Abstract
Korean red ginseng (KRG), the steamed root of Panax ginseng Meyer, has a variety of biological properties, including anti-inflammatory, antioxidant and anticancer effects. Aflatoxin B1 (AFB1) produced by the Aspergillus spp. causes acute hepatotoxicity by lipid peroxidation and oxidative DNA damage, and induces liver carcinoma in humans and laboratory animals. This study was performed to examine the protective effects of KRG against hepatotoxicity induced by AFB1 using liver-specific serum marker analysis, histopathology, and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling. In addition, to elucidate the possible mechanism of hepatoprotective effects, superoxide dismutase, catalase, glutathione peroxidase, and malondialdehyde were analyzed. Rats were treated with 250 mg/kg of KRG (KRG group) or saline (AFB1 group) for 4 weeks and then received 150 μg/kg of AFB1 intraperitoneally for 3 days. Rats were sacrificed at 12 h, 24 h, 48 h, 72 h, or 1 wk after AFB1 treatment. In the KRG pre-treatment group, serum alanine aminotransferase, aspartate aminotransferase, and malondialdehyde levels were low, but superoxide dismutase, catalase, and glutathione peroxidase activities were high as compared to the AFB1 alone group. Histopathologically, AFB1 treatment induced necrosis and apoptosis in hepatocytes, and led to inflammatory cells infiltration in the liver. KRG pre-treatment ameliorated these changes. These results indicate that KRG may have protective effects against hepatotoxicity induced by AFB1 that involve the antioxidant properties of KRG.
Keywords: Panax ginseng, Korean red ginseng, Aflatoxin B1, Antioxidant enzymes
INTRODUCTION
Aflatoxin B1 (AFB1) is a metabolite produced by fungi, Aspergillus flavus and Aspergillus parasiticus, which contaminate grains. AFB1 causes hepatotoxicity and liver carcinoma in humans and laboratory animals [1]. It is metabolized by cytochrome P-450 monooxygenases into reactive aflatoxin B1-8,9-epoxide, which binds to cellular macromolecules and causes injury to the periportal regions of the liver. This damage appears as hemorrhage, parenchymal cell necrosis, and injury to intrahepatic bile ducts as well as elevated alanine and aspartate amino transferase levels [2-5]. In addition, AFB1-induced toxicity is due to lipid peroxidation and oxidative DNA damage [6,7]. The main active components of P. ginseng are ginsenosides which have been shown to have a variety of biological properties including anti-inflammatory, antioxidant, and anticancer effects. In addition, ginseng extract clearly reduce liver damage induced by certain chemicals including alcohol [8] or carbon tetrachloride [9,10]. Another study suggested that ginseng and/or ginsenoside can induce antioxidant enzymes essential for maintaining cell viability by lowering the level of oxygen radicals generated from intracellular metabolism [11].
This study was performed to examine the protective effects of Korean red ginseng (KRG) against hepatotoxicity induced by AFB1. We evaluated sequential pathological characteristics and liver-specific serum markers. In addition, antioxidant enzyme activities were analyzed in the liver to elucidate the mechanisms underlying the hepatoprotective effects of KRG.
MATERIALS AND METHODS
Animals and chemicals
Thirty-three 4-week-old male Sprague-Dawley rats weighing 85-100 g were obtained from Nara Biotech (Seoul, Korea). The rats were housed in polycarbonate cages at 23±1℃ with 55±5% humidity and were maintained on a 12 h light/dark cycle. The animals had access to rodent chow (Jeil Feed Co., Daejeon, Korea) and water ad libitum. KRG extract was donated from KT&G (Seoul, Korea). The extracts were freshly dissolved in distilled water immediately before use. AFB1 was purchased from Sigma-Aldrich (St. Louis, MO, USA). All animal studies were approved by the Institutional Animal Ethical Committee of Chungnam National University (approval no. 2009-1-26).
Experimental procedures
After 1 wk of acclimation, the rats were randomly divided into three groups: control group (n=3), KRG group (n=15), and AFB1 group (n=15). The KRG group was given oral doses of KRG (250 mg/kg/d) or saline for 4 wk orally, and then received 150 μg/kg/d of AFB1 intraperitoneally for 3 d. Control rats (n=3) received an equivalent volume of corn oil. The rats were sacrificed while under ether anesthesia at 12 h, 24 h, 48 h, 72 h, or 1 wk after receiving the last dose of AFB1 (n=3 at each time point).
Immediately after sacrificing, the liver from each rat was removed and the left lobe was placed in 10% neutral buffered formalin for histopathological examination and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL). The others lobes were used for antioxidant enzyme activity and thiobarbituric acid reactive substances (TBARS) assays.
Blood chemistry
Blood was collected from the inferior vena cava while the rats were under ether anesthesia. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using a Hitachi 7150 Chemistry analyzer (Hitachi Ltd., Tokyo, Japan).
TUNEL
Apoptotic cells were detected by TUNEL using an ApopTag-peroxidase Kit (Intergen Co., Purchase, NY, USA). The procedure was performed according to the manufacturer’s instructions.
Hepatic antioxidant enzyme activity assay
To elucidate the possible mechanism underlying the hepatoprotective effects of KRG, the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) were analyzed. The enzyme source fraction from liver tissue was prepared as follows. Liver tissue (1 g) was homogenized in a five-fold volume of the 0.25 mol/L sucrose buffer, centrifuged at 600 ×g for 20 min to remove any cell debris, and then the supernatant was centrifuged at 10,000 ×g for 10 min to remove the mitochondria. Finally, the supernatant was removed and further centrifuged at 105,000 ×g for 60 min to obtain the cytosolic fraction. The amount of protein in the mitochondrial and cytosolic fractions was measured using Bradford method [12] with bovine serum albumin as the standard.
Superoxide dismutase activities assay
SOD activity was measured as described by Marklund and Marklund [13] with a slight modification. The cytosol supernatant (100 L) was mixed with 1.5 mL of Tris-EDTA-HCl buffer (pH 8.5), then 100 L of 7.2 mmol/L pyrogallol was added and the mixture was incubated at 25℃ for 10 min. The reaction was terminated by the addition of 50 L of 1.0 M/L HCl and the absorbance was measured at 420 nm. One unit was defined as the amount of enzyme that inhibited the oxidation of pyrogallol by 50%. The activity was expressed as U/mg protein.
Catalase activities assay
CAT activity was measured using the method by Aebi [14] with a slight modification. The mitochondria pellet was dissolved in 1.0 mL of a 0.25 M/L sucrose buffer. The mitochondria solution (10 L) was then added to a cuvette containing 2.89 mL of a 50 mmol/L potassium phosphate buffer (pH 7.4), and the reaction initiated by the addition of 0.1 mL of 300 M/L H2O2 in a final volume of 3.0 mL at 25℃. The decomposition of H2O2 was measured at 240 nm for 5 min using a spectrophotometer. A molar extinction coefficient of 0.041 (mmol/L)-1 ·cm-1 was used to determine the CAT activity. One unit of CAT activity was defined as the amount of enzyme which oxidized 1.0 μmol H2O2 per min per mg protein.
Glutathione peroxide activities assay
GPX activity was measured using the Paglia and Valentine method [15] with a slight modification. The reaction mixture contained 2.6 mL of a 0.1 M/L of Tris-HCl (pH 7.2) buffer, 100 L of 30 mmol/L glutathione, and 100 L of 6.0 mmol/L NADPH. The cytosolic supernatant (100 L) was added to 2.9 mL of the reaction mixture and incubated at 25℃ for 5 min. The reaction was initiated by the addition of 100 L of 7.5 mmol/L H2O2 and the absorbance was measured at 340 nm for 5 min. A molar extinction coefficient of 6.22×103 (mmol/L)-1·cm-1 was used to determine the activity. One unit of GPX was defined as the amount of enzyme which oxidized 1.0 μmol NADPH per min per mg protein.
ThioBarbituric acid reactive substances assay
Malondialdehyde (MDA) quantitation was performed using an OxiSelectTM TBARS assay kit (Cell Biolabs Inc., San Diego, CA, USA). Samples containing unknown quantities of MDA or MDA standards were first incubated with thiobarbituric acid at 95℃. After the brief incubation, the samples and standards can be read spectrophotometrically or fluorometrically. The unknown MDA content in the samples was determined by comparison with the MDA standard curve.
Statistical analysis
The results are expressed as the mean±SD. Comparison between groups were carried out using a two-tailed Student’s t-test. The threshold of significance was set a p<0.05.
RESULTS
Blood chemistry
In the AFB1 and KRG groups, the AST and ALT levels were increased starting at 12 h compared to the control group. In AFB1 group, AST and ALT levels peaked at 48 h and then decreased. KRG pre-treatment ameliorated these changes (Fig. 1).
Fig. 1. Liver specific serum biomarker analysis in rats treated with Aflatoxin B1 (AFB1) with or without Korean red ginseng (KRG). Serum alanine aminotransferase (A) and aspartate aminotransferase (B) were relatively low in group pretreated with KRG compared to the group treated only with AFB1.

Histopathological evalutaion
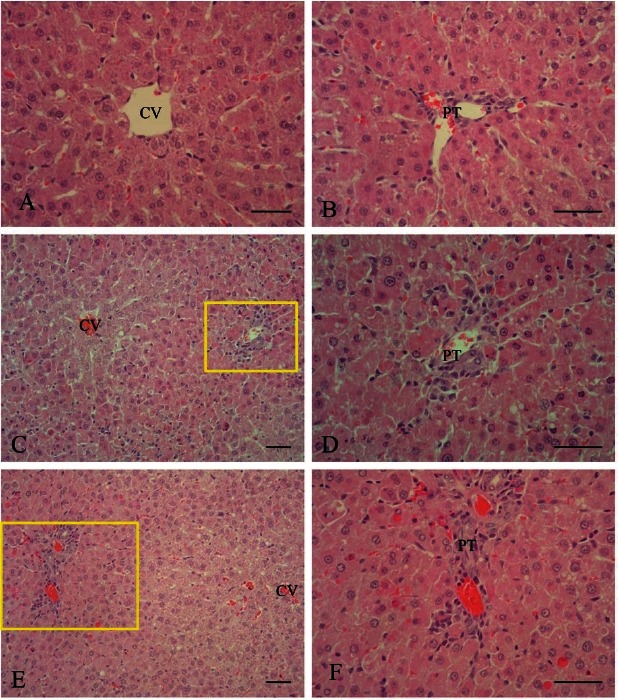
Microscopically, significant pathologic changes in the liver were detected in all AFB1-treated rats. Histopathological changes were characterized by hepatic parenchymal cell necrosis, loss of hepatic cords, fatty changes, congestion, and hemorrhage in the centrilobular and periportal regions. In addition, inflammatory cells infiltration in the periportal region was observed. In the AFB1 group, the appearance of severe liver lesions were observed after 12 h and 24 h of AFB1 treatment. Liver lesions were still present at 48 h, but were less extensive and almost completely disappeared 1 wk after AFB1 treatment. KRG pre-treatment significantly ameliorated these changes (Fig. 2).
Fig. 2. Representative photomicrograph of liver lesions in rats treated with Aflatoxin B1 (AFB1) with or without Korean red ginseng (KRG) pretreatment. H&E stain. (A,B) Centrilobular (CV) and periportal (PT) zone of the control group. Bar=50 μm. (C) Hepatic lesion found in the AFB1-treated group after 12 h; severe necrosis of hepatocytes and loss of hepatic cords were observed around the portal region. Bar=100 μm. (D) Higher magnification of (C). Severe hepatocyte necrosis was noted around the portal triad. Bar=50 μm. (E) Hepatic lesions of the KRG pretreated group after 12 h; mild necrosis in hepatocytes and loss of hepatic cords were observed around the portal region. Bar=100 μm. (F) Higher magnification of (E). Mild hepatocyte necrosis was noted around the portal triad. Bar=50 μm.
TUNEL assay
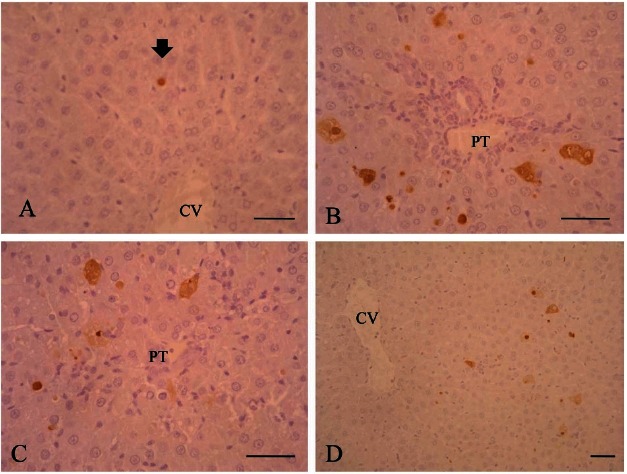
Positive TUNEL reactions were detected in hepatic parenchymal cells scattered throughout the liver in the AFB1 rats. Apoptosis rates peaked at 12 h and remained elevated until 48 h after the AFB1 treatment, but then gradually decreased until the end of the follow-up period. The number of apoptotic cells was decreased by KRG pre-treatment (Fig. 3).
Fig. 3. Apoptosis in liver of rats treated with Aflatoxin B1 (AFB1) with or without Korean red ginseng (KRG) pretreatment. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) stain. Bar=50 μm. (A) Control group; few TUNEL-labeled apoptotic cells (arrow) were noted. (B) AFB1 group; at 12 h many apoptotic cells were observed. (C) KRG pretreated group; at 12 h the number of apoptotic cells decreased compared to the AFB1 group. (D) AFB1 group; at 48 h the number of apoptotic cells decreased. CV, central vein; PT, portal triad.
Hepatic antioxidant enzymes activities
AFB1 treatment slightly increased antioxidant enzyme activity compared to the control group. SOD, CAT, and GPX activity levels were higher in the KRG group than in the AFB1 group (Fig. 4). However, the hepatic MDA level was increased by treatment with AFB1 compared to the control, and was significantly lower in KRG group than in AFB1 group (Fig. 5).
Fig. 4. Level of hepatic antioxidant enzymes in rats treated with Aflatoxin B1 (AFB1) with or without Korean red ginseng (KRG) pretreatment. Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) enzyme activity was increased after 12 h in the KRG and AFB1 groups compared to the control group. These enzyme activities were relatively higher in the KRG group compared to the AFB1 group.

Fig. 5. The levels of malondialdehyde (MDA), a natural byproduct of lipid peroxidation, in the plasma and liver of rats treated with Aflatoxin B1 (AFB1) with or without Korean red ginseng (KRG) pretreatment. Plasma MDA levels were higher in the AFB1 group compared to the control and KRG group at each time point except at 72 h. In addition, MDA levels in the liver were higher in the AFB1 group than the control and KRG groups at 12 and 24 h. *Significantly different from the control and KRG group.

DISCUSSION
AFB1, a mycotoxin produced by the fungi Aspergillus flavus and Aspergillus paracticus, is known to be a potent hepatotoxin and hepatocellular carcinogen in experimental animals [16-18]. In the present study, AFB1 treatment increased serum ALT and AST levels; they both increased until 48 h after AFB1 treatment and then decreased in a time-dependent manner. These results concurred with our histopathological findings. After 12 h and 24 h of AFB1 treatment, severe histopathological changes in the liver were noted. In addition, AFB1 treatment resulted in significant increases in apoptosis after 12 h after which the number of apoptotic cells decreased in a time-dependent manner. The present results clearly indicate that AFB1 induces damage in the liver during an early stage of exposure in which apoptosis of hepatocytes is involved. KRG pre-treatment clearly ameliorated these changes. Apoptosis is a form of cell death that permits the elimination of damaged or unwanted cells in multicellular organisms. The protective effect of Panax ginseng extract against the apoptotic cell death induced by PCB52 in human neuronal SK-N-MC cells has been previously reported [19]. Likewise, ginsenoside Rh2 induced apoptotic cell death in human breast cancer cell lines [20]. Further studies are now necessary to elucidate the effects of KRG on apoptotic cell death and the mechanisms underlying the protective effects of KRG against AFB1-induced hepatocyte apoptosis.
Lipid peroxidation is a well-defined mechanism of cellular damage in animals and plants. Oxidative modification of lipids can be induced in vitro by a wide array of pro-oxidant agents and occurs in vivo during aging and under certain pathologic conditions [21,22]. It was hypothesized that AFB1-induced hepatotoxicity is due to lipid peroxidation and oxidative DNA damage [6,7]. Lipid peroxides are indicators of cellular oxidative stress that decompose to form more complex and reactive compounds such as MDA and 4-hydroxynonenal. These aldehydic secondary byproducts of lipid peroxidation are generally accepted markers of oxidative stress. The enzymatic antioxidant system is one of the protective mechanisms including SOD which can be found in various cellular compartments and catalyze the disproportion of superoxide anions to hydrogen peroxide and oxygen [21,23]. H2O2 is eliminated by various antioxidant enzymes such as CAT [21,24,25] and GPX [22,26] which convert H2O2 into water. Toxic O2-, H2O2, and OH radicals are efficiently eliminated by non-enzymatic (α-tocopherol, б-carotene, phenolic compounds, ascorbate, glutathione) and enzymatic antioxidants [27,28]. Red ginseng extracts are potent antioxidants that exert protective effects against the progression of oxidative stress-induced DNA damage [11,23,29]. In the present study, CAT, GPX, and SOD enzyme activities were higher in the KRG pretreatment group compared to the AFB1 group. However, the level of MDA was lower in the KRG pretreated group. These results indicate that KRG prevented AFB1-induced hepatotoxicity through its antioxidant effects by increasing SOD, CAT, and GPX activity and reducing lipid peroxidation. In conclusion, KRG may be used to protect hepatocyte from oxidative injury caused by AFB1.
Acknowledgments
This study was supported by the grant 2009 from the Korean Society of Ginseng funded by Korea Ginseng Corporation.
References
- 1.Chao TC, Maxwell SM, Wong SY. An outbreak of aflatoxicosis and boric acid poisoning in Malaysia: a clinicopathological study. J Pathol. 1991;164:225–233. doi: 10.1002/path.1711640307. [DOI] [PubMed] [Google Scholar]
- 2.Ueng YF, Shimada T, Yamazaki H, Guengerich FP. Oxidation of aflatoxin B1 by bacterial recombinant human cytochrome P450 enzymes. Chem Res Toxicol. 1995;8:218–225. doi: 10.1021/tx00044a006. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe SA, Colley PJ, Neal GE. A comparison of the effects of pretreatment with phenobarbitone and 3-methylcholanthrene on the metabolism of aflatoxin B1 by rat liver microsomes and isolated hepatocytes in vitro. Chem Biol Interact. 1981;35:145–157. doi: 10.1016/0009-2797(81)90139-3. [DOI] [PubMed] [Google Scholar]
- 4.Judah DJ, Hayes JD, Yang JC, Lian LY, Roberts GC, Farmer PB, Lamb JH, Neal GE. A novel aldehyde reductase with activity towards a metabolite of aflatoxin B1 is expressed in rat liver during carcinogenesis and following the administration of an anti-oxidant. Biochem J. 1993;292(Pt 1):13–18. doi: 10.1042/bj2920013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelick NJ. Risk assessment for aflatoxin. I. Metabolism of aflatoxin B1 by different species. Risk Anal. 1990;10:539–559. doi: 10.1111/j.1539-6924.1990.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 6.Manson MM, Hudson EA, Ball HW, Barrett MC, Clark HL, Judah DJ, Verschoyle RD, Neal GE. Chemoprevention of aflatoxin B1-induced carcinogenesis by indole-3-carbinol in rat liver: predicting the outcome using early biomarkers. Carcinogenesis. 1998;19:1829–1836. doi: 10.1093/carcin/19.10.1829. [DOI] [PubMed] [Google Scholar]
- 7.Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, Qian GS, Kuang SY, Zarba A, Egner PA, et al. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People’s Republic of China. J Natl Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- 8.Zuin M, Battezzati PM, Camisasca M, Riebenfeld D, Podda M. Effects of a preparation containing a standardized ginseng extract combined with trace elements and multivitamins against hepatotoxin-induced chronic liver disease in the elderly. J Int Med Res. 1987;15:276–281. doi: 10.1177/030006058701500503. [DOI] [PubMed] [Google Scholar]
- 9.Jeong TC, Kim HJ, Park JI, Ha CS, Park JD, Kim SI, Roh JK. Protective effects of red ginseng saponins against carbon tetrachloride-induced hepatotoxicity in Sprague Dawley rats. Planta Med. 1997;63:136–140. doi: 10.1055/s-2006-957630. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Chun YJ, Park JD, Kim SI, Roh JK, Jeong TC. Protection of rat liver microsomes against carbon tetrachloride-induced lipid peroxidation by red ginseng saponin through cytochrome P450 inhibition. Planta Med. 1997;63:415–418. doi: 10.1055/s-2006-957724. [DOI] [PubMed] [Google Scholar]
- 11.Chang MS, Lee SG, Rho HM. Transcriptional activation of Cu/Zn superoxide dismutase and catalase genes by panaxadiol ginsenosides extracted from Panax ginseng. Phytother Res. 1999;13:641–644. doi: 10.1002/(SICI)1099-1573(199912)13:8<641::AID-PTR527>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 14.Aebi H. Catalase. In: Bergmeyer HU, ed. Methods of enzymatic analysis. Academic Press; New York: 1974. pp. 673–698. [Google Scholar]
- 15.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 16.Towner RA, Hashimoto H, Summers PM. Non-invasive in vivo magnetic resonance imaging assessment of acute aflatoxin B1 hepatotoxicity in rats. Biochim Biophys Acta. 2000;1475:314–320. doi: 10.1016/s0304-4165(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 17.Gopalan P, Tsuji K, Lehmann K, Kimura M, Shinozuka H, Sato K, Lotlikar PD. Modulation of aflatoxin B1-induced glutathione S-transferase placental form positive hepatic foci by pretreatment of rats with phenobarbital and buthionine sulfoximine. Carcinogenesis. 1993;14:1469–1470. doi: 10.1093/carcin/14.7.1469. [DOI] [PubMed] [Google Scholar]
- 18.Toskulkao C, Yoshida T, Glinsukon T, Kuroiwa Y. Potentiation of aflatoxin B1 induced hepatotoxicity in male Wistar rats with ethanol pretreatment. J Toxicol Sci. 1986;11:41–51. doi: 10.2131/jts.11.41. [DOI] [PubMed] [Google Scholar]
- 19.Lee JY, Kim JW, Cho SD, Kim YH, Choi KJ, Joo WH, Cho YK, Moon JY. Protective effect of ginseng extract against apoptotic cell death induced by 2,2’,5,5’-tetrachlorobiphenyl in neuronal SK-N-MC cells. Life Sci. 2004;75:1621–1634. doi: 10.1016/j.lfs.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Choi S, Oh JY, Kim SJ. Ginsenoside Rh2 induces Bcl-2 family proteins-mediated apoptosis in vitro and in xenografts in vivo models. J Cell Biochem. 2011;112:330–340. doi: 10.1002/jcb.22932. [DOI] [PubMed] [Google Scholar]
- 21.Scandalios JG. Oxygen stress and superoxide dismutases. Plant Physiol. 1993;101:7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Gara LD, de Pinto MC, Tommasi F. The antioxidant systems vis-á-vis reactive oxygen species during plant-pathogen interaction. Plant Physiol Biochem. 2003;41:863–870. doi: 10.1016/S0981-9428(03)00135-9. [DOI] [Google Scholar]
- 23.Hegedus A, Erdei S, Horvath G. Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci. 2001;160:1085–1093. doi: 10.1016/S0168-9452(01)00330-2. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez JA, Campillo A, Jimenez A, Alarcon JJ, Sevilla F. Response of antioxidant systems and leaf water relations to NaCl stress in pea plants. New Phytol. 1999;141:241–251. doi: 10.1046/j.1469-8137.1999.00341.x. [DOI] [PubMed] [Google Scholar]
- 25.Kono Y, Fridovich I. Inhibition and reactivation of Mn-catalase. Implications for valence changes at the active site manganese. J Biol Chem. 1983;258:13646–13648. [PubMed] [Google Scholar]
- 26.Jablonski PP, Anderson JW. Light-dependent reduction of hydrogen peroxide by ruptured pea chloroplasts. Plant Physiol. 1982;69:1407–1413. doi: 10.1104/pp.69.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 28.Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 29.Choi HJ, Han HS, Park JH, Son JH, Bae JH, Seung TS, Choi C. Antioxidantive, phospholipase A2 inhibiting, and anticancer effect of polyphenol rich fractions from Panax ginseng C. A. Meyer. J Korean Soc Agric Chem Biotechnol. 2003;46:251–256. [Google Scholar]


