 seconds, for a stimulus presentation of 1.25 seconds with an interstimulus interval of 0.5 seconds, yielding a total run time of 6 minutes and 36 seconds. One number was always different from the other two (distractor) numbers, and subjects were instructed to report the identity of the number that differed from the distractors using a button box. During control trials, distractor numbers were always zeros and the target number was always presented in a matching position to the corresponding button on the button box (i.e. 100, 020, 003). During the interference condition, distracters were numbers other than zero and the position of the target number was never the same as its identity (i.e. 211, 232, 331, etc.). The entire task was comprised of four blocks of control trials alternating with four blocks of interference trials. Each block consisted of 24 presentations of number sets with a total of 192 number sets presented. Stimuli were generated from a laptop computer running E-Prime software and presented via a high-resolution, rear-projection system onto a translucent screen located at the rear of the scanner (Resonance Technology, Inc.) and viewed through a mirror mounted on the head coil. Once inside the magnet, subjects were given a practice session of the task prior to scanning to familiarize them with the task and button box. Performance on the task was quantified by 1) percent accuracy on each of the task conditions; 2) reaction time for each response; and 3) errors of omission (no response given) and commission (incorrect response) per task condition.
Imaging Methods
Imaging was performed on a Siemens Trio whole body 3T MRI scanner (Siemens Corporation, Erlangen, Germany) using a quadrature RF head coil; 40 contiguous coronal slices were acquired from each subject, providing whole brain coverage (5 mm, 0 mm skip), and images were collected every 3 seconds using a single shot, gradient pulse echo sequence (TR=3000 ms; TE =30 ms, flip angle =90, with a 20 cm field of view and a 64 × 64 acquisition matrix; in plane resolution 3.125 × 3.125 × 3.125 mm). A total of 132 images per slice were collected.
Image Processing and Analysis
FMRI images were analyzed using SPM8 (Wellcome Trust Center for Neuroimaging, UK). Blood oxygen level dependent (BOLD) fMRI data were corrected for motion, normalized to Montreal Neurological Institute (MNI) stereotactic space, and spatially smoothed using an isotropic Gaussian kernel 6mm full width at half maximum (FWHM). Statistical parametric images were calculated individually for each subject and each task, using a general linear model that accounted for task-related changes, with each condition modeled as a block design with a boxcar waveform. At the first level, three regressors were fit to the data, including the baseline fixation condition, the control condition, and the interference condition. The control and interference conditions each included four active blocks each comprised of 42 second stimulation periods. Activation was averaged across these blocks, and no attempt was made to adjust for individual item performances. A direct contrast between the fixation versus the control condition was calculated for each subject. These contrast images were subsequently entered into second level model, subjected to a voxel-wise t-tests to assess statistical significance using 1-sample t-tests. The non-MJ smoking HCs and the chronic, heavy MJ smokers were compared using between group t-tests. Chronic MJ smokers were further divided into early onset MJ and late onset MJ groups for comparison. Because our previous work revealed specific differences between HCs and MJ smokers within the anterior cingulate cortex (ACC), we applied an ACC region of interest (ROI) mask using the Wake Forest University Pickatlas utility [16] to restrict analyses to this area. Voxel-wise comparisons restricted to this ROI were evaluated at p < .005 (uncorrected), k ≥ 15 contiguous voxels. In addition, only clusters that exceeded a false discovery rate (FDR) correction of p < .05 are included in Table 1.
Table 1.
Demographic and Behavioral Data
| Variable | Healthy Controls |
MJ Smokers |
P (2-tailed) |
Early MJ Onset (before age 16) |
Late MJ Onset (Age 16 or after) |
P (2-tailed) |
|---|---|---|---|---|---|---|
| N | 16 (7M, 9F) | 23(16M, 7F) | - | 9 (6M, 3F) | 14(10M, 4F) | - |
| Handedness | 16(15R, 1L) | 23 (22R, 1L) | - | 9 (9R, 0L) | 14(13R, 1L) | - |
| Age | 22.75±2.82 | 22.43±5.29 | NS | 21.44±3.57 | 23.07±6.20 | NS |
| Full Scale IQ (FSIQ) | 124.25±8.13 | 119.22±14.23 | NS | 116.89±13.81 | 120.64±14.90 | NS |
| Verbal IQ (VIQ) | 126.88±6.01 | 120.09±17.83 | NS | 118.67±16.76 | 121.00±19.24 | NS |
| Performance IQ (PIQ) | 116.56±11.01 | 114.50±9.42 | NS | 112.22±8.48 | 116.08±10.04 | NS |
| MJ-related Variables |
P (2-tailed) |
P (2-tailed) |
||||
| THC Concentration | - | 611.45±876.78 | - | 405.67±318.36 | 743.74±1091.07 | NS |
| Age of MJ Onset | - | 16.61±2.37 | - | 14.56±0.53 | 17.93±2.13 | <0.001 |
| Smokes per Week | - | 15.72±7.35 | - | 16.05±8.02 | 15.51±7.18 | NS |
| Grams per week | - | 7.27±5.51 | - | 8.23±5.63 | 6.66±5.56 | NS |
| Duration of Use | - | 5.83±4.09 | - | 6.89±3.48 | 5.14±4.42 | NS |
|
Multisource Interference Task (MSIT): Control Condition |
P (2-tailed) |
P (2-tailed) |
||||
| Percent Accuracy | 97.92±3.29 | 98.23±2.44 | NS | 98.61±2.02 | 97.99±2.72 | NS |
| Reaction Time (msec) | 633.85±97.61 | 608.91 ±63.38 | NS | 593.31 ±51.90 | 618.95±69.73 | NS |
| # Commission Errors | 0.13±0.34 | 0.52±1.16 | NS | 0.33±0.71 | 0.64±1.39 | NS |
| # Omission Errors | 1.88±3.18 | 1.17±1.34 | NS | 1.00±1.32 | 1.29±1.32 | NS |
|
Multisource Interference Task (MSIT): Interference Condition |
P (2-tailed) |
P (2-tailed) |
||||
| Percent Accuracy | 86.26±14.13 | 85.55±17.13 | NS | 87.73±11.90 | 84.15±20.10 | NS |
| Reaction Time (msec) | 884.19±63.62 | 866.62±82.03 | NS | 839.10±77.44 | 884.31 ±82.68 | NS |
| # Commission Errors | 3.75±3.73 | 4.35±4.17 | NS | 5.11±4.11 | 3.86±4.29 | NS |
| # Omission Errors | 9.44±11.90 | 9.04±13.53 | NS | 6.56±7.70 | 10.64±16.32 | NS |
Results
Behavioral Data
As noted in Table 1, subjects were well matched, and did not differ with regard to age or IQ measures derived from the four-factor WASI. Performance data from both the Control and Interference conditions of the MSIT suggest that subjects were actively focused and engaged, as total percent accuracy for both conditions far exceeded chance levels. Overall, no significant differences were detected between HCs and MJ smokers on the control or interference conditions of the task, although MJ smokers tended to have faster reaction times and higher errors of commission relative to control subjects. Within the MJ group, early onset smokers had faster reaction times and higher errors of commission relative to their later onset smoking counterparts, although this did not reach statistical significance.
fMRI Data
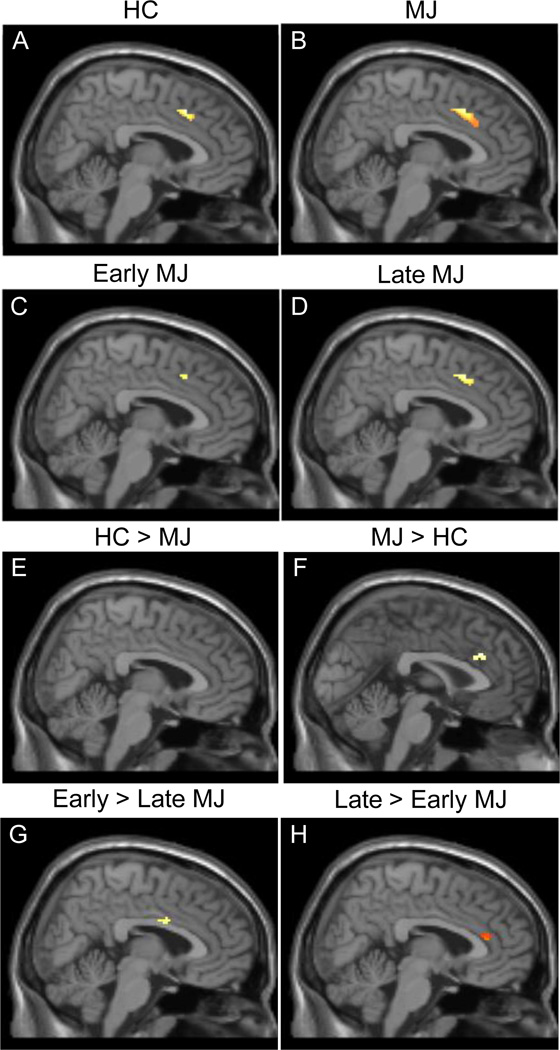
Despite similar task performance, results from the single sample analyses demonstrated that within the ACC, HCs demonstrated a smaller, more focal region of activation during the Interference condition minus the Control condition contrast relative to MJ smokers (Figures 1A and 1B; Table 2), with HCs activating a total of 163 voxels and MJ smokers activating 458 voxels. For the same contrast, early onset MJ smokers activated a total of 153 voxels while late onset MJ smokers activated 196 voxels (Figure 1C and 1D; Table 2).
Figure 1.
Early onset MJ smokers activated a more focal region of ACC during the MSIT, and appeared more similar to the single sample analysis of healthy controls relative to the late onset smokers, who demonstrated a pattern more similar to MJ smokers overall
Table 2.
Local Maxima for Group Comparisons with Anterior and Middle Cingulate Gyrus Regional of Interest (ROI).
|
Comparison Group Region |
Cluster Size (Voxels) |
Cluster p Uncorrected |
X | y | z | SPM {t} | Voxel p Uncorrected |
|---|---|---|---|---|---|---|---|
| 1-sample t-tests against null | |||||||
| Healthy Controls | |||||||
| Mid Right Cingulum (BA32) | 163 | 0.002 | 8 | 16 | 44 | 5.27 | <0.001 |
| Mid Left Cingulum | −10 | 10 | 44 | 4.20 | <0.001 | ||
| Mid Right Cingulum (BA32) | 12 | 6 | 44 | 3.05 | 0.002 | ||
| All MJ Smokers | |||||||
| Mid Right Cingulum | 458 | <0.001 | 8 | 14 | 44 | 5.08 | <0.001 |
| Mid Left Cingulum | −10 | 12 | 44 | 4.81 | <0.001 | ||
| Mid Left Cingulum | −10 | 18 | 38 | 3.95 | <0.001 | ||
| Early MJ Smokers | |||||||
| Mid Left Cingulum | 153 | 0.002 | −10 | 12 | 44 | 3.90 | <0.001 |
| Mid Right Cingulum (BA32) | 8 | 16 | 44 | 3.73 | <0.001 | ||
| Mid Right Cingulum (BA32) | 14 | 12 | 38 | 3.38 | <0.001 | ||
| Late MJ Smokers | |||||||
| Mid Right Cingulum | 196 | 0.001 | 10 | 14 | 44 | 4.18 | <0.001 |
| Mid Left Cingulum | −10 | 12 | 44 | 3.42 | <0.001 | ||
| Mid Left Cingulum | −6 | 20 | 38 | 3.26 | 0.001 | ||
| Between-group Comparisons | |||||||
| HC > All Smokers | |||||||
| None | - | - | - | - | - | ||
| All MJ Smokers > HC | |||||||
| Mid Left Cingulum (BA9) | 31 | 0.007 | −4 | 30 | 34 | 3.49 | 0.001 |
| Early > Late MJ Smokers | |||||||
| Mid Right Cingulum | 29 | 0.035 | 6 | 4 | 30 | 2.65 | 0.006 |
| Late > Early MJ Smokers | |||||||
| Mid Right Cingulum | 84 | 0.049 | 12 | 36 | 12 | 3.69 | 0.003 |
Between-group contrasts are illustrated in Figure 1, and Table 2 presents voxel locations and data for local maxima. While no significant activation was detected within the ROI for the HC > MJ smokers contrast (see Fig 1E), significant activation was detected for the MJ smokers > HC contrast, specifically within the anterior right cingulum (see Fig 1F). Interestingly, contrast analyses for early > late MJ smokers revealed an area of increased activation within the mid right cingulum (see Fig 1G), while the contrast of late > early MJ smokers revealed areas within a far more anterior portion of the mid left cingulum (see Fig 1H) region.
Discussion
As hypothesized, significant differences in activation patterns were detected between chronic, heavy MJ smokers and HCs for the interference minus control contrast of the MSIT, with MJ smokers activating a larger number of voxels during the completion of the task despite no significant performance differences between the groups. Further, early onset MJ smokers appeared to activate the mid right cingulum during the MSIT when directly compared to late onset smokers, while late onset MJ smokers demonstrated activation in a more anterior region of the mid left cingulum. Within-group analyses suggest that during the task, early onset smokers activate a smaller, more focal region of mid ACC relative to late onset MJ smokers, who demonstrate an increased number of voxels in a more anterior area of the region.
Findings from this study are consistent with previous reports of dorsal anterior cingulate cortex (dACC) activation in control subjects during the interference condition of the MSIT [4–5], and studies of MJ smokers reporting alterations in ACC activation relative to controls during tasks requiring higher cognitive processing [8,13]. Studies have also reported increased activation during Go/No Go tasks in chronic MJ smokers relative to controls despite similar task performance, and that earlier age of onset and higher lifetime use were associated with reduced inhibitory response [23]; significant relationships have also been detected between age of onset of MJ use and reduced inhibitory function [14] and conflict-related processes [1], suggesting that earlier regular MJ use is related to poorer inhibitory control. To our knowledge, however, this is the first study to directly compare early to late onset MJ smokers during an inhibitory task resulting in a striking pattern of differences. Early onset MJ smokers activated a more focal region of ACC during the task, and in fact appeared more similar to the single sample analysis of healthy controls relative to late onset smokers, who demonstrated a pattern more similar to MJ smokers overall. Despite this finding, which may suggest increased neural efficiency during the task in early onset MJ smokers, these subjects also responded more quickly and made more errors of commission than later onset smokers, suggesting difficulty with inhibition of inappropriate responses. While this difference did not reach statistical significance, it is consistent with previous reports of poorer inhibitory function in early onset MJ smokers relative to those who begin smoking later [2,10–11,18]
While intriguing,results should be interpreted in light of several limitations. The sample acquired was moderate in size, with 23 MJ smokers and 16 control subjects, which may limit generalizability of study findings. Additionally, the MJ-smoking sample was comprised of individuals who, despite heavy, frequent MJ use, met diagnostic criteria for MJ abuse but not dependence. Marijuana smokers willingly and openly reported their level of use, (highly correlated with their urinary cannabinoid levels assessed using GC-MS, adding credibility to their reporting), yet did not report significant negative impact of their smoking on social, physical, educational or occupational function, and had no significant tolerance or withdrawal symptoms. Study findings, therefore, may be limited to individuals who are chronic marijuana smokers but who do not endorse the negative effects of marijuana use, and to those who do not meet for dependence, despite frequent, heavy use. Finally, although we required MJ abstinence for a minimum of 12 hours, we cannot be certain that subjects fulfilled this requirement. All smokers reported a minimum of 12–16 hours abstinence and expected investigators to be able to tell if they had used the drug since the previous evening, Further, subjects were able complete the tasks, as well as a neurocognitive assessment, and no subject endorsed clinical ratings suggestive of intoxication or withdrawal from MJ. One strength of the current study is the naturalistic design, which assesses marijuana smokers who are not asked to complete an extended period of abstinence. Study findings are therefore more likely to be reflective of chronic marijuana smokers in everyday life and not those constrained by laboratory-based situations. Future studies should include longitudinal, repeated assessments of brain activation, as the current study used a cross sectional design which although powerful, is strengthened by repeated assessments over time.
Taken together, data from the current investigation suggests greater activation during an inhibitory task in chronic MJ smokers relative to healthy controls, and a pattern of greater midcingulate brain activation in early vs late onset MJ smokers. Interestingly, despite no significant between group differences in performance, early onset MJ smokers perform more quickly yet make more commission errors relative to late onset MJ smokers, suggesting difficulty with inhibition of inappropriate responses. This finding, in conjunction with the smaller, more focal pattern of midcingulate activation in early vs late MJ onset smokers is intriguing, as it may suggest a potential neural compensation for early exposure to MJ during a period of neurodevelopmental vulnerability. One possible interpretation is that early onset smokers, who are repeatedly exposed to MJ prior to age 16, a critical period of neurodevelopment, adapt to having the MJ ‘on board’ and experience a neural adaptation early on. Since the brain is still developing, with both large scale organization and fine tuning of gray and white matter distribution, brain response is similar to those who do not smoke MJ; their brains are simply used to the MJ and respond accordingly. Conversely, chronic, heavy exposure after age 16, when the brain is more fully developed, appears to result in a different pattern of neural response, perhaps attributable to the well-established finding that critical connections within the frontal cortex are more well-established at older ages, and exposure to MJ appears to disrupt the normal neural response. Findings suggest a potential neural adaptation for early exposure to MJ during a period of neurodevelopmental vulnerability despite increased difficulty in executing cognitive control. Further investigation is warranted, as early exposure to MJ may result in reorganization of critical brain regions.
Research Highlights.
Despite similar MSIT performance, MJ users had significantly different activation than controls
During the MSIT, MJ smokers had greater activation within cingulate cortex than controls
Early onset smokers had more focal activation but made more errors relative to later onset smokers
Findings may suggest neural compensation in early onset MJ smokers despite inhibitory difficulty
Acknowledgments
This project was supported by the National Institute of Drug Abuse (NIDA) 5 R21 DA021241 awarded to Dr. Gruber.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology (Berl.) 2010;2012:613–624. doi: 10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- 2.Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Dauman J. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 3.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 4.Bush G, Shin LM. The mulit-source interference task: an fMRI task that reliability activates the cingulo-frontal-parietal cognitive/attention network. Nat. Protoc. 2006;1:308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- 5.Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The multi-source interference task: Validation study with fMRI in individual subjects. Mol. Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- 6.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends Cogn. Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schiling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 8.Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 9.First MB, Spitzer RL, Gibbon M, Williams BW. Structured clinical interview for axis I DSM IV disorder, patient edition (SCID-I/P), Version 2.0. Biometric Research Department, NY State Psychiatric Institute; New York: 1994. [Google Scholar]
- 10.Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Hungerman F, Laranjeira RR, Bressnan RA, Lacerda ALT. Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry. 2011;198:442–447. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- 11.Gruber SA, Sagar KA, Dahlgren MK, Racine MT, Lukas SE. Age of onset of marijuana use and executive function. Psych. Add. Behav. 2011 doi: 10.1037/a0026269. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber SA, Silveri MM, Dahlgren MK, Yugelun-Todd DA. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp. Clin. Psychopharmacol. 2011;19:231–242. doi: 10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruber SA, Yugelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: A Pilot Investigation. Brain Res. Cogn. Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulated cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92:559–565. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maldijan JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 17.McHale S, Hunt N. Executive function deficits in short-term abstinent cannabis users. Human Psychopharmacolog. 2008;23:409–415. doi: 10.1002/hup.941. [DOI] [PubMed] [Google Scholar]
- 18.Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: What is the nature of the association,? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 19.Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict. Biol. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- 20.Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- 21.Solowij N, Stephens RS, Roffman RA, Babor T, Kaden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 22.Substance Abuse and Mental Health Services Administration, Office of Applied Studies, Results from the 2007 National Survey on Drug Use and Health. National Findings (NSDUH Series H-34, DHHS Publication No. SMA 08-4343); Rockville: 2008. [Google Scholar]
- 23.Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl.) 2007;94:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]