Abstract
A case of canine discospondylitis and epidural empyema due to Salmonella species is reported. The history, clinical signs, and magnetic resonance imaging were suggestive of discospondylitis and empyema, which was subsequently confirmed by blood cultures. To the authors’ knowledge, this is the first reported case of canine discospondylitis due to Salmonella species.
Résumé
Cas de discospondylite canine et d’empyème épidural causé par l’espèceSalmonella. Un cas de discospondylite canine et d’empyème épidural causé par l’espèce Salmonella est présenté. L’anamnèse, les signes cliniques et l’imagerie par résonance magnétique suggéraient une discospondylite et l’empyème, ce qui a été subséquemment confirmé par des hémocultures. À la connaissance des auteurs, il s’agit du premier cas signalé de discospondylite canine causée par l’espèce Salmonella.
(Traduit par Isabelle Vallières)
Discospondylitis is an infectious, inflammatory disease of the intervertebral disc, the adjacent endplates, and vertebral bodies (1,2). The most common cause of discospondylitis in dogs is Staphylococus pseudintermedius. Other frequently isolated bacteria include Brucella canis, Streptococcus spp., Escherichia coli, and less commonly Actinomyces viscosus, Bacteroides spp., Bordetella spp., Coccidioides immitis, Corynebacterium spp., Erysipelothrix rhisiopathiae, Mycobacterium avium, Nocardia spp., Pasteurella multocida, and Proteus spp. (3–7). Fungal species have also been identified in canine discospondylitis (8).
Salmonella species as a cause of discospondylitis has been reported in human medicine (9–14) but to the authors’ knowledge this is the first report in a dog.
Case description
A 7-year-old, intact male boxer dog was referred to the neurology and neurosurgery service for investigation of a 2-week history of lethargy, inappetence, pyrexia, spinal pain, and paraparesis. Complete blood cell count, serum biochemical profile, and spinal radiographs at the referring veterinary surgeon prior to presentation were unremarkable and treatment was initiated with meloxicam, fentanyl patches, and antibiotics (amoxicillin/clavulanate). After an initial improvement, the dog started deteriorating and a referral was arranged.
On presentation, physical examination was unremarkable apart from marked lethargy. Rectal temperature was 38.6°C. Neurological examination revealed a non-ambulatory paraparesis with absent postural reactions in the pelvic limbs. Myotatic and withdrawal reflexes in all limbs were intact. Spinal palpation revealed severe hyperesthesia at the level of T4–T6. Neuroanatomical localization was consistent with the T3–L3 spinal cord segments.
Complete blood cell count demonstrated mild neutrophilia [13.1 × 109/L; reference range (RR): 3 to 11 × 109/L], reduced red blood cell count (4.16 × 109/L; RR: 5.5 to 8.5 × 109/L), reduced hematocrit (31.5%; RR: 37% to 45%), and reduced hemoglobin (107 g/L; RR: 120 to 180 g/L). Serum biochemical profile demonstrated hypoalbuminemia (24.6 g/L; RR: 29 to 39 g/L), hyperphosphatemia (3.67 mmol/L; RR: 0.8 to 2.00 mmol/L), and elevated creatine kinase activity (1039 U/L; RR: 61 to 394 U/L). Urine analysis was unremarkable.
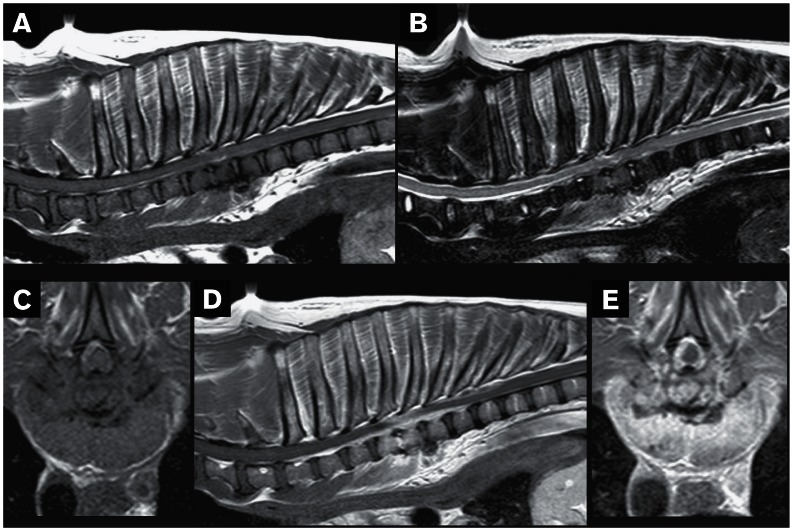
Radiographs of the thoracic spine revealed marked spondylosis deformans at the level of T4–T9, intervertebral disc space narrowing and end plate sclerosis at the levels of T3–T4, and T4–T5. Thoracic cavity radiographs, abdominal ultrasonography, and echocardiography were within normal limits. Magnetic resonance (MR) images of the thoraco-lumbar spine were obtained with a 1.5 Tesla MRI unit (Philips Medical Systems, Eindhoven, the Netherlands). T2-weighted images (T2WI) and T1-weighted images (T1WI) pre- and post-contrast administration [intravenous administration of gadoteric acid 27.9 mg/kg body weight (BW)] were obtained in sagittal and transverse planes. Also, short-T1 inversion recovery (STIR) images were obtained in a dorsal plane. The end plates of T4, T5, and T6 vertebral bodies appeared markedly hypointense compared to adjacent vertebral bodies on T1WI and hyperintense on T2WI (Figures 1A, B). On T1 and T2-weighted transverse images there was hyperintense (to the spinal cord parenchyma) and mildly compressive extradural material on the left side, displacing the spinal cord to the right (Figure 1C). On T1WI post-contrast administration there was diffuse contrast uptake at the vertebral bodies of T4, T5, and T6, and marked contrast uptake at the T4–T5 intervertebral disc, T4–T7 extradural material, and surrounding paraspinal soft tissues (Figures 1D, E). On STIR images the vertebral bodies of T4, T5, and cranial end plate of T6 appeared hyperintense compared with adjacent vertebral bodies. The MR imaging was suggestive of discospondylitis and epidural empyema at the level of T4–T6 (15,16).
Figure 1.
Magnetic resonance images of a dog with a discospondylitis lesion affecting its thoracic vertebral column. A — Sagittal T1-weighted image of the thoracic spine; the end plates of T4, T5, and T6 vertebral bodies appear markedly hypointense to adjacent vertebral bodies. The hypointensity is extending into the ventral aspect of the same vertebral bodies causing loss of definition. B — Sagittal T2-weighted image of the thoracic spine; the caudal end plate of T4 and the whole of T5 vertebral body appear markedly hyperintense to adjacent vertebral bodies, the intervertebral disc (IVD) space appears narrowed and the T4–T5 IVD are more hyperintense compared to the other IVDs. The soft tissues on the ventral aspect of T3–T8 vertebral bodies appear markedly hyperintense to the surrounding soft tissues. C — Transverse T1-weighted image at the level of T4–T5 IVD space; there is hyperintense (to spinal cord parenchyma) and mildly compressive extradural material on the left side, displacing the spinal cord to the right. D — Sagittal T1-weighted post-contrast image of the thoracic spine reveals diffuse contrast uptake at the vertebral bodies of T4, T5, and T6, marked contrast uptake at the T4–T5 IVD, T4–T7 extradural material and surrounding paraspinal soft tissues. E — Transverse T1-weighted post-contrast image at the level of T4–T5 IVD space; marked contrast enhancement of the extradural material, IVD and longus colli muscle.
Cerebrospinal fluid (CSF) was collected by lumbar puncture and cytology revealed 4 total nucleated cells and 13 red blood cells per mm3. The nucleated differential count was 65% large mononuclear cells and 35% small lymphocytes. Protein was markedly increased (1.32 g/L, reference value: < 0.4 g/L) which was consistent with proteinocytological dissociation. A sample was also submitted for aerobic and anaerobic bacterial culture.
Urine and blood samples were collected aseptically for aerobic and anaerobic bacterial cultures. Urine was collected by cysto-centesis and a blood sample of 10 mL was taken by venipuncture every hour for 3 h, each sample from a different site (left saphenous, right and left jugular veins). The blood was added to a commercially available bacterial culture medium (Signal blood culture system; Oxoid, Basingstoke, Hampshire, UK).
Subcutaneous cephalexin (Ceporex; Schering-Plough Animal Health, Uxbridge, Middlesex, UK), 25 mg/kg BW, q24h and intravenous (IV) enrofloxacin (Baytril; Bayer, Animal Division, Newbury, Berkshire, UK), 7.5 mg/kg BW, q24h were initiated. Analgesia was provided with meloxicam (Metacam; Boehringer Ingelheim, Bracknell, Berkshire, UK), 0.1 mg/kg BW, IV, q24h and methadone (Physeptone; Martindale Pharmaceuticals, Romford, Essex, UK), 0.2 mg/kg BW, IV, q4h. Urine and CSF bacterial cultures were negative after 48 h of incubation but all 3 blood samples yielded Salmonella species sensitive to cephalexin and enrofloxacin. The dog failed to improve clinically and 5 d after the initiation of medical treatment it was decided to perform a left-sided T3–T5 hemilaminectomy. During the surgery, distinct areas of fragile, yellow-gray adipose subcutaneous tissue were removed and submitted for culture and histopathology. The spinal cord did not appear compressed and macroscopically no other changes could be identified. The epidural space was flushed rigorously with sterile saline and a routine closure was performed in 4 layers. Bacterial cultures from the subcutaneous adipose tissue were negative and histopathology was consistent with suppurative panniculitis and cellulitis.
The day after surgery the patient started improving, analgesia was gradually stopped and he was discharged 1 wk later with ambulatory paraparesis and marked pelvic limb ataxia. Antibiotic treatment was continued in oral form and follow-up examinations at 1 and 2 mo revealed gradual improvement of the clinical signs. Six months later the antibiotics were discontinued but the dog was still exhibiting mild paraparesis and severe pelvic limb ataxia. During that time, the patient developed recurrent urinary tract infections due to E. coli initially and Enterococcus species which were successfully managed with antibiotic therapy.
Discussion
Salmonella serotypes are known to cause severe gastrointestinal disease in dogs, humans, and other species; they also cause septicemia, and/or sudden death especially in young, immuno-compromised or geriatric patients (17). Despite several reports of discospondylitis and epidural empyema due to Salmonella species in immunocompromised humans (9–14), to the authors’ knowledge this has not been previously reported in the dog.
The route of infection in this patient remains unknown and interestingly he did not show any gastrointestinal clinical signs. Common sources of Salmonella are contaminated foods (especially poultry and eggs) and feces (17). This dog was fed commercially prepared dog food.
We assume that Salmonella species was the cause of discospondylitis and empyema in this dog, based on all 3 positive blood bacterial cultures. Intervertebral disc aspirates were not obtained initially and when surgery was performed after 5 d of antibiotic treatment it was thought that it would be of a low yield. Antibodies against Brucella species were not investigated as the dog did not have scrotal and/or testicular abnormalities (scrotal dermatitis, testicular edema, or atrophy), and brucellosis is not an endemic disease in dogs in the United Kingdom.
The MR imaging findings in this dog were similar to the findings of discospondylitis and empyema that have previously been described (15,16,18) with the exception of lack of T2WI increased signal in the spinal cord gray matter, which was a consistent finding in De Stefani’s study (16). Two patterns of T1WI contrast enhancement have been previously described in epidural empyema (16). One pattern is characterized by peripheral enhancement surrounding pockets of homogeneous non-enhancing tissue and the other, by diffuse enhancement of the epidural fat, which reflects best the contrast enhancement in our patient.
Various treatment options have been reported in the veterinary literature for discospondylitis and epidural empyema. Long-term antibiotic therapy, after culture and sensitivity test, remains the core treatment but it has also been combined with decompressive hemilaminectomy or dorsal laminectomy and stabilization, when necessary, (16,19,20), fluoroscopy-guided percutaneous discectomy (21) or aspiration (22). With the exception of the last technique, which had poor outcome in 2 dogs, surgical intervention has been associated with a positive outcome. Good outcome has also been reported in 5 medically treated dogs with epidural empyema (23,24). Whilst the time for surgical intervention remains controversial for the treatment of spinal infections, it was decided to treat our patient medically initially. However, due to lack of clinical improvement after 5 d of specific antibiotic and analgesic treatment and after careful evaluation, a hemilaminectomy was performed. Very soon after surgery our patient started improving clinically, but it remains unknown whether surgery was necessary for this dog, considering that the spinal cord did not seem compressed during examination at surgery.
In humans, spinal empyemas are treated mainly surgically (25). However, some selected cases can be treated medically, without surgical intervention, but there have been no prospective, randomized treatment trials comparing the one against the other. In veterinary literature, until recently, surgical drainage in combination with medical treatment was the only reported treatment. Although surgical drainage in combination with medical management remains the treatment of choice in animals with spinal empyema, 2 recent reports (23,24) suggest that medical management only, in carefully selected cases, can be an option. Unfortunately, there are no criteria reported (neither in humans, nor in animals) to indicate which patients can be treated only medically and which require surgical intervention too. The decision is made usually upon individual assessment, severity of clinical signs, imaging findings, and clinician’s preference.
In conclusion, this is the first report of canine discospondylitis and epidural empyema due to Salmonella species. This bacterium should also be included in the list of causative agents of discospondylitis in small animals and extra care should be taken in suspected animals, considering the zoonotic character of this pathogen.
Acknowledgments
The authors acknowledge the referring veterinary surgeon and the owner of our patient, as well as all the staff of the Royal Veterinary College Small Animal Referral Hospital. The authors also thank the Research Office of the Royal Veterinary College for assessing the manuscript according to the Royal Veterinary College’s code of good research practice (authorization number: VCS_00482). CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Thomas WB. Diskospondylitis and other vertebral infections. Vet Clin North Am Small Anim Pract. 2000;30:169–182. doi: 10.1016/s0195-5616(00)50008-4. [DOI] [PubMed] [Google Scholar]
- 2.Tipold A, Stein VM. Inflammatory diseases of the spine in small animals. Vet Clin North Am Small Anim Pract. 2010;40:871–879. doi: 10.1016/j.cvsm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Kerwin SC, Lewis DD, Hribernik TN, Partington B, Hosgood G, Eilts BE. Diskospondylitis associated with Brucella canis infection in dogs: 14 cases (1980–1991) J Am Vet Med Assoc. 1992;201:1253–1257. [PubMed] [Google Scholar]
- 4.Betbeze C, Mclaughlin R. Canine diskospondylitis: Its etiology, diagnosis, and treatment. Vet Med. 2002;97:673–681. [Google Scholar]
- 5.Cherubini GB, Cappello R, Lu D, Targett M, Wessmann A, Mantis P. MRI findings in a dog with discospondylitis caused by Bordetella species. J Small Anim Pract. 2004;45:417–420. doi: 10.1111/j.1748-5827.2004.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 6.Burkert BA, Kerwin SC, Hosgood GL, Pechman RD, Fontenelle JP. Signalment and clinical features of diskospondylitis in dogs: 513 cases (1980–2001) J Am Vet Med Assoc. 2005;227:268–275. doi: 10.2460/javma.2005.227.268. [DOI] [PubMed] [Google Scholar]
- 7.Golini L, Morgan JP, Glaus T, Steffen F. Successful medical treatment of Erysipelothrix rhusiopathiae-induced lumbosacral diskospondylitis in a dog. Vet Rec. 2012;170:543b. doi: 10.1136/vr.100657. [DOI] [PubMed] [Google Scholar]
- 8.Dallman MJ, Dew TL, Tobias L, Doss R. Disseminated aspergillosis in a dog with diskospondylitis and neurologic deficits. J Am Vet Med Assoc. 1992;200:511–513. [PubMed] [Google Scholar]
- 9.Barkai G, Leibovitz E, Smolnikov A, Tal A, Cohen E. Salmonella diskitis in a 2-year old immunocompetent child. Scand J Infect Dis. 2005;37:232–235. doi: 10.1080/00365540410020767. [DOI] [PubMed] [Google Scholar]
- 10.Zebouh M, Loïez C, Marceau L, Vieillard MH, Izard D, Courcol RJ. Spondylodiscitis due to Salmonella enteritica serotype Typhi. Ann Biol Clin (Paris) 2005;63:517–518. [PubMed] [Google Scholar]
- 11.Ozturk C, Tezer M, Mirzanli C, Erkal Bilen F, Aydogan M, Hamzaoglu A. An uncommon cause of paraplegia: Salmonella spondylodiskitis. J Spinal Cord Med. 2006;29:234–236. doi: 10.1080/10790268.2006.11753879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amritanand R, Venkatesh K, Sundararaj GD. Salmonella spondylodiscitis in the immunocompetent: Our experience with eleven patients. Spine. 2010;35:1317–1321. doi: 10.1097/BRS.0b013e3181e87afe. [DOI] [PubMed] [Google Scholar]
- 13.Suwanpimolkul G, Nilgate S, Suankratay C. Typhoid spondylodiscitis: The first reported case in Southeast Asia and review of the literature. J Med Assoc Thai. 2010;93:137–141. [PubMed] [Google Scholar]
- 14.Shkurti-Leka K, Kraja D, Leka N, Vreto G, Melyshi K, Kasmi G. Spondylodiscitis due to Salmonella in an immunocompetent patient. Med Arh. 2011;65:252–3. doi: 10.5455/medarh.2011.65.252-253. [DOI] [PubMed] [Google Scholar]
- 15.Carrera I, Sullivan M, McConnell F, Gonçalves R. Magnetic resonance imaging features of discospondylitis in dogs. Vet Radiol Ultrasound. 2011;52:125–131. doi: 10.1111/j.1740-8261.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 16.De Stefani A, Garosi LS, Mcconnell FJ, Llabres Diaz FJ, Dennis R, Platt SR. Magnetic resonance imaging features of spinal epidural empyema in five dogs. Vet Radiol Ultrasound. 2008;49:135–140. doi: 10.1111/j.1740-8261.2008.00339.x. [DOI] [PubMed] [Google Scholar]
- 17.Willard MD. Disorders of the intestinal tract. In: Nelson RW, Couto CG, editors. Small Animal Internal Medicine. 3rd ed. St Louis, Missouri: Mosby; 2003. pp. 437–438. [Google Scholar]
- 18.Kraft SL, Mussman JM, Smith T, Biller DS, Hoskinson JJ. Magnetic resonance imaging of presumptive lumbosacral discospondylitis in a dog. Vet Radiol Ultrasound. 1998;39:9–13. doi: 10.1111/j.1740-8261.1998.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 19.Cherrone KL, Eich CS, Bonzynski JJ. Suspected paraspinal abscess and spinal epidural empyema in a dog. J Am Anim Hosp Assoc. 2002;38:149–151. doi: 10.5326/0380149. [DOI] [PubMed] [Google Scholar]
- 20.Lavely JA, Vernau KM, Vernau W, Herrgesell EJ, Lecouteur RA. Spinal epidural empyema in seven dogs. Vet Surg. 2006;35:176–185. doi: 10.1111/j.1532-950X.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 21.Kinzel S, Koch J, Buecker A, et al. Treatment of 10 dogs with discospondylitis by fluoroscopy-guided percutaneous discectomy. Vet Rec. 2005;156:78–81. doi: 10.1136/vr.156.3.78. [DOI] [PubMed] [Google Scholar]
- 22.Dewey CW, Kortz GD, Bailey CS. Spinal epidural empyema in two dogs. J Am Anim Hosp Assoc. 1998;34:305–308. doi: 10.5326/15473317-34-4-305. [DOI] [PubMed] [Google Scholar]
- 23.Escriou C, Duchene LS, Gibert S, Seurin MJ. Spinal epidural infection medically treated in 3 dogs: MRI features and follow-up, clinical findings and outcome. Proceedings of the European Society of Veterinary Neurology; 22–24 September 2011; Trier, Germany. [Google Scholar]
- 24.Monteiro S, Parker J, Freeman P, Rousset N, Soares C, Vanhaesebrouck A. Non-surgical treatment in two dogs with suspected spinal epidural empyema. Proceedings of European Society of Veterinary Neurology; 13–15 September 2012; Ghent, Belgium.. p. 148. [Google Scholar]
- 25.Nussbaum ES, Rigamonti D, Standiford H, Numaguchi Y, Wolf AL, Robinson WL. Spinal epidural abscess: A report of 40 cases and review. Surg Neurol. 1992;38:225–231. doi: 10.1016/0090-3019(92)90173-k. [DOI] [PubMed] [Google Scholar]