Abstract
The human ether-a-go-go-related gene (HERG) cardiac K+ channels are one of the representative pharmacological targets for development of drugs against cardiovascular diseases such as arrhythmia. Panax ginseng has been known to exhibit cardioprotective effects. In a previous report we demonstrated that ginsenoside Rg3 regulates HERG K+ channels by decelerating deactivation. However, little is known about how ginsenoside metabolites regulate HERG K+ channel activity. In the present study, we examined the effects of ginsenoside metabolites such as compound K (CK), protopanaxadiol (PPD), and protopanaxatriol (PPT) on HERG K+ channel activity by expressing human α subunits in Xenopus oocytes. CK induced a large persistent deactivating-tail current (Ideactivating-tail) and significantly decelerated deactivating current decay in a concentration-dependent manner. The EC50 for persistent Ideactivating-tail was 16.6±1.3 μM. In contrast to CK, PPT accelerated deactivating-tail current deactivation. PPD itself had no effects on deactivating-tail currents, whereas PPD inhibited ginsenoside Rg3-induced persistent Ideactivating-tail and accelerated HERG K+ channel deactivation in a concentration-dependent manner. These results indicate that ginsenoside metabolites exhibit differential regulation on Ideactivating-tail of HERG K+ channel.
Keywords: Panax ginseng, Ginsenoside metabolites, Human ether-a-go-go-related gene K+ channel, Human heart
INTRODUCTION
K+ channels play critical roles in a wide variety of physiological processes, including the regulation of neurotransmitter release, neuronal excitability, heart rate, muscle contraction, hormone secretion, epithelial electrolyte transport, cell volume, and cell proliferation [1]. Cardiomyocytes contain two kinds of delayed rectifier K+ channels, which are important for cardiac repolarization after cardiac action potential and shorten the action potential duration [2]. Thus, the human ether-a-go-go-related gene (HERG, IKr) and KVLQT (KCNQ) (IKs) K+ channels are mainly responsible for repolarization of the heart cardiac action potential [3]. The genetic or pathological dysfunctions of HERG or KCNQ K+ channels are one of the primary triggers of cardiac diseases such as arrhythmias. Since arrhythmias are a major cause of sudden cardiac death in the world [2], HERG as well as KCNQ K+ channels are the principal pharmacological targets for development of therapeutic drugs against cardiovascular diseases including arrhythmias.
Ginseng, the root of Panax ginseng Meyer, has been used as a general tonic to promote longevity and enhance bodily functions against stress, fatigue, diseases, cancer and diabetes mellitus [4]. Among various ginseng components, ginsenosides (also called ginseng saponins) exhibit anti-hypertension and cardio-protective effects [5]. In a previous study, we demonstrated that ginsenoside Rg3 (Rg3) enhanced cardiac IKs channel currents, which consist of KCNQ1 plus KCNE1 subunits in both concentration- and voltage-dependent manners [6]. In addition, we also demonstrated that Rg3 enhanced outward currents (IHERG) and transient tail currents (Itail). Rg3 induced a large persistent deactivating-tail current (Ideactivataing-tail) and significantly decelerated deactivating current decay [7].
On the other hand, ginsenosides administered via the oral route can pass into the large intestine without being broken down by either gastric juices or digestive enzymes [8]. But protopanaxadiol (PPD) ginsenosides are metabolized by intestinal microorganisms into compound K (CK) with one glucose at the C-20 position and are further metabolized to form PPD without glucose, whereas protopanaxatriol (PPT) ginsenosides are metabolized to PPT that only maintains the backbone structure of ginsenosides without any carbohydrate component (Fig. 1). The purpose of this study was to investigate how ginsenoside metabolites affect HERG K+ channel activity. We report here that CK decelerated HERG K+ channel deactivation, whereas PPT accelerated HERG K+ channel deactivation. PPD itself had no effect on HERG K+ channel activity but antagonized Rg3-mediated HERG K+ channel regulations. These results indicate that ginsenoside metabolites exhibit differential regulation on HERG K+ channel activity.
Fig. 1. Chemical structures of ginsenoside Rg3 and ginsenoside metabolites used in this study. CK, compound K; PPD, protopanaxadiol; PPT, protopanaxatriol; Glc, glucopyranoside.
MATERIALS AND METHODS
Materials
The individual ginsenoside Rg3 and ginsenoside metabolites such as PPD, PPT, and CK were provided by AMBO Institute (Seoul, Korea) (Fig. 1A). The cDNAs for human HERG K+ channels (accession no. U04270) were kindly provided by Dr. Pongs (University of Hamburg, Germany). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of Xenopus oocytes and microinjection
Xenopus laevis frogs were purchased from Xenopus I (Ann Arbor, MI, USA). Their care and handling were in accordance with the highest standards of institutional guidelines of Konkuk University. For isolation of oocytes, frogs were anesthetized with an aerated solution of 3-amino benzoic acid ethyl ester followed by removal of ovarian follicles. The oocytes were treated with collagenase and then agitated for 2 h in Ca2+-free medium containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, 100 units/mL penicillin and 100 μg/mL streptomycin. Stage V-VI oocytes were collected and stored in ND96 medium (in mM: 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES, pH 7.5) supplemented with 50 μg/mL gentamicin. The oocyte-containing solution was maintained at 18℃ with continuous gentle shaking and renewed daily. Electrophysiological experiments were performed within 5-6 days of oocyte isolation, with ginsenoside Rg3 or ginsenoside metabolites added to the bath. For HERG K+ channel experiments, HERG K+ channel-encoding cRNAs (40 nL) were injected into the animal or vegetal pole of each oocyte one day after isolation, using a 10 μl microdispenser (VWR Scientific, San Francisco, CA, USA) fitted with a tapered glass pipette tip (15-20 μm in diameter) [9].
Data recording
A custom-made Plexiglas net chamber was used for two-electrode voltage-clamp recordings as previously reported [7]. The oocytes were impaled with two microelectrodes filled with 3M KCl (0.2-0.7 MΩ), and electrophysiological experiments were carried out at room temperature using an Oocyte Clamp (OC-725C; Warner Instruments, Hamsden, CT, USA). Stimulation and data acquisition were controlled with a pClamp 8 (Axon Instruments, Union City, CA, USA). For most electrophysiological experiments, oocytes were initially perfused with ND96 solution (in mM: 96 NaCl, 3 KCl, 2 CaCl2, 5 HEPES, pH 7.4 with NaOH) to obtain control current recordings. The oocytes were then clamped at a holding potential of –90 mV, the membrane potential was depolarized to 0 mV for 4 s, followed by repolarization to –60 mV at 20 s intervals, and current values were recorded.
Data analysis
To obtain the concentration-response curve of the respective ginsenoside metabolites effects on the K+ current from the HERG K+ channel, the peak amplitudes at various concentrations of the respective ginsenoside metabolites were plotted, and Origin software (Origin, Northampton, MA, USA) was used to fit the plot to the Hill equation: y/ymax=[A]nH/([A]nH+[IC50]nH), where y is the peak current at a given concentration of ginsenoside metabolite, ymax is the maximal peak current, EC50 is the concentration of the respective ginsenoside metabolite producing a half-maximal effect, [A] is the concentration of the respective ginsenoside metabolite, and nH is the Hill coefficient. All values were presented as the mean±SEM. The significance of differences between mean control and treatment values was determined using Student’s t-test, where p<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Effects of CK on HERG K+ channel currents
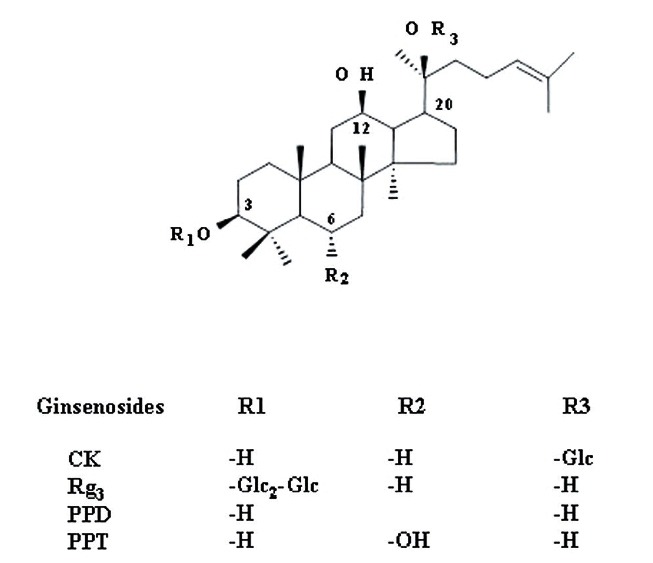
We first examined the effect of intermediate ginsenoside metabolite CK on the HERG K+ channel currents using a Xenopus oocyte gene expression system. HERG K+ channel currents were recorded using the two-electrode voltage-clamp recording technique at room temperature [10]. Throughout these experiments, the holding potential was maintained at –90 mV and repolarized to –60 mV after depolarizing pulses from 0 mV, unless otherwise indicated. Fig. 2A gives an example of a voltage-clamp recording with the representative current traces of IHERG at end of depolarization, transient peak Itail, and slow Ideactivating-tail as indicated by arrows in the absence or presence of various concentrations of CK. CK had almost no effect on IHERG and Itail (Fig. 2A). Interestingly, CK showed a major effect on slow Ideactivating-tail. Thus in the presence of CK, Ideactivating-tail failed to decay, and instead, a large persistent outward current developed. CK (100 μM) increased slow IHERG by an average of 97.8±8.13% compared to control (Fig. 2A). CK response was completely reversible upon washing the oocytes with ND96 (data not shown). Next, we examined the concentration-dependent effect of CK on IHERG, Itail, and slow Ideactivating-tail. As shown in Fig. 2B, CK had a negligible effect on both IHERG and Itail. CK also increased a persistent Ideactivating-tail in a concentration-dependent manner. The EC50 value was16.6±1.3 μM for slow Ideactivating-tail.
Fig. 2. Effects of compound K (CK) on IHERG, Itail, and Ideactivating-tail. (A) Representative current traces on human ether-a-go-go-related gene (HERG) K+ channel enhancements by various concentrations of CK. Currents were in response to 4-s voltage steps to 0 mV from a holding potential of –90 mV, followed by repolarization to –60 mV. IHERG was obtained at the end of depolarization; Itail was obtained at beginning of repolarization; slow Ideactivating-tail was obtained at the end of repolarization as indicated by arrow in (A). (B) Concentration-response curves for the activation of HERG K+ currents by CK on IHERG, Itail, and Ideactivating-tail. Solid lines were fitted to the Hill equation. CK was potent for the enhancement of Ideactivating-tail. Bars represent the means±SEM (n=5-7). (C) The representative control (upper traces) and 100 μM CK-mediated currents (down traces) in I-V relationship. Currents were recorded at test potential from –60 to + 50 mV. Itail and Ideactivating-tail were recorded after repolarization to –60 mV. (D) I-V relationships for HERG K+ currents measurement at the end of the 4-s test pulse before and after application of 3, 10, and 30 μM CK (n=5). Currents were normalized to the control current at 0 mV for each oocyte. Data are represented by the means±SEM (n=7). (E) Effects of CK on the steady-state activation curve for HERG K+ channel. Itail were normalized to the peak current under each condition, and the data were fitted with a Boltzmann function. Treatment of CK (10, 30, and 100 μM) did cause a leftward shift. Con, control.
Next, we investigated the HERG K+ current-voltage (I-V) relationship using CK (Fig. 2C, D). The amplitude of IHERG measured at the end of depolarization increased with increasing positive voltage steps, reaching a maximum at –10 mV and then progressively decreased with further voltage steps in the control, indicating that HERG K+ channel is activated with rectified manner (Fig. 2C, D). When the amplitude of IHERG was normalized to the maximum amplitude of the IHERG obtained under the control conditions and was plotted against the potential of the step depolarization (Fig. 2C, D), the presence of various concentrations of CK did not show a significant increase of IHERG even at high concentrations of CK, indicating that CK mainly affects slow Ideactivating-tail.
We next examined whether CK affects steady-state activation (Fig. 2E). Treatment of 10 and 30 μM CK caused significant leftward shifts in steady-state activation curves compared to control oocytes. The V1/2 values were –25.5±0.39 mV in the control and –24.6±0.43, –30.9±0.50 and –32.4±0.82 mV in the presence of 3, 10, and 30 μM CK-treated groups, respectively (p<0.05 at 10 and 30 μM Rg3 compared to control, n=5). The slope factors (kg) were not significantly changed, yielding values of 6.7±0.34 in control and 6.3±0.37, 6.1±0.44 and 6.5±0.70 in the presence of 3, 10 and 30 μM CK-treated groups, respectively. We also examined CK (30 μM) and observed that it did not affect steady-state inactivation (data not shown). Therefore, these results indicate that CK affects the activation gating rather than inactivation gating of HERG K+ channels.
Effects of PPT on HERG K+ channel currents
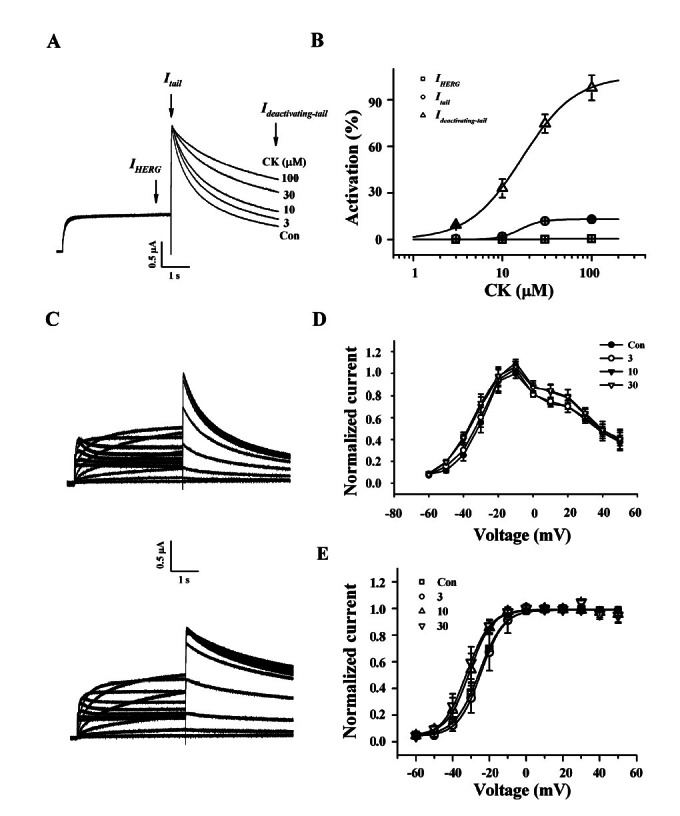
PPT had no effects on IHERG and Itail as observed with CK. However, PPT showed a significant effect on slow Ideactivating-tail. As a result, in the presence of PPT, Ideactivating-tail accelerated to decay in contrast to CK. PPT (100 μM) decreased slow Ideactivating-tail by an average of 88.2±1.3% compared to control (Fig. 3A). PPT response was also completely reversible upon washing the oocytes with ND96 (data not shown). Next, we examined the concentration-dependent effect of PPT on IHERG, Itail, and slow Ideactivating-tail. As shown in Fig. 3B, PPT showed a slight inhibition on Itail but a negligible effect on IHERG, however PPT primarily inhibited Ideactivating-tail in a concentrationdependent manner. Interestingly, PPT had a considerable effect on Ideactivating-tail compared to IHERG and Itail. The IC50 value was 27.5±2.3 μM for slow Itail, while the IC50 value was 10.6±0.5 μM for slow Ideactivating-tail.
Fig. 3. Effects of protopanaxatriol (PPT) on IHERG, Itail, and Ideactivating-tail. (A) Representative current traces on human ether-a-go-go-related gene (HERG) K+ channel inhibitions by various concentrations of PPT. Currents were in response to 4-s voltage steps to 0 mV from a holding potential of –90 mV, followed by repolarization to –60 mV. IHERG was obtained at the end of depolarization; Itail was obtained at beginning of repolarization; slow Ideactivating-tail was obtained at the end of repolarization as indicated by the arrow. (B) Concentration-response curves for the inhibition of HERG K+ currents by PPT on IHERG, Itail, and Ideactivating-tail. Solid lines were fitted to the Hill equation. PPT was potent for the inhibition of Ideactivating-tail. Bars represent the means±SEM (n=5-7). (C) The representative control (upper traces) and 100 μM PPT-mediated currents (down traces) in I-V relationship. Currents were recorded at test potential from –60 to +50 mV. Itail and Ideactivating-tail were recorded after repolarization to –60 mV. (D) I-V relationships for HERG K+ currents measurement at the end of the 4-s test pulse before and after application of 3, 10, and 30 μM PPT (n=5). Currents were normalized to the control current at 0 mV for each oocyte. Data are represented by the means±SEM (n=7). (E) Effects of PPT on the steady-state activation curve for HERG K+ channel. Itail were normalized to the peak current under each condition, and the data were fitted with a Boltzmann function. Treatment of PPT (3, 10, and 30 μM) did not cause a leftward shift. Con, control.
Next, we examined the HERG K+ current-voltage (I-V) relationship using PPT (Fig. 3C, D). The amplitude of IHERG measured at the end of depolarization increased with increasing positive voltage steps, reaching a maximum at –10 mV and then progressively decreased with further voltage steps in the control, indicating that HERG K+ channel rectifies (Fig. 3C, D). When the amplitude of IHERG was normalized to the maximum amplitude of the IHERG obtained under the control conditions and was plotted against the potential of the step depolarization (Fig. 3C, D), the presence of various concentrations of PPT did not show a significant increase of IHERG even at high concentrations of PPT, indicating that PPT mainly affects slow Ideactivating-tail.
We next set forth to determine whether PPT affects steady-state activation (Fig. 3E). Treatment of 10 and 30 μM PPT did induce slightly but not significant leftward shifts in steady-state activation curves compared to control oocytes. The V1/2 values were –22.6±0.37 mV in the control and –28.7±1.35, –29.1±1.43 and –30.1±0.46 mV in the presence of 3, 10, and 30 μM PPT-treated groups, respectively (p<0.08 at 10 and 30 μM Rg3 compared to control, n=5). The slope factors (kg) were not significantly changed, yielding values of 7.9±0.33 in control and 4.8±0.09, 5.2±1.29 and 6.3±0.39 in the presence of 3, 10 and 30 μM PPT-treated groups, respectively. We also examined PPT (100 μM) and observed that it did not affect steady-state inactivation (data not shown). Therefore, these results indicate that PPT affects the activation gating rather than inactivation gating of HERG K+ channels.
Effects of PPD on HERG K+ channel currents
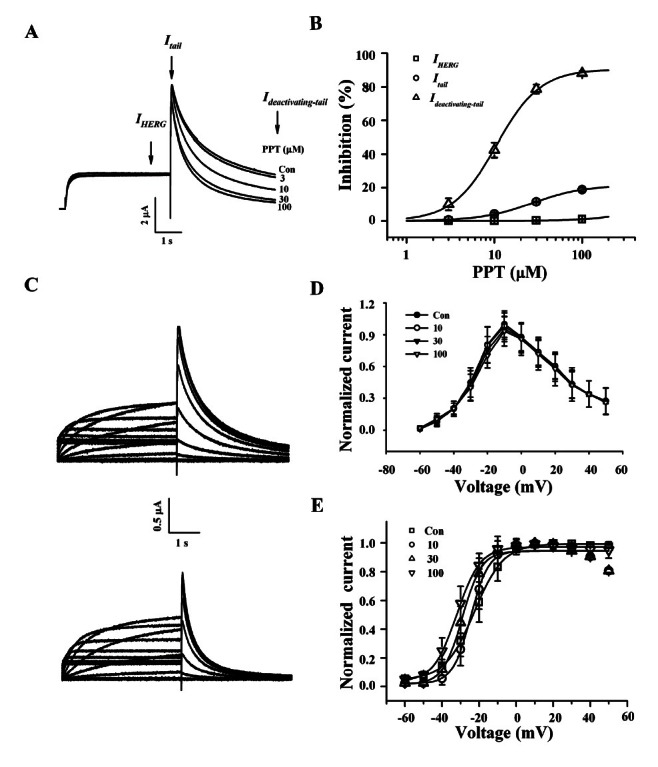
PPD itself had nearly no effects on IHERG, Itail, and slow Ideactivating-tail (Fig. 4A). However, PPD showed an inhibition on Rg3-mediated enhancement on slow Ideactivating-tail. Hence in the presence of PPD, Rg3-induced persistent Ideactivating-tail was greatly attenuated. PPD effect on Rg3-induced persistent Ideactivating-tail was completely reversible upon washing the oocytes with ND96 (data not shown). We subsequently examined the concentration-dependent effect of PPD on Rg3-induced persistent Ideactivating-tail. As shown in Fig. 4B, PPD showed a negligible effect on IHERG and Itail but PPD greatly attenuated Rg3-induced persistent Ideactivating-tail in a concentration-dependent manner. Interestingly, PPD caused a rightward shift of Rg3-induced persistent Ideactivating-tail from 0.5±0.04 to 2.9±0.57 μM for slow Ideactivating-tail (Fig. 4C).
Fig. 4. Effects of protopanaxadiol (PPD) on Rg3-mediated IHERG, Itail, and Ideactivating-tail. (A) Representative current traces on human ether-a-go-go-related gene (HERG) K+ channel inhibitions by various concentrations of PPD itself (left panel). Currents were in response to 4-s voltage steps to 0 mV from a holding potential of –90 mV, followed by repolarization to –60 mV. IHERG was obtained at the end of depolarization; Itail was obtained at beginning of repolarization; slow Ideactivating-tail was obtained at the end of repolarization as indicated by the arrow. Concentration-dependent inhibitions of HERG K+ currents by PPD on Rg3-mediated IHERG, Itail, and Ideactivating-tail (right panel). (B) I-V relationships for HERG K+ currents measurement at the end of the 4-s test pulse before (upper traces) and after application of 1 μM Rg3 plus 100 μM PPD (down traces). Currents were normalized to the control current at 0 mV for each oocyte (right panel). Data are represented by the means±SEM (n=7). (C) Concentration-response curves for the activation of HERG K+ currents by Rg3 in the absence or presence of PPD on IHERG, Itail, and Ideactivating-tail. Solid lines were fitted to the Hill equation. PPD significantly affected Ideactivating-tail. Bars represent the means±SEM (n=5-7). (D) Effects of PPD on Rg3-mediated the steady-state activation curve for HERG K+ channel. Itail were normalized to the peak current under each condition, and the data were fitted with a Boltzmann function. Treatment of 100 μM PPD in the presence of 0.3, 1, or 3 μM Rg3 did not cause a leftward shift. Con, control.
We then studied the HERG K+ current-voltage (I-V) relationship using PPD on Rg3-induced enhancement of IHERG (Fig. 4B). The amplitude of IHERG measured at the end of depolarization increased with increasing positive voltage steps, reaching a maximum at –10 mV and then progressively decreased with further voltage steps in the control, indicating that HERG K+ channel rectifies (Fig. 4B). When the amplitude of IHERG was normalized to the maximum amplitude of the IHERG obtained under the control conditions and was plotted against the potential of the step depolarization (Fig. 4B), the presence of various concentrations of PPD did not show a significant increase of IHERG even at high concentrations of PPD, indicating that PPD mainly affects Rg3-induced persistent Ideactivating-tail.
Our following goal was to examine whether PPD affects steady-state activation (Fig. 3E). In contrast to CK or PPT, co-treatment of 10 and 30 μM PPD with Rg3 did not affect on Rg3-caused leftward shifts in steady-state activation curves compared to control oocytes. The V1/2 values were –21.7±0.18 mV in the control and –21.2±0.35, –24.6±0.27 and –24.6±0.28 mV in the presence of 3, 10, and 30 μM PPD-treated groups, respectively (p<0.07 at 10 and 30 μM Rg3 compared to control, n=5). The slope factors (kg) were not significantly changed, yielding values of 7.9±0.16 in control and 8.7±0.31, 7.5±0.24 and 7.5±0.25 in the presence of 3, 10 and 30 μM PPD-treated groups, respectively. We also examined PPD (100 μM) and observed that it did not affect steady-state inactivation (data not shown). These results suggest PPD affects the activation gating rather than inactivation gating of HERG K+ channels.
Ginsenosides consist of aglycone and carbohydrates portions. The aglycone is the backbone of the ginsenoside, with a hydrophobic four-ring steroid-like structure that may be non-polar, whereas the carbohydrates on carbons-3, 6, and 20 of the backbone are polar (Fig. 1A). In vitro and in vivo studies have shown that ginsenosides administered by an oral route were metabolized and finally become an agylcone such as CK which has one glucose at the C-20, PPD and PPT [8]. Recent report showed that these ginsenoside metabolites might also induce apoptosis of the cancer cells and play a role as an anti-cancer agent [8,11], suggesting that ginsenosides are pro-drugs of these metabolites. However, relatively little is known regarding how ginsenoside metabolites regulate ion channel or receptor activity.
In the present study, we report three major findings. First, we observed that CK decelerated deactivating time constants in both concentration- and voltage-dependent manners, whereas CK had no effects on IHERG and Itail, Second, we found that PPT accelerated Ideactivating-tail deactivation in both concentration- and voltage-dependent manners without affecting on IHERG and Itail. Third, PPD itself had no effect on IHERG, Itail, and Ideactivating-tail. However, co-treatment of PPD with Rg3 blocked Rg3-mediated decelerating effects on Ideactivating-tail. Thus, the major findings of the present study are that ginsenoside metabolites had no significant effects on IHERG and Itail of HERG K+ channel but they did affect Ideactivating-tail in differing manners, indicating that ginsenoside metabolites show a differential regulation of Ideactivating-tail with respect to the HERG K+ channel.
On the other hand, we demonstrated in previous studies that ginsenoside metabolites such as CK, PPD and PPT regulate ion channels and receptors. For example, CK but not PPT caused strong inhibition of the voltage-dependent α1G-type Ca2+ channel [12]. Similarly, we have also found that CK but not M4 (PPT) inhibited a neuronal Na+ (Nav1.2) channel [13]. We demonstrated that M4, but not CK, exhibited an inhibitory effect on 5-HT3A receptor-mediated currents [14]. In addition, CK and PPT exhibited an inhibitory effect on α3β4 nicotinic acetylcholine receptor-mediated currents [15]. All these findings indicate that ginsenoside metabolites as well as ginsenosides have regulatory effects on voltage-dependent ion channel and receptor activities.
Since the HERG K+ channel regulators are clinically important for treatment of cardiac diseases such as arrhythmia, there are numerous reports on the development of HERG K+ channel regulators from natural compounds [16]. In the previous study, we found that Rg3 increased IHERG and peak Itail. In addition, Rg3 profoundly delayed deactivation kinetics by inducing a persistent Ideactivating-tail and caused a leftward shift of steady-state voltage-dependent activation but not inactivation, leading to the possibility that Rg3 is an activator to open HERG K+ channel at a more negative voltage step than control and that Rg3 mainly works by slowing closure of the HERG K+ channel once it has opened. In the present study, when we examined the effects of ginsenoside metabolites on HERG K+ channel currents, we found that CK induced a persistent Ideactivating-tail and caused a leftward shift of steady-state voltage-dependent activation (Fig. 2). However, the effective concentration of CK was higher than that of Rg3. In contrast to CK, PPT caused an acceleration of Ideactivating-tail decay (Fig. 3). Interestingly, co-treatment of PPD with Rg3 blocked Rg3-mediated deceleration of deactivation process. It is noteworthy that since Rg3 became PPD after metabolic process, PPD-mediated antagonism of Rg3 action on HERG K+ channel activity show the possibility that aglycone, PPD, ginsenoside Rg3 and other PPD ginsenosides might interfere with in vivo Rg3 actions on HERG K+ channel activity.
Taken together, we found that CK decelerated the deactivation process by inducing a persistent Ideactivating-tail with delayed deactivation in HERG K+ channel without affecting IHERG and Itail. In contrast, PPT accelerated the deactivation process by inducing an inhibition of Ideactivating-tail without affecting IHERG and Itail. PPD itself had no observable effects on IHERG, Itail, and Ideactivating-tail but co-treatment of PPD with Rg3 blocked Rg3-mediated a persistent Ideactivating-tail with delayed deactivation in HERG K+ channel. These novel findings provide an insight into the molecular action of ginsenoside metabolites on HERG K+ channel activity.
Acknowledgments
This study was supported by The grant 2010 from the Korean Society of Ginseng funded by Korea Ginseng Corporation, Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093824), and BK21 to S. Y. Nah.
References
- 1.Hille B. Ion channels of excitable membranes. Sinauer Associates; Sunderland: 2001. [Google Scholar]
- 2.Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001;90:1–19. doi: 10.1016/S0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- 3.Tristani-Firouzi M, Sanguinetti MC. Structural determinants and biophysical properties of HERG and KCNQ1 channel gating. J Mol Cell Cardiol. 2003;35:27–35. doi: 10.1016/S0022-2828(02)00286-9. [DOI] [PubMed] [Google Scholar]
- 4.Nah SY. Ginseng, recent advances and trends. Korean J Ginseng Sci. 1997;21:1–12. [Google Scholar]
- 5.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Shin TJ, Lee BH, Chu DH, Choe H, Pyo MK, Hwang SH, Kim BR, Lee SM, Lee JH, et al. Ginsenoside Rg3 activates human KCNQ1 K+ channel currents through interacting with the K318 and V319 residues: a role of KCNE1 subunit. Eur J Pharmacol. 2010;637:138–147. doi: 10.1016/j.ejphar.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Choi SH, Shin TJ, Lee BH, Hwang SH, Lee BC, Park CS, Nah SY. Ginsenoside Rg3 decelerates HERG K channel deactivation through Ser631 residue interaction. Eur J Pharmacol. 2011 doi: 10.1016/j.ejphar.2011.05.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol Pharm Bull. 2002;25:861–866. doi: 10.1248/bpb.25.861. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Lee BH, Choi SH, Yoon IS, Pyo MK, Shin TJ, Choi WS, Lim Y, Rhim H, Won KH, et al. Ginsenoside Rg3 inhibits human Kv1.4 channel currents by interacting with the Lys531 residue. Mol Pharmacol. 2008;73:619–626. doi: 10.1124/mol.107.040360. [DOI] [PubMed] [Google Scholar]
- 10.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 1998;246:725–730. doi: 10.1006/bbrc.1998.8690. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Jeong SM, Kim JH, Lee BH, Yoon IS, Lee JH, Choi SH, Lee SM, Park YS, Lee JH, et al. Effects of ginsenosides and their metabolites on voltage-dependent Ca(2+) channel subtypes. Mol Cells. 2006;21:52–62. [PubMed] [Google Scholar]
- 13.Kim JH, Hong YH, Lee JH, Kim DH, Nam G, Jeong SM, Lee BH, Lee SM, Nah SY. A role for the carbohydrate portion of ginsenoside Rg3 in Na+ channel inhibition. Mol Cells. 2005;19:137–142. [PubMed] [Google Scholar]
- 14.Lee BH, Jeong SM, Lee JH, Kim DH, Kim JH, Kim JI, Shin HC, Lee SM, Nah SY. Differential effect of ginsenoside metabolites on the 5-HT3A receptor-mediated ion current in Xenopus oocytes. Mol Cells. 2004;17:51–56. [PubMed] [Google Scholar]
- 15.Lee JH, Jeong SM, Lee BH, Kim DH, Kim JH, Kim JI, Lee SM, Nah SY. Differential effect of bovine serum albumin on ginsenoside metabolite-induced inhibition of alpha3beta4 nicotinic acetylcholine receptor expressed in Xenopus oocytes. Arch Pharm Res. 2003;26:868–873. doi: 10.1007/BF02980034. [DOI] [PubMed] [Google Scholar]
- 16.Scholz EP, Zitron E, Kiesecker C, Luck S, Thomas D, Kathofer S, Kreye VA, Katus HA, Kiehn J. Schoels W et al. Inhibition of cardiac HERG channels by grapefruit flavonoid naringenin: implications for the influence of dietary compounds on cardiac repolarisation. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:516–525. doi: 10.1007/s00210-005-1069-z. [DOI] [PubMed] [Google Scholar]


