Abstract
Difficulty monitoring and inhibiting impulsive behaviors has been reported in marijuana (MJ) smokers; neuroimaging studies, which examined frontal systems in chronic MJ smokers, have reported alterations during inhibitory tasks. Diffusion tensor imaging (DTI) provides a quantitative estimate of white matter integrity at the microstructural level. We applied DTI, clinical ratings, and impulsivity measures to explore the hypotheses that chronic, heavy MJ smokers would demonstrate alterations in white matter microstructure and a different association between white matter measures and impulsivity relative to nonsmoking control subjects (NS). Fractional anisotropy (FA), a measure of directional coherence, and trace, a measure of overall diffusivity, were calculated for 6 locations including bilateral frontal regions in 15 chronic MJ smokers and 15 NS. Subjects completed clinical rating scales, including the Barratt Impulsivity Scale (BIS). Analyses revealed significant reductions in left frontal FA in MJ smokers relative to NS and significantly higher levels of trace in the right genu. MJ smokers also had significantly higher BIS total and motor subscale scores relative to NS, which were positively correlated with left frontal FA values. Finally, age of onset of MJ use was positively correlated with frontal FA values and inversely related to trace. These data represent the first report of significant alterations in frontal white matter tracts associated with measures of impulsivity in chronic MJ smokers. Early MJ use may result in reduced FA and increased diffusivity, which may be associated with increased impulsivity, and ultimately contribute to the initiation of MJ use or the inability to discontinue use.
Keywords: diffusion tensor imaging, marijuana, impulsivity, white matter, age of onset
Marijuana (MJ) remains the most widely used illicit drug in the United States, with an estimated 14.4 million past month users; 72.8% of current illicit drug users report using MJ, and 53.3% report that it is the only drug they use (SAMHSA, 2008). Moreover, numerous investigations have reported reductions in cognitive functions, specifically behavioral response inhibition, a process mediated by the frontal cortex, in individuals who abuse MJ (Bolla, Rothman, & Cadet, 1999; Gruber & Yurgelun-Todd, 2005; Pope & Yurgelun-Todd, 1996; Ramaekers et al., 2006; Vadhan et al., 2007), making this a critical area of investigation. In a recent neuroimaging study by Gruber and Yurgelun-Todd (2005), processing deficits were demonstrated during frontally mediated cognitive tasks in MJ smokers that resulted in altered response inhibition when compared to nonsmoking controls. These findings are consistent with studies of neurocognitive function, which have reported alterations in frontal/executive function in MJ smokers (Pope & Yurgelun-Todd, 1996; Solowij et al., 2002; Verdejo-Garcia, Bechara, Recknor, & Perez-Garcia, 2006; Whitlow et al., 2004). Pope and Yurgelun-Todd (1996) reported lower performance scores in MJ smokers relative to control subjects on tests designed to measure mental flexibility and the ability to shift attention, processes mediated by frontal cortical regions. Solowij and colleagues (2002) reported significantly worse performance in heavy MJ smokers relative to both lighter smokers and nonsmoking control subjects on a battery of measures including assessments of attention, memory, and executive function. Taken together, these findings underscore the likelihood of alterations within the frontal system of these subjects.
Investigations of the cognitive effects of MJ following a brief abstinence period also have reported that heavy MJ use is associated with deficits in cognitive tasks mediated by the frontal system (Bolla, Brown, Eldreth, Tate, & Cadet, 2002; Fletcher et al., 1996; McHale & Hunt, 2008; Pope & Yurgelun-Todd, 1996). Bolla et al. (2002) reported persistent, dose-related cognitive decrements on a battery of neurocognitive tasks, including frontal/executive measures in heavy MJ smokers despite a full 28-day abstinence period. In a study of chronic MJ smokers abstinent for 24 hr, McHale and Hunt (2008) reported deficits in executive function in the smoking cohort as compared to both a nonsmoking and a former smoking cohort. Results from neuroimaging studies also support frontal/executive dysfunction in adult MJ smokers. Using positron emission tomography (PET) techniques, Eldreth, Matochik, Cadet, and Bolla (2004) examined abstinent MJ smokers between the ages of 21 and 35 during the performance of a modified Stroop test and reported hypoactivity of the anterior cingulate cortex (ACC) and lateral prefrontal cortex (LPFC) and increased hippocampal activity relative to control subjects, despite a lack of performance differences between the groups (Eldreth et al., 2004), suggesting that MJ smokers may recruit alternative networks as a compensatory strategy for the completion of the task. Bolla, Eldreth, Matochik, and Cadet (2005) administered the Iowa Gambling Task (Bechara et al., 1994) to abstinent MJ smokers during PET scanning and reported both poorer task performance and reduced activation in dorsolateral prefrontal and orbitofrontal regions as compared to control subjects. Overall, results from these investigations are in agreement with those from other studies (Gruber & Yurgelun-Todd, 2005; Kanayama, Rogowska, Pope, Gruber, & Yurgelun-Todd, 2004; Pillay et al., 2004), which provide evidence for the view that heavy MJ smoking individuals demonstrate alterations in frontal function, most notably during tasks that require executive control, inhibition, and decision making.
A complex and multidimensional construct, impulsivity has been well-documented in individuals with substance use problems (Brady et al., 1998; Heil et al., 2006; Vitaro et al., 1998). Higher levels of both behavioral impulsivity and risk taking have previously been reported in substance abusing individuals (Gruber et al., 2005; Lejuez et al., 2002, 2003), and an impulsive personality style has previously been identified as both a risk factor and predictor of substance abuse and dependence (Guy et al., 1994). Although few studies have focused on the specific relationship between impulsivity and MJ use, Vangsness et al. (2005) examined the role of positive and negative MJ related expectancies in MJ users and reported that individuals with higher levels of impulsivity held fewer negative expectancies related to MJ, and in turn, used MJ more often than those with lower levels of impulsivity. In a recent study of delay discounting, widely considered a behavioral index of impulsivity, Johnson and colleagues (2010) reported that MJ dependent subjects demonstrated a trend toward increased delay discounting relative to controls and former MJ smokers. Further, the dependent MJ users scored significantly higher than controls on the impulsiveness subscale of the Eyesenk Impulsiveness–Venturesomeness–Empathy (IVE) questionnaire (Eysenck & Eysenck, 1978). Taken together with the previously described findings from neuroimaging studies of MJ smokers, which report alterations in response inhibition as well as decision making, impulsivity may in fact reflect a stable trait in those who smoke MJ relative to those who do not.
Diffusion tensor imaging (DTI) has evolved into a reliable technique for acquiring quantitative information regarding white matter tract integrity. Two DTI measures that have been shown to have physiological relevance include the apparent diffusion coefficient (ADC) or “trace,” which averages diffusion over multiple directions and fractional anisotropy (FA), which measures the degree of directionality and coherence of the fibers. FA has been used as an index of white matter tracts, or coherence of a fiber bundle, as the high degree of directionality of white matter tissue reflects axonal direction, while trace, a measure of the magnitude of molecular motion, has been used successfully to identify ischemia. Thus, the visualization of anisotropic diffusion within the brain based on the application of DTI methods allows for noninvasive examination of white matter microstructure in vivo in chronic heavy MJ users.
To date, a limited number of investigations have utilized DTI in studies of substance abusers, and the number of studies that have focused on heavy MJ smoking adults is even more limited. In an investigation conducted at 3T in which BOLD fMRI data and DTI measures were acquired in chronic, heavy MJ smoking adults and nonsmoking healthy controls, we found that MJ smokers demonstrated a trend toward lower FA and higher trace values relative to the controls in the genu and splenium of the corpus callosum and bilateral anterior cingulate white matter regions (Gruber & Yurgelun-Todd, 2005). These findings suggested that white matter fiber tracts may be altered in MJ smokers and may affect functional activity and behavior associated with these regions. In a more recent investigation that utilized tract based spatial statistics (TBSS) to examine DTI data from young adult heavy MJ smokers and control subjects, Arnone et al. (2008) reported significantly increased mean diffusivity (i.e., trace) in the prefrontal area of the corpus callosum in the smokers relative to the control subjects. Further, a trend toward a positive correlation between diffusivity levels and length of MJ use was noted, suggesting the possibility of a cumulative effect of MJ on white matter integrity over time. The authors suggested that the finding of increased diffusivity in the prefrontal white matter bundles in the MJ smokers could be an expression of the micropathology affecting this region in smokers relative to control subjects. These few findings reflect the paucity of DTI studies conducted to date aimed at identifying the consequence of heavy MJ use on white matter fiber tract integrity in adults and suggest further investigation is warranted. In the current study, we utilized DTI and administered clinical rating scales and measures of impulsivity to evaluate the potential relationship between measures of white matter integrity and behavioral changes in adult MJ smokers compared to non-MJ smoking control subjects, which to our knowledge has not previously been done. We hypothesized that MJ smokers would demonstrate alterations in white matter microstructure relative to nonsmoking controls, have higher levels of reported impulsivity, and exhibit a different pattern of association between white matter measures and measures of impulsivity.
Method
Fifteen adult chronic, heavy MJ smokers who had smoked at least 3,000 joints in their lifetime, smoked at least four of the last 7 days and tested positive for urinary cannabinoids and 15 non-MJ smoking control subjects who were age, sex, and education matched were included in the study (see Table 1). Subjects were recruited from the greater Boston, MA area, and all subjects received the Structured Clinical Interview for DSM–IV (SCID–P; First et al., 1994) to ensure that no Axis I pathology was present (except for MJ abuse, a requirement for the MJ smoking group) and had no history of head trauma or medical condition. No subjects met diagnostic criteria for current or previous alcohol abuse or dependence; subjects were excluded if they reported more than 10 lifetime episodes of using any category of illicit drugs (including sedative-hypnotics, stimulants, cocaine, opioids, hallucinogens, and Methylenedioxymethamphetamine (MDMA)) except for MJ in the case of the smokers. Subjects were required to provide a urine sample to be tested for MJ, amphetamines, opioids, phencyclidine, barbiturates, benzodiazepines, and cocaine (TRIAGE, Biosite Diagnostics, San Diego, CA) just prior to imaging to ensure that subjects did not test positive for other substances of abuse, to determine whether subjects had used MJ recently enough to have a positive urine screen, and to encourage subjects, as requested, to abstain from MJ from the previous evening until arriving at the laboratory so that subjects were not acutely intoxicated at the time of the visit. Subjects were repeatedly reminded that they would be tested for MJ use on their arrival at the lab, and were led to believe that we would be able to determine if they had smoked within the previous 12 hr. An aliquot of the sample was sent to an outside laboratory for quantification of urinary cannabinoid concentration via gas chromatography mass spectrometry (Quest Diagnostics, Cambridge, MA). All study subjects completed clinical rating scales, which included the Addiction Severity Index (ASI; McLellan et al., 1980), Positive and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988), Beck Depression Inventory (BDI; Beck, 1987), Hamilton Anxiety Scale (HAM–A; Hamilton, 1959), and the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1971) prior to their scanning sessions to evaluate their clinical state at time of testing. A subgroup of subjects also completed the Barratt Impulsivity Scale (BIS; Patton, Stanford, & Barratt, 1995), an instrument that measures impulsivity, and provides subscales covering the domains of attention, motor, and planning as well as a total impulsivity score. Prior to their participation in any study related activity, study procedures were explained and all subjects were required to read and sign an informed consent form, which described in detail the scanning procedures and had been approved by the McLean Hospital Institutional Review Board.
Table 1.
Subject Demographics
| Variable | Normal controls (n = 15) |
MJ smokers (n = 15) |
p value |
|---|---|---|---|
| Age | 25.2 ± 8.4 | 25 ± 8.7 | .81 |
| Education | 15.5 ± 2.9 | 13.8 ± 2.1 | .32 |
| Sex (male/female) | 14/1 | 14/1 | — |
| Handedness (right/left) | 13/2 | 13/2 | — |
| Verbal IQ | 116.15 ± 12.9 | 113.33 ± 14.4 | .59 |
| Performance IQ | 121.71 ± 17.8 | 123.67 ± 19.5 | .78 |
| MJ smokers | |||
| Average age of onset (years) of use | — | 14.9 ± 2.5 | — |
| Average smokes per week (joints) | — | 25.5 ± 27.8 | — |
| Average duration (years) of use | — | 10.1 ± 9.7 | — |
| Average urinary THC concentration (ng/ml) | — | 505.8 ± 734.7 | — |
| Alcohol use (days/month) | 4.0 ± 4.5 | 9.6 ± 5.4 | .05 |
| Alcohol use to intoxication (days/month) | 2.3 ± 3.0 | 4.8 ± 4.1 | .07 |
Note. Scores are averages plus or minus standard deviations. MJ = marijuana.
Images were acquired using a 3.0T Siemens magnetic resonance scanner (Siemens Medical Solutions, Malvern, PA) and an eight channel phased array coil. Sagittal scout images were acquired for alignment and localization using a fast spin echo sequence (FSE) with the following imaging parameters: repetition time (TR) = 2 ms, echo time (TE) = 75 ms, field of view (FOV) = 240 mm, matrix size = 256 × 256, slice thickness = 5 mm, and flip angle = 90°. DTI data were acquired in the axial plane using a diffusion weighted standard single shot, double spin echo, echo planar protocol. Multiple-diffusion-weighted images were acquired using a single diffusion “b” weighting value of 1,000 s/mm2. MRI acquisition parameters were: TE/TR = 81ms/5 s; matrix = 128 × 128 on a 210 mm FOV; slice thickness = 5 mm with a gap of 0. The DTI six-direction gradient scheme used was a modification of the method described previously (Basser & Pierpaoli, 1998; directions [1, 0, 1], [−1, 0, 1], [0, 1, 1], [0, 1, −1], [1, 1, 0], [−1, 1, 0]). Eight averages were collected using an image magnitude averaging mode. Apparent diffusion coefficient tensor values were calculated using a pixel-wise least squares fit to log images.
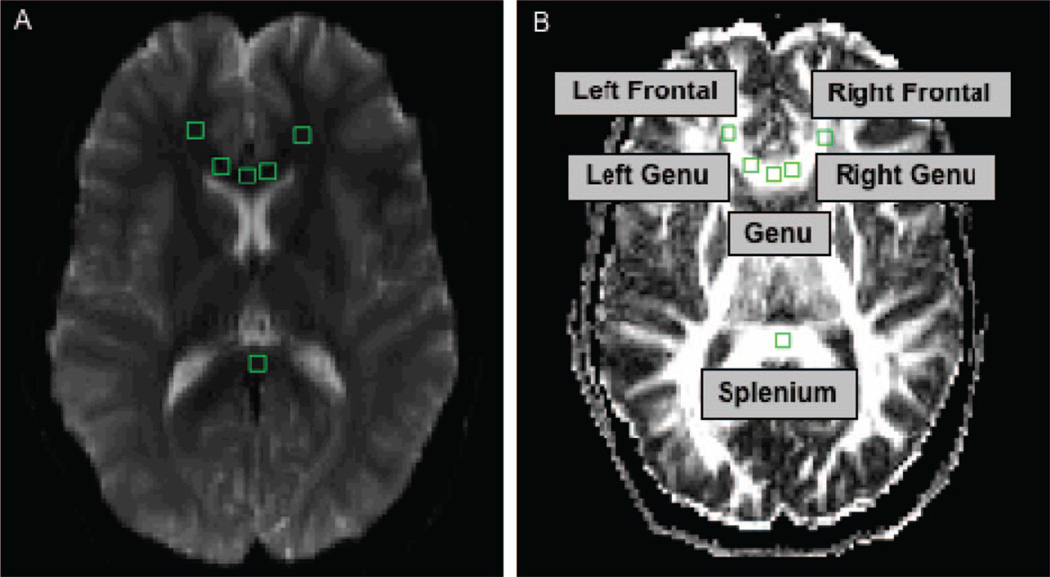
The FA measure of Basser and Pierpaoli (1996) and the trace (diffusivity) of the calculated tensor were values calculated in pixel-wise maps determined from axial images. Diffusion-weighed image data were converted into pixel-wise FA and diffusivity maps using an in house Interactive Data Language IDL-based fMRI processing program (fMRI Analysis Tool [FAT]; Maas, Frederick, & Renshaw, 1997). FA, a measure of intravoxel coherence, ranges from 0 (lowest FA) to 1 (highest FA) and represents a fractional measure of directional diffusion in white matter, which is influenced by fiber organization, myelination, and integrity. Trace, a measure of the total magnitude of water diffusion in three dimensions, was expressed as millimeters per second (mm/s). Using a region of interest (ROI) approach, 3 × 3 pixel regions were drawn by trained raters, with a total ROI voxel volume per region = .12 cm3 (or 121.5 mm3), calculated as 2.7 mm2 (in-plane resolution) × 5 mm (slice thickness) × 9 pixels. Drawing and placement of the ROIs was done by two individuals with high interrater reliability in voxel placement (intraclass coefficient = 0.96, p < .001) and who had considerable experience with DTI data analyses. Regions were drawn on a single slice of the echoplanar image in the genu of the corpus callosum, and two bilateral regions of the forward projecting arms of the white matter adjacent to the anterior cingulate cortex (see Figure 1). A 3 × 3 region also was drawn in the midline of the posterior corpus callosum (splenium). Image analysis was performed using callosal tract regions to avoid confounds of fiber tract crossing, as occurs in more lateral white matter regions (i.e., dorsolateral prefrontal cortex; Tuch, Reese, Wiegell, & Wedeen, 2003). ROIs were selected with reference to an anatomic atlas (Kretschmann et al., 1992), and placements were made on the basis of gyral boundaries and structural landmarks that were visible on the T1 weighted magnetic resonance images (Damasio et al., 1989).
Figure 1.
Diffusion tensor imaging regions of interest (ROI). Axial images illustrating the placement of .12-cm3 ROIs in left and right frontal regions, left and right forward-projecting arms of the genu, genu and splenium on the (A) echoplanar image and (B) corresponding ROI placements with labels on the fractional anisotropy map.
Results
Demographic and clinical variables for all subjects are included in Tables 1 and 2. The subject groups did not differ with regard to any demographic or clinical variable, and no subject tested positive on the urine toxicology screen for illicit substances, with the exception of MJ in the case of the smokers. MJ smokers had an average age of onset of 14.9 years, smoked an average of 25.5 joints per week and had a mean urinary cannabinoid concentration, normalized to their creatinine level, of 505.8 ng/ml on the day of scanning. One subject within each of the study groups endorsed using tobacco in an occasional fashion. Subjects did not differ on their past use of illicit substances, no subject in either group met diagnostic criteria for past abuse or dependence for any illicit substance (other than MJ in the case of the MJ smokers), and no subject in either group reported use of any illicit substance greater than 10 times in their lives. However, the chronic, heavy MJ smokers reported using alcohol more days per month (9.6 days) than the non-MJ smoking control subjects (4.0 days). This difference was statistically significant (p < .05), yet there were no significant differences between the groups for the number of days intoxicated in the past month (chronic heavy MJ smokers = 4.8 days/month; non-MJ smoking control subjects = 2.3 days/month), no subject in either group met diagnostic criteria for alcohol abuse or dependence, and analyses did not reveal any significant relationship between any of the DTI variables and alcohol use. With regard to clinical state, as seen in Table 2, no significant between-groups differences were detected for the BDI, PANAS, or POMS, or HAM–A, suggesting that the samples were equally matched for clinical state at the time of testing. No subject in either study group demonstrated clinically elevated scores on any of the clinical scales, and overall, scores for both groups suggested that the study samples were affectively stable at the time of testing. Given that one female subject was included in each sample, we completed analyses with and without these subjects. As all measures were unchanged with the female subjects excluded, we report data for the entire sample intact.
Table 2.
Clinical Measures
| Variable | Normal controls | MJ smokers | p value two-tailed |
|---|---|---|---|
| Beck Depression Index | |||
| Total score | 1.85 ± 3.4 | 3.08 ± 4.8 | .46 |
| Positive and Negative Affect Scale | |||
| Positive Item Score | 36.23 ± 4.2 | 34.00 ± 4.9 | .20 |
| Negative Item Score | 11.92 ± 2.8 | 13.37 ± 3.5 | .28 |
| Profile of Mood States | |||
| Vigor | 21.96 ± 3.3 | 20.93 ± 3.9 | .48 |
| Anger | 3.67 ± 5.0 | 6.14 ± 3.8 | .16 |
| Confusion | 6.67 ± 2.8 | 7.57 ± 2.3 | .38 |
| Tension | 6.29 ± 4.6 | 7.71 ± 2.3 | .32 |
| Fatigue | 3.63 ± 3.6 | 4.36 ± 2.4 | .54 |
| Depression | 2.79 ± 3.3 | 4.93 ± 3.5 | .12 |
| Total | 45.50 ± 17.9 | 51.64 ± 11.5 | .26 |
| Hamilton Anxiety Scale | |||
| Total score | 2.42 ± 3.3 | 3.00 ± 2.5 | .61 |
| Barratt Impulsivity Scale | |||
| Attention | 15.00 ± 3.5 | 17.90 ± 3.4 | .07 |
| Motor | 19.36 ± 5.1 | 24.60 ± 4.01 | .02 |
| Planning | 24.64 ± 5.1 | 27.30 ± 4.6 | .23 |
| Total | 59.00 ± 9.7 | 69.80 ± 9.6 | .02 |
Note. Scores are averages plus or minus standard deviations. MJ = marijuana.
As seen in Table 2, significant differences between the groups were demonstrated for measures from the BIS. In general, as compared to the non-MJ smoking control subjects, MJ smokers had higher BIS scores for all domains, which reached statistical significance for both the Motor subscore (p = .02) and total BIS score (p = .02).
DTI values for both groups are reported in Table 3 and include measurements of both FA and trace. As hypothesized, a significant difference in FA was detected between the groups in the left frontal region, F(29) = 4.43, p = .04, with chronic MJ smokers having reduced FA relative to the nonsmoking controls. Further, FA within the right genu approached statistical significance, with MJ smokers having reduced right genu values relative to the nonsmoking controls, F(29) = 3.20, p = .08. With regard to overall diffusivity or trace, MJ smokers demonstrated an increase in the right genu relative to nonsmoking controls, F(29) = 4.38, p = .04.
Table 3.
DTI Measures
| Variable | Region | Normal controls | MJ smokers | F(29) | p value |
|---|---|---|---|---|---|
| FA | Genu | 0.81 ± 0.07 | 0.82 ± 0.08 | 0.28 | .60 |
| Left | 0.85 ± 0.08 | 0.83 ± 0.10 | 0.19 | .67 | |
| Right | 0.94 ± 0.09 | 0.88 ± 0.11 | 3.20 | .08 | |
| Left Frontal | 0.50 ± 0.10 | 0.43 ± 0.07 | 4.43 | .04 | |
| Right Frontal | 0.50 ± 0.09 | 0.46 ± 0.08 | 1.73 | .20 | |
| Splenium | 0.92 ± 0.05 | 0.89 ± 0.06 | 2.78 | .11 | |
| Trace | Genu | 2.44 ± 0.22 | 2.51 ± 0.21 | 0.910 | .35 |
| Left | 2.55 ± 0.43 | 2.60 ± 0.26 | 0.146 | .71 | |
| Right | 2.40 ± 0.28 | 2.64 ± 0.35 | 4.387 | .04 | |
| Left Frontal | 2.46 ± 0.15 | 2.47 ± 0.12 | 0.028 | .87 | |
| Right Frontal | 2.49 ± 0.15 | 2.48 ± 0.12 | 0.145 | .71 | |
| Splenium | 2.08 ± 0.20 | 2.14 ± 0.15 | 0.830 | .37 |
Note. Scores are averages plus or minus standard deviations. DTI = diffusion tensor imaging; MJ = marijuana; FA = fractional anisotropy.
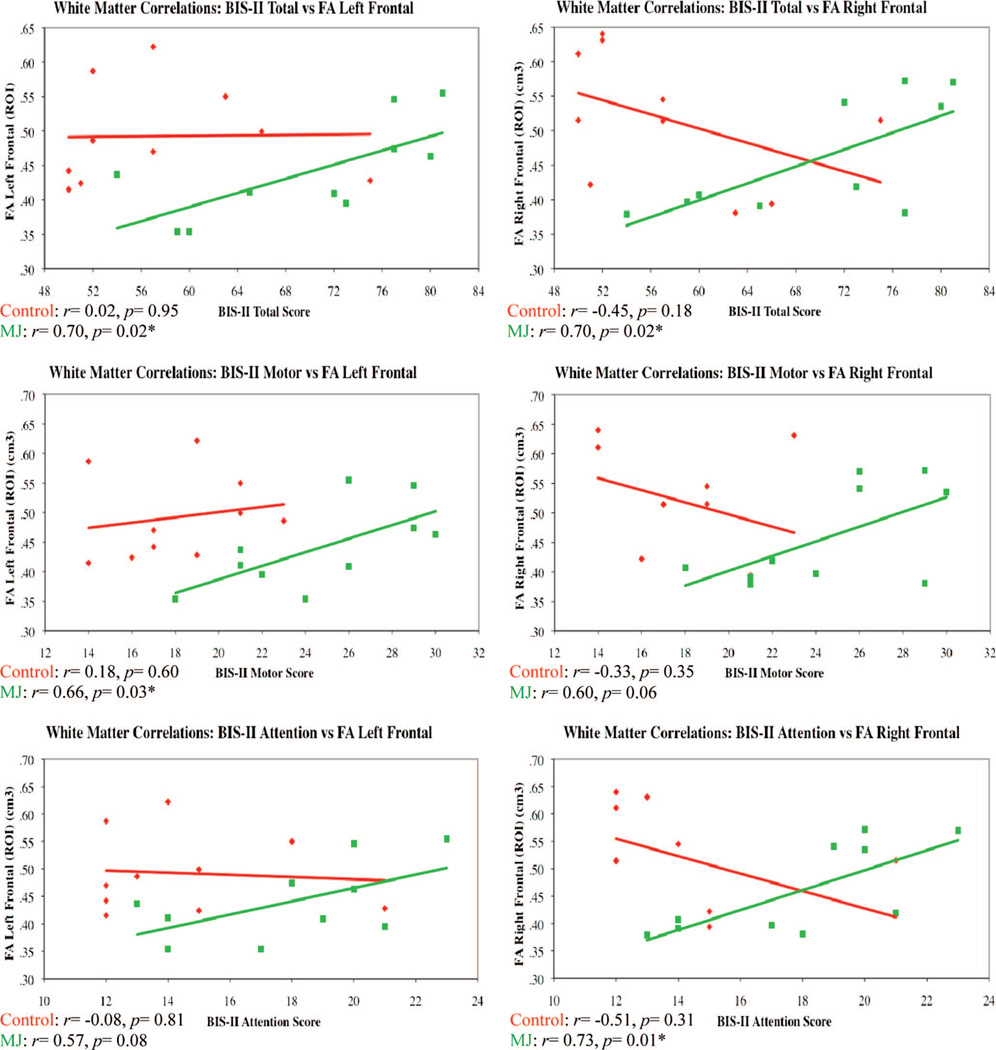
To examine the potential relationship between impulsivity and measures of white matter, we completed correlations for each of the BIS subscores and the measures of FA and trace for both subject groups. As illustrated in Figure 2, a significant positive relationship was detected for FA in the left frontal region and the BIS Total and Motor subscores for the MJ smoking subjects (BIS Total r = .70, p = .02; BIS Motor r = .66, p = .03) but not for the nonsmoking control subjects. Further, measures of FA in the right frontal region were also significantly associated with BIS Total and Attention subscores for the MJ smokers (BIS Total r = .70, p = .02; BIS Attention r = .73, p = .01), but not for the nonsmoking control subjects. Specifically, for both the left and right frontal regions of chronic MJ smokers, higher measures of FA were positively correlated with higher levels of impulsivity.
Figure 2.
BIS–II = Barratt Impulsivity Scale–II; FA = fractional anisotropy; ROI = region of interest. Correlation scatterplots for impulsivity and white matter in frontal regions.
To assess the potential impact of age of onset of first regular MJ use on measures of white matter, we completed correlation analyses on the MJ smoking subjects, who were asked to report the age of their first regular MJ use, which ranged from 11 to 20 years of age. As seen in Figure 3, within the left frontal region, age of onset of MJ use was significantly positively correlated with FA values (r = .43, p = .05) and inversely correlated with trace (r = −.52, p = .02). Similarly, within the right genu, age of onset was significantly positively correlated with FA values (r = .58, p = .01) and inversely correlated with trace (r = −.55, p = .02), indicating lower white matter and higher diffusivity in these regions. It is of note that duration of MJ use was inversely correlated with FA values (r = −.65, p = .01) and positively correlated with trace (r = .64, p = .01) within the right genu, suggesting a relationship between length of MJ use and white matter measures in this region.
Figure 3.
MJ = marijuana; FA = fractional anisotropy; ROI = region of interest. *p ≤ .05. Correlation scatterplots for age of MJ onset and white matter in frontal regions.
Discussion
As hypothesized, analyses of the DTI data revealed significant reductions in frontal FA, particularly on the left side, reflective of lower white matter fiber tract integrity in MJ smokers relative to healthy, nonsmoking control subjects. In addition, increased trace or calculated overall diffusivity was detected within the right genu of MJ smokers relative to nonsmoking controls. MJ smokers had significantly higher BIS scores, both for the Motor subscale and the total BIS score, indicating higher levels of impulsivity in MJ smokers compared to healthy nonsmoking controls, despite the fact that no significant between-groups differences were detected for any clinical mood rating. Furthermore, a significant relationship was detected between the BIS scores and FA measures for the MJ smokers but not for the control subjects, suggesting that the observed alterations in white matter are associated with levels of impulsivity in MJ smoking individuals.
Findings from this investigation are consistent with the previous studies of white matter measures in MJ smokers. As noted above, Arnone et al. (2008) utilized TBSS techniques in young adults and reported increased mean diffusivity (i.e., trace) in the prefrontal area of the corpus callosum in the MJ smokers relative to the control subjects and a trend toward a positive correlation between diffusivity levels and length of use. Ashtari et al., (2009) used both voxel-wise and tractography techniques to examine white matter in heavy MJ smoking adolescents and young adults and reported reduced FA and increased trace in fronto-temporal regions relative to non-MJ smoking control subjects. It is of note, however, that a subset of MJ smokers in this study also met criteria for alcohol abuse, which, although unlikely, may have impacted the findings. Bava and colleagues (2009) recently reported decreases in FA in adolescents who used both MJ and alcohol relative to control subjects in 10 regions, which included inferior frontal and temporal areas. The authors concluded that the fronto-parietal network in adolescents may be heavily impacted by the use of both MJ and alcohol, and that the between-groups differences detected may be a reflection of altered axonal and myelin maturation. It is of note, however, that subjects in the Bava et al. investigation were classified as those who used both alcohol and MJ or were nonusing control subjects; no pure MJ using group was included.
In a related DTI study, Jacobus et al. (2009) also used TBSS methods to examine adolescents who were classified as binge drinkers, binge drinkers who were also heavy MJ users, or control subjects. The authors reported significant between-groups differences in the binge drinkers as compared to the controls in several white matter regions. Adolescents who were both binge drinkers and MJ smokers showed a smaller difference in white matter measures as compared to the pure binge-drinking sample. In fact, increasing FA values were associated with more MJ use in the left superior longitudinal fasiculus and left superior corona radiata, raising the question of the potential effect of both substances on the developing brain. The authors hypothesize that MJ use may yield neuroprotective properties in reducing alcohol related oxidative stress or excitotoxic cell death, which has previously been reported in animal models of neuronal death (Crews & Nixon, 2009).
Although these investigations included adolescent subjects, and the current study is focused on adults, they lend support to the finding of white matter alterations in MJ smokers. Studies of other substance abusing populations also lend support to the finding of reduced FA in frontal regions that are associated with measures of impulsivity in the MJ smokers. An early study by Lim, Choi, Pomara, Wolkin, and Rotrosen (2002) reported reductions in inferior frontal FA in cocaine users relative to nonusing control subjects, consistent with the idea that cocaine dependence involves disruptions of orbitofrontal connectivity, critical for decision making. Moeller and colleagues (2005) reported reduced FA in both the genu and rostral body of the anterior corpus callosum in cocaine dependent subjects, as well as increased BIS scores and errors of impulsivity on a continuous performance task relative to controls. The authors concluded that reductions in white matter coherence were associated with impulsivity and consistent with prior theories regarding frontal cortical involvement in impaired inhibitory control in cocaine dependence. In a recent DTI study of methamphetamine (MA) abusers, Salo et al. (2009) examined FA and trace in the genu and splenium of the corpus callosum and also administered the Stroop color word test, a measure of cognitive control and inhibitory function. Compared to control subjects, MA abusers had lower levels of FA in the genu relative to the control subjects, which were also correlated with poorer performance on the interference condition of the task; no differences were detected within the splenium. These findings lend support to the growing hypothesis that substance abuse is related to white matter microstructural changes, which are associated with compromised ability to perform tasks requiring cognitive control and greater difficulty regulating impulse control.
Given the importance of frontal regions for the completion of tasks requiring cognitive control and inhibition, it is not surprising that the MJ smokers exhibited lower levels of white matter FA in this region. Numerous studies have reported that MJ smokers demonstrate alterations in frontal function, most notably during tasks that require executive control, inhibition, and decision making (Eldreth et al., 2004; Gruber & Yurgelun-Todd, 2005; Pillay et al., 2004; Tapert et al., 2007). Further, fibers that cross through the genu connect the left and right dorsolateral prefrontal cortex (DLPFC), which has strong interconnections to the ACC (Pandya & Seltzer, 1982; Park et al., 2008). Both the ACC and DLPFC are components of the cingulo-fronto-parietal cognitive attention network, which plays a critical role in executive control, attention, target and error detection, response selection, and inhibition as well as feedback-based decision making (Bush et al., 2008). The finding of increased diffusivity or trace within the right genu and a trend toward significantly lower FA in the MJ smokers relative to the controls in this region may be due to the fact that fibers within the genu are thinner than those in other regions, such as the splenium, and may therefore be more vulnerable to damage following long term exposure to MJ or other substances of abuse (Aboitiz, Rodriguez, Olivarez, & Zaidel, 1996).
The significant association between BIS scores and FA and trace white matter measures in the MJ smokers but not the healthy control subjects observed in the present study underscores the importance of understanding the structure of the BIS and the content for each of the subscales. The BIS provides scores for three subscale domains, attention, motor, and nonplanning as well as an overall total score. It appears that some of the items within each of the subscales are more indicative of what is commonly considered impulsivity, and that the subscale headings (i.e., Motor) may not accurately describe the behaviors assessed by that subscale. For example, within the Motor subscore, items such as “I do things without thinking” or “I act on impulse” are included. These items represent impulsive behaviors that are not primarily or solely motor in nature. Scores on the Motor subscale were found to be significantly different between the groups and may index what is commonly referred to as general impulsivity. Regardless of the categorization, MJ smokers produced significantly higher scores of impulsivity, which appear to be related to white matter, a relationship that was not detected in the control subjects.
The significant relationship detected between FA and age of onset of MJ use for both the left frontal and right genu may help to explain the significant differences in FA and trace between the MJ smokers and controls. During development, age appropriate myelination is represented by progressive increases in FA and decreases in trace (Morriss, Zimmerman, Bilaniuk, Hunter, & Haselgrove, 1999). These changes over time are thought to parallel the dynamic process of cognitive development, with notable improvements in executive function, memory and emotional processing. Although MJ may be neuroprotective and prevent oligodendrocyte death (Molina-Holgado, Molina-Holgado, Guaza, & Rothwell, 2002), early or chronic exposure to MJ may cause alterations of CB1 receptor function or myelination disturbances during neurodevelopment. Cannabinoids have been demonstrated to bind to myelin basic protein with high affinity (Nye, Voglmaier, Martenson, & Snyder, 1988), and exposure to MJ during developmentally vulnerable periods may result in lasting morphologic alterations, as has previously been shown in animal (Cha, White, Kuh, Wilson, & Swartzwelder, 2006; Schneider & Koch, 2003) and human studies (Wilson et al., 2000; Yucel et al., 2008). In a study that examined whole brain morphometry and MJ use, Wilson et al. (2000) reported larger percentage white matter brain volumes (adjusted for whole brain volume) in subjects who began smoking prior to age 17 relative to those who had a later onset of use. In a more recent morphometric study, which included gyrification indexes and measures of cortical thickness, Mata and colleagues (2010) reported significantly flatter sulci and decreased sulcal cortical thickness in the frontal lobes of chronic MJ smokers compared to controls. In addition, measures of cortical thickness in the MJ smokers did not show the expected dependency on age, and instead, smokers appeared to have flatter, thinner sulci at earlier ages relative to the control subjects. The authors concluded that MJ use during adolescence may result in premature alterations in cortical gyrification and proposed a disruption of normal neurodevelopment.
An effect of age of onset for MJ use has also been suggested in fMRI based studies. Tapert and colleagues (2007), examined adolescents with and without a history of MJ use during the performance of an inhibitory task, and reported increased frontal activation in MJ users relative to nonusers despite 28 days of abstinence from the drug and no significant performance differences between the groups. They noted that increased activation may reflect a neural compensation for successful task completion in MJ smokers, and in fact, a significant inverse relationship was detected between activation during the task and age of onset of MJ use, duration of use and number of MJ hits per month. In an fMRI study designed to assess the impact of age of onset of MJ use, Becker, Wagner, Gouzoulis-Mayfrank, Spuentrup, and Daumann (2010) reported increased activation in adults with early onset of MJ use (prior to age 16) relative to those who began smoking later on one condition of the N-back task. The authors suggested that as the maturing brain may be more vulnerable to the effects of MJ, early MJ use may result in suboptimal cortical efficiency during cognitive tasks. Our finding of a positive relationship between age of onset of MJ use and measures of FA, as well as an inverse relationship between age of onset of MJ use and trace suggests that early use may result in altered white matter and increased diffusivity in specific brain regions. This is further supported by the relationship noted between duration of MJ use and white matter measures within the right genu; years of MJ use was inversely correlated with FA and positively correlated with trace. It is therefore not surprising that the regions reported to be different between the MJ smokers and controls are also those that are impacted by age of onset of MJ use. These data support the perspective that in some individuals with early onset of MJ smoking, brain changes emerge during development that either predate drug use or result from MJ use and result in both functional and structural changes in MJ smokers. Although our sample of MJ smokers was not large enough to directly compare early onset smokers to those who began smoking later, future investigations should focus on this important area of study.
It should be noted that the current investigation has several limitations. First, although we did not find any statistically significant difference between the subject groups on any measure of clinical state or demographic variable, it is possible that the groups differed on measures that we did not assess, including social style and personality traits. In addition, our MJ smoking sample reported more alcohol use than the control subjects; however, no significant difference was detected for number of days of intoxication between the groups. Further, no subject in either group met diagnostic criteria for alcohol abuse or dependence, therefore, this difference in alcohol consumption is likely related to lifestyle differences and is not expected to account for the study findings. This is underscored by the fact that we found no significant association between any of the alcohol-related variables and the DTI measures. In addition, the current study utilized a ROI approach rather than whole brain analyses, and a DTI scheme that acquired data from only six directions. Future investigations should capitalize on the evolution of DTI methodology and include ROI and whole brain methods as well as TBSS and tractography. Nevertheless, the methods used in the current study have been successfully utilized by others in previous investigations (Silveri et al., 2006; Yurgelun-Todd, Silveri, Gruber, Rohan, & Pimental, 2007), and have allowed the study of the hypothesis of between-groups white matter differences and the association with measures of impulsivity. Our sample size (15 per group) is moderate in size, which limits the generalizability of the study findings. It is of note, however, that our findings are consistent with other DTI studies of substance abusing individuals, and the first to report a significant association between measures of white matter and impulsivity in chronic MJ smokers. Finally, although only a subset of subjects in each group received the BIS (N = 10), the associations between white matter and impulsivity are only present in the MJ smoking sample, and are statistically significant, underscoring the importance of examining the potential relationship between impulsivity and white matter measures in future studies.
Although we hypothesized a priori differences in white matter measures between the subject groups, and in fact demonstrated that they are present in frontal regions critical for regulation of inhibitory processes and impulsive behavior, the direction of the association between white matter and impulsivity may at first seem counterintuitive. Fractional anisotropy is a measure of white matter fiber tract coherence, and because frontal brain regions play a role in the regulation of impulsive behavior, the expectation would likely be that lower levels of FA would be correlated with higher levels of behavioral impulsivity, as measured by the BIS. It would seem that measures of lower tissue integrity or coherence could easily result in a reduced ability to successfully modulate frontally mediated processes, which include those related to inhibition. Data from the current investigation, which reports a positive association between FA levels and impulsivity in the MJ smokers, may therefore be suggestive of a neurodevelopmental alteration of MJ use. As previously noted, because the developing brain is vulnerable to exogenous agents during adolescence, it is possible that early exposure to MJ results in anomalous myelination patterns and/or a potential failure to prune within some brain regions.
These data represent the first report of significant alterations in frontal white matter fiber tract integrity that are associated with self-report measures of impulsivity in chronic, heavy MJ smokers, and appear to be related to age of onset of MJ use. The reductions in FA observed may be a reflection of demyelination or axonal damage that occur following chronic exposure to MJ or may reflect delayed brain development in MJ smokers. Future investigations should include additional measures of behavioral impulsivity and their relationship to age of onset of MJ use to more fully explore the potential neurodevelopmental aspects of white matter changes in MJ smokers. Findings from this study suggest that changes in white matter microstructure may be predictive or associated with increased impulsivity, and may ultimately contribute to the initiation of MJ use or the inability to discontinue use.
Acknowledgments
This project was supported by the National Institute of Drug Abuse (NIDA 1R03 DA016695–01) to Staci A. Gruber. As the sponsor of the project, NIDA did not have a role in the collection, analysis or interpretation of the data, writing of the manuscript, or the decision to submit such for publication. We gratefully acknowledge the efforts of Kelly Sagar and Megan Racine for their assistance with preparation and proofreading of the manuscript as well as data checking and scoring.
Contributor Information
Staci A. Gruber, Cognitive and Clinical Neuroimaging Core and Brain Imaging Center, McLean Hospital, and Department of Psychiatry, Harvard Medical School
Marisa M. Silveri, Brain Imaging Center, McLean Hospital, and Department of Psychiatry, Harvard Medical School
Mary Kathryn Dahlgren, Cognitive and Clinical Neuroimaging Core and Brain Imaging Center, McLean Hospital.
Deborah Yurgelun-Todd, Brain Imaging Center, McLean Hospital and Department of Psychiatry, Harvard Medical School, The Brain Institute at the University of Utah.
References
- Aboitiz F, Rodriguez E, Olivares R, Zaidel E. Age-related changes in fibre composition of the human corpus callosum: Sex differences. Neuroreport. 1996;7:1761–1764. doi: 10.1097/00001756-199607290-00013. [DOI] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: Preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. NeuroImage. 2008;41:1067–1074. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Kurma S. Diffusion abnormalities in adolescent and young adults with a history of heavy cannabis use. Journal of Psychiatric Research. 2009;43:189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusiontensor mri. Journal of Magnetic Resonance B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magnetic Resonance in Medicine. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank R, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance abusers. Psychiatry Research: Neuroimaging. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck depression inventory: Manual. New York, NY: Psychological Corporation; 1987. [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Progress in Neuro-Pyschopharmacology & Biological Psychiatry. 2010;34:837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet J. Dose related neurobehavioral effects of chronic cocaine use. Journal of Neuropsychiatry and Clinical Neurosciences. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Brady KT, Myrick H, McElroy S. The relationship between substance use disorders, impulse control disorders, and pathological aggression. The American Journal on Addictions. 1998;7:221–230. [PubMed] [Google Scholar]
- Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, Biederman J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi source interference task. Archives of General Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuh CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacology, Biochemistry, and Behavior. 2006;83:448–455. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and Alcoholism. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. Localization in neuropsychology. New York, NY: Oxford University Press; 1989. [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Eysenck SG, Eysenck HJ. Impulsiveness and Venturesomeness: Their position in a dimensional system of personality description. Psychological Reports. 1978;43:1247–1255. doi: 10.2466/pr0.1978.43.3f.1247. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams BW. Structured clinical interview for Axis I DSM–IV disorder, patient edition (SCID–I/P) (Version 2.0) New York, NY: Biometric Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, Satz P. Cognitive correlates of long-term cannabis use in Costa Rican men. Archives of General Psychiatry. 1996;53:1051–1057. doi: 10.1001/archpsyc.1996.01830110089011. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: A pilot investigation. Cognitive Brain Research. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Guy SM, Smith GM, Bentler PM. Consequences of adolescent drug use and personality factors on adult drug use. Journal of Drug Education. 1994;24:109–132. doi: 10.2190/X4WU-BV3X-Q483-Y5BT. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Heil SH, Johson MW, Higgins ST, Bicknel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addictive Behaviors. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Experimental and Clinical Psychopharmacology. 2010;18:99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: A functional magnetic resonance imaging study. Psychopharmacology (Berlin) 2004;176:239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kretschmann HJ. Cranial neuroimaging and clinical neuroanatomy. New York, NY: Verlag; 1992. [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence. 2003;26:475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of behavioral measure of risk taking: The Balloon Analogue Risk Task (BART) Journal of Experimental Psychology, Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: A controlled diffusion tensor imaging study. Biological Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Maas LC, Frederick BD, Renshaw PF. Decoupled automated rotational and translational registration for functional MRI time series data: The DART registration algorithm. Magnetic Resonance in Medicine. 1997;37:131–139. doi: 10.1002/mrm.1910370119. [DOI] [PubMed] [Google Scholar]
- Mata I, Perez-Iglesias R, Roiz-Santianez R, Tordesillas-Gutierrez D, Pazos A, Gutierrez A, Crespo-Facorro B. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Research. 2010;1317:297–304. doi: 10.1016/j.brainres.2009.12.069. [DOI] [PubMed] [Google Scholar]
- McHale S, Hunt N. Executive function deficits in short-term abstinent cannabis users. Human Psychopharmacology. 2008;23:409–415. doi: 10.1002/hup.941. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, O’Brien CP, Woody GE. An improved diagnostic instrument for substance abuse patients: The Addiction Severity Index. Journal of Nervous & Mental Diseases. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: Diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Molina-Holgado E, Guaza C, Rothwell NJ. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. Journal of Neuroscience Research. 2002;67:829–836. doi: 10.1002/jnr.10165. [DOI] [PubMed] [Google Scholar]
- Morriss MC, Zimmerman RA, Bilaniuk LT, Hunter JV, Haselgrove JC. Changes in brain water diffusion during childhood. Neuroradiology. 1999;41:929–934. doi: 10.1007/s002340050869. [DOI] [PubMed] [Google Scholar]
- Nye JS, Voglmaier S, Martenson RE, Snyder SH. Myelin basic protein is an endogenous inhibitor of the high-affinity cannabinoid binding site in brain. New Zealand Medical Journal. 1988;101:418–419. doi: 10.1111/j.1471-4159.1988.tb10589.x. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. Journal of Comparative Neurology. 1982;204:196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Human Brain Mapping. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Rogowska J, Kanayama G, Jon DI, Gruber S, Simpson N, Yurgelun-Todd DA. Neurophysiology of motor function following cannabis discontinuation in chronic cannabis smokers: An fMRI study. Drug and Alcohol Dependence. 2004;76:261–271. doi: 10.1016/j.drugalcdep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. Journal of the American Medical Association. 1996;275:521–527. [PubMed] [Google Scholar]
- Ramaekers JG, Kauret G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Buonocore MH, Natsuaki Y, Waters C, Moore CD, Leamon MH. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: A diffusion tensor imaging study. Biological Psychiatry. 2009;65:122–128. doi: 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ration task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelun-Todd DA. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magnetic Resonance Imaging. 2006;24:833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. Journal of the American Medical Association. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: SAMHSA; 2008. (NSDUH Series H–34, DHHS Publication No. SMA 08–4343) [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Wedeen VJ. Diffusion MRI of complex neural architecture. Neuron. 2003;40:885–895. doi: 10.1016/s0896-6273(03)00758-x. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, van Gorp WG, Gunderson EW, Haney M, Foltin RW. Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. Journal of Clinical and Experimental Neuropsychology. 2007;29:357–364. doi: 10.1080/13803390600693615. [DOI] [PubMed] [Google Scholar]
- Vangsness L, Bry BH, LaBouvie EW. Impulsivity, negative expectancies, and marijuana use: A test of the acquired preparedness model. Addictive Behaviors. 2005;30:1071–1076. doi: 10.1016/j.addbeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction. Journal of the International Neuropsychological Society. 2006;12:405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Gendreau PL, Tremblay RE, Oligny P. Reactive and proactive aggression differentially redict later conduct problems. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1998;39:377–385. [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn DM, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug and Alcohol Dependence. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: A magnetic resonance and positron emission tomography study. J Addict Des. 2000;19(1):1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Archives of General Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Silveri MM, Gruber SA, Rohan ML, Pimental PJ. White matter abnormalities observed in bipolar disorder: A diffusion tensor imaging study. Bipolar Disorder. 2007;9:504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]