Abstract
The antioxidant activities of fermented red ginseng (FRG) were investigated in vitro and in vivo. The contents of total polyphenol and total flavonoid in FRG extracts were 17.01±2.00 μg/mg and 18.42±3.97 μg/mg, respectively. These extracts were capable of directly scavenging α, α-diphenyl-picrylhydrazyl free radicals. The antioxidative effects of the FRG extracts in streptozotocin (STZ)-induced diabetic rats were also investigated. The activities of plasma alanine transaminase, aspartate transaminase, and γ-glutamyltransferase were significantly decreased by extract administration as compared to an STZ control group. Hepatic glutathione content depleted by STZ treatment was significantly increased by treatment of the FRG extracts, but the elevation of lipid peroxide content induced by STZ was significantly decreased by the extracts. Activities of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase decreased after STZ-treatment were recovered by the treatment of the FRG extracts. These results indicate that FRG extracts have antioxidative effets in STZ-induced diabetic rats.
Keywords: Panax ginseng, Red ginseng, Fermentation, Antioxidant effect, Streptozotocin
INTRODUCTION
According to a recently published World Health Organization report, it is estimated that over one million seven thousand people around the world were affected by diabetes in 2000, and diabetes incidence will only continue to increase in the future, affecting approximately three million six thousand people by 2030 [1]. It is expected that the number of diabetic patients will dramatically increase in Korea as well, owing to bad lifestyle and dietary choices in addition to a sharp increase in the obese population [2,3].
Diabetes mellitus is a chronic metabolic disease that is characterized by high serum glucose levels. The insufficiency of insulin secretion or increase in insulin resistance seen in this disease state leads to dysfunction of not only glucose metabolism, but also the metabolic regulation of protein, lipids, and electrolytes [4]. In particular, the free radicals generated in diabetic patients through sustained chronic high blood glucose levels and associated glycosylation processes, promote lipid peroxidation and increase endogenous oxidative stress, inducing cellular damage and engendering hyperlipidaemia, hypertension, coronary atherosclerosis, diabetic retinopathy, and many other diabetic complications [5,6].
Oxidative stress is one of the most important mechanisms of diabetic complication genesis, and the imbalance between the production of active oxygen and its opposing protective mechanisms is known to be associated with even greater cellular damage, a phenomenon that is seen in diabetic patients. Furthermore, free radicals are reported to destroy beta cells that produce and secrete insulin [7,8].
On the other hand, antioxidants can decrease diabetic complications by protecting the body from oxidative stress, and examples of anti-oxidating protective agents include anti-oxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), as well as anti-oxidant nutrients such as vitamin C, vitamin E, and selenium [9]. Even as anti-oxidant enzymes represent an important protective mechanism against the toxicity of active oxygen, decreased levels of antioxidant enzyme activity are seen in diabetic patients, contributing to the genesis of diabetic complications [10]. This decreased antioxidant enzyme activity in diabetic patients is thought to be due to the glycosylation of enzymes and depletion by oxygen free radicals, and inefficient glycosylation of anti-oxidant enzymes in the hyperglycaemic state is known to induce changes in enzymatic structure and function, effectively reducing activity levels [11].
Ginseng has long been known to possess a variety of beneficial pharmacological effects, such as enhancement of immunity, and hyperlipidaemia and diabetes prevention [12,13]. Red ginseng possesses enhanced pharmacological effects through its production process involving the washing of raw ginseng followed by steaming, drying, and red ginseng formation, allowing numerous chemical changes such as saponin deformation, amino acid changes, and browning reactions, in order to concentrate the active principles [14,15]. The pharmacological components of red ginseng include saponin-type ginsenosides and non-saponin type polyacetylene panaxatriol, panaxadiol, oxidative polysaccharides, and amino acids. These components are known to have beneficial effects in immunity enhancement, diabetes mellitus, cancer, fatigue and stress, and hyperlipidaemia [16-20].
Recent studies on the effectiveness of saponin components that are newly produced and converted in red ginseng fermentation [21] have shown that 30 types of metabolic factors, including Rb1, Rb2, Rc, and Rd, are converted to chemical components such as compound K to enable endogenous absorption by intestinal microbial flora. Compound K has also been associated with anti-cancer activities, and anti-diabetes and immune-enhancing effects [22]. Fermentation can also increase the effectiveness of pharmacological factors in red ginseng through easier and more effective endogenous absorption via degradation into small molecules, as well as disintegration of any toxic, heavy metal, or pesticide residues, and attenuation of their toxicity [23].
Our research team previously established that more types of ginsenosides were present in red ginseng fermented by Lactobacillus fermentum (Lac. fermentum) NUC-C1 NUC-C1 than normal red ginseng, and that contents of Rg3 and Rh2, which have anti-diabetic activities, greatly increased [24]. Treatment of fermented red ginseng (FRG) resulted in higher oral glucose tolerance test levels in streptozotocin (STZ)-induced diabetic rats as compared to normal red ginseng, as well as decreased levels of serum intermediate fats and total cholesterol content and significantly decreased serum glucose concentration and disaccharidase activity levels in the small intestine. It also significantly increased serum insulin concentration, indicating it had anti-diabetic effects [24].
Administration of STZ decreases the activity levels of enzymes by producing endogenous reactive oxygen species (ROS) and directly attacking antioxidant enzymes [25]. In particular, ginsenoside Rh2 is known to contribute to the ability of red ginseng saponins to induce anti-oxidation [26]. Considering the greatly increased level of Rh2 content in red ginseng fermented by Lac. fermentum NUC-C1, FRG is thought to also contribute to increased activities of antioxidant enzymes in STZ-administered rats.
Therefore, this study aimed to assess the in vitro and in vivo anti-oxidative abilities of FRG extracts prepared from red ginseng solution fermented with Lac. fermentum NUC-C1. We assessed total polyphenol and total flavonoid contents and α, α-diphenyl-picrylhydrazyl (DPPH) scavenging effects of FRG extracts, as well as evaluated the effects the FRG extracts had on antioxidant enzyme activities in STZ-induced diabetic rats.
MATERIALS AND METHODS
Samples
The FRG used in this study was produced at the Bio Research Institute of NUC Electronics Co., Ltd. (Daegu, Korea). A 2 L amount of purified water was added to 300 g of 6-year-old white dried ginseng, purchased from Geumsan Insam Nong-Hyup (Geumsan, Korea), and steamed for 24 h. A further 4 L of distilled water was added, and the ginseng solution was steamed at 90℃ for 48 h to make the red ginseng (RG) extracts. The RG extracts were then used to make FRG extracts by the addition of 0.1% Lac. fermentum NUC-C1(KCCM10929P) and 12 h of fermentation at 40℃. The RG and FRG extracts were both dried in vacuo (Eyela; Tokyo Rikakikai Co., Tokyo, Japan), freeze-dried and pulverized, and finally stored at -20℃ until used for experiments.
The total polyphenol and total flavonoid contents of fermented red ginseng
The total polyphenol contents were measured using the Folin-Denis method [27] adapted to 96-well plates, and total polyphenol concentration was calculated from a calibration curve generated using tannic acid as a standard. The results were expressed as mg/L of tannic acid equivalents.
Also, total flavonoid contents of the samples were determined using the method of Moreno et al. [28] with some modifications. Total flavonoid content was calculated by comparisons with a calibration curve generated using quercetin as a standard. The results were expressed as mg/L of quercetin equivalents.
DPPH radical scavenging effects
The free radical scavenging activities of samples were determined by measuring the reducing power of the stable radical DPPH. This involved dissolving each sample in 99% methanol to different concentrations, and combining 800 μL of diluted solution with 200 μL of 0.15 mM DPPH solution that had been dissolved in methanol. The reagent solution was then left at room temperature for 30 min, followed by measurement of optical density at 517 nm. The free radical scavenging activity of each sample solution was presented as an RC50 value, which is the concentration of sample needed to change the optical density level of a control group without sample addition to 1/2. The free radical scavenging activity was calculated from a standard curve of known concentration of butylated hydroxyanisole.
Induction of diabetes in experimental animals
Sprague-Dawley rats (Orient Bio Inc., Seongnam, Korea) weighing 180±10 g were used. Their cages were maintained at 23±2℃, with a humidity of 60±5%, and 12/12-h light/dark cycles. The rats were fed solid rat food and water ad libitum for 1 week to adjust them to the environment; they were then used in the subsequent 3 weeks, with close adherence to the university’s ethical guidelines for animal experiments. The animals were divided into one normal control group (NC) and three diabetes induction groups. The diabetes induction groups were further divided into diabetes control (DM) and FRG groups (FRG administered at 100 mg/kg [FRG100] and FRG administered at 200 mg/kg [FRG200]). Six rats were maintained for 3 wk in each group. The NC and DM groups were fed only a commercial standard rat diet (Sam Yang Co., Seoul, Korea), whereas the FRG groups were orally administered 100 or 200 mg/kg FRG extracts dissolved in water, at 10 a.m. daily. The sample concentrations were determined in preliminary experiments. The rats that had undergone a 1-week adjustment period for diabetes induction were fasted for at least 12 h, and then a peritoneal injection of STZ, diluted in 0.01 M citrate buffer, was given at 60 mg/kg. In the NC group, the same concentration of normal saline was used for the peritoneal injection. Blood collected from tail veins was used to confirm diabetes induction, and the rats with fasting blood glucose levels above 300 mg/dL were used in experiments.
Preparation of enzyme source
At the end of the 3 wk treatment period after an overnight fast, the rats were sacrificed under ether anesthesia, and blood samples were collected in heparinized tubes. The collected blood was centrifuged at 3,000 rpm for 15 min, and the plasma was stored at -80℃ until processed. After blood collection, the liver was removed immediately, washed in ice-cold saline, and weighed after blotting on filter paper. The liver was dissected and homogenized in 0.1 M phosphate buffer (pH 7.4). The homogenates were then centrifuged at 600 ×g for 10 min at 4℃ to remove nuclear fractions, and the remaining separated supernatant (microsomal fraction) was re-centrifuged at 10,000 ×g for 20 min at 4℃ to collect the mitochondrial fraction (pellet) for a CAT assay. The supernatant was ultra-centrifuged at 100,000 ×g for one h at 4℃ to isolate the cytosolic fraction for SOD, GPx, and GR assays. The supernatant was used to assess the activities of antioxidant enzymes as described below, and the amount of protein was measured using BSA as a standard, at 660 nm [29].
Determination of hepatic glutathione and lipid peroxide contents
Hepatic glutathione (GSH) content was determined by the method of Ellman [30] based on the development of a yellow color when 5,5’-dithio-bis-2-nitrobenzoic acid is added to compounds containing sulfhydryl groups. Reduced GSH was used as a standard to calculate micromoles of –SH content/g tissue. Lipid peroxidation in the microsomes, which were prepared from the liver, was estimated using the thiobarbituric acid-reactive substance (TBARS) method as described by Ohkawa et al. [31] and was expressed in terms of malonaldehyde formed per milligram of protein.
Measurement of enzyme activity
The activities of plasma alanine transaminase (ALT) and aspartate transaminase (AST) were measured as a marker of liver injury using the method of Reitman and Frankel [32]. For the analysis of ALT and AST, plasma was made to react with 100 mM phosphate buffer (pH7.4), 800 mM L-alanine, 0.18 mM nicotinamide adenine dinucleotide (NADH), and 3.7 U/mL ALT and then allowed to stand for 3 min. This was followed by the addition of 18 mM 2-oxoglutatrate. ALT and AST activities were measured as the rate of loss of ß-NADH absorption at 340 nm for 2 min. Plasma γ-glutamyltransferase (GGT) activity was also measured with Szasz’s method [33]. The plasma was added ot 40 mM glycine buffer (pH 8.2) and then activity was measured by the formation of p-nitroaniline at 405 nm.
CAT was assayed on a UV spectrophotometer at 240 nm by monitoring the decomposition of H2O2 as described by Aebi [34]. The specific activity of CAT was expressed as nmoles of H2O2 reduced/min/mg protein. SOD was assayed by the method of Marklund and Marklund [35]. The activity level of SOD was measured by the amount of enzyme that inhibited 50% of cytochrome C defined as 1 unit. GPx was measured by the coupled assay method as described by Paglia and Valentine [36]. One unit of enzyme activity has been defined as nmoles of NADPH consumed/min/mg protein based on an extinction coefficient of 6.66 mM/cm. Glutathione reductase (GR) activity was determined using Mize and Langdon’s method [37]. For measurement of enzymatic activity, the amount of glutathione created by 1 mg of protein per min was presented in mM.
Statistics
The means and standard deviations of the data were calculated. Evaluations of each group’s significance were performed using SAS ver. 8 (SAS Institute, Cary, NC, USA) at the 5% significance level with Duncan’s multiple range test.
RESULTS AND DISCUSSION
Total polyphenol and total flavonoid contents, and DPPH scavenging effects
Polyphenol compounds which exist in plants, can act as natural antioxidants. The total polyphenol and flavonoid contents present in red ginseng and FRG extracts were measured using tannic acid and quercetin as standard samples, respectively (Fig. 1).
Fig. 1. Total polyphenol and flavonoid contents of water extracts from red ginseng (RG) and fermented red ginseng (FRG). Micrograms of total polyphenol and total flavonoid content/mg of samples based on tannic acid and quercetin as standard, respectively. Each value is mean±SD (n≥3).
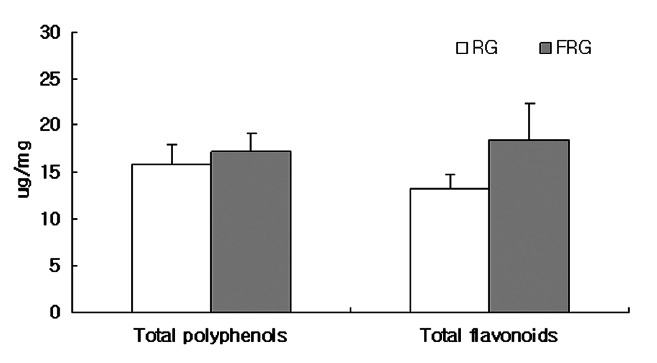
According to the results, the total polyphenol (TP) and total flavonoid (TF) contents of the red ginseng extracts were 15.79±15 μg/mg and 13.20±1.63 μg/mg, respectively. While TP and TF contents of the FRG extracts were 17.01±2.00 μg/mg and 18.42±3.97 μg/mg, respectively, showing higher levels than the red ginseng extracts; however, there were no significant differences. Furthermore, the RC50 values for the DPPH scavenging effects of the red ginseng and FRG extracts were 484.46±21.98 μg/mL and 259.42±2.13 μg/mL, respectively, indicating that the FRG had superior DPPH scavenging effects (Table 1). It is thought that the increased total polyphenol and flavonoid contents of FRG as compared to red ginseng lead to greater DPPH scavenging effects.
Table 1.
Scavenging effects of water extracts from RG and FRG, and BHA on DPPH•
| Sample | Concentration ((μg/mL) | Scavenging effect (%) | RC501) ((μg/mL) |
|---|---|---|---|
|
| |||
| RG | 300 | 34.93±5.08 | 484.46±21.98 |
| 500 | 50.60±5.55 | ||
| 700 | 55.57±2.26 | ||
| FRG | 100 | 32.37±7.50 | 259.42±2.13 |
| 300 | 58.65±4.59 | ||
| 500 | 61.12±4.31 | ||
| BHA | 1 | 32.83±1.13 | 3.33±1.12 |
| 5 | 79.20±2.98 | ||
| 10 | 90.26±4.53 | ||
Each value is mean±SD (n≤3).
RG, red ginseng; FRG, fermented red ginseng; BHA, butylated hydroxyanisole; DPPH•, α,α-diphenly-β-picrylhydrazyl radicals.
1)Concentration required for 50% reduction of DPPH• at 30 minutes after starting the reaction.
The ethanol extracts of 5-year-old ginseng and 10-year-old cultured wild ginseng were reported to have DPPH scavenging effects with RC50 values of 14.55 and 4.86 mg/mL, respectively [38]. Considering this, it can be noted that RG and FRG are created by the steaming and maturation of ginseng for ‘FRG manufacturer’. RG ethanol extracts made from the steaming and maturation of water ginseng have greater anti-oxidant activity [39], and red ginseng’s ginsenosides Rb1 and Rg1 have lipid oxidant suppression and antioxidant activities [19]. Furthermore, red ginseng’s ginsenoside Rh2 can significantly increase antioxidant enzymatic activity, thus showing antioxidant effects [26,40]. FRG has more various types of ginsenosides compared to RG, and although Rb1 and Rh2 which are known to have antioxidant activities could not be detected in RG, FRG showed Rb1 and Rh2 levels of 0.14 mg% and 2.57 mg%, respectively. Similarly, while Rg3 was found to be present in ginseng at 23.62 mg%, in FRG it increased greatly to 95.11 mg% [24].
Therefore, even though antioxidant effects are present in both FRG and RG, FRG has a much greater level of antioxidant activity than RG.
ALT, AST, and GGT levels in plasma
The activities of ALT and AST, which are liver-specific enzymes detected in plasma, were measured from animals that were fed for three weeks after oral administration of FRG extracts to STZ-induced diabetic mice. Plasma ALT and AST activities were increased significantly in the STZ- induced DM compared to the NC group as shown in Fig. 2, and decreased significantly upon oral administration of the FRG extracts. When 200 mg/ kg of FRG extract was administered as compared to 100 mg/ kg, ALT and AST activities decreased to a greater extent; however with no significant differences. Since the administration of FRG brought about decreases in AST and ALT activity, which had previously been increased due to STZ administration, it is thought that the FRG extracts decreased aminotransferase activity, thus having a protective effect against STZ-induced liver damage.
Fig. 2. Effect of fermented red ginseng (FRG) extracts on the activities of plasma aspartate transaminase (A) and alanine transaminase (B) in streptozotocin-induced rats. NC (n=6), normal control group; DM (n=6), diabetic mellitus group; FRG100 (n=6), diabetic group fed with 100 mg/kg of FRG; FRG200 (n=6), diabetic group fed with 200 mg/kg of FRG. a,b,cMean values with different superscripts in the same column are significantly different (p˂0.05).
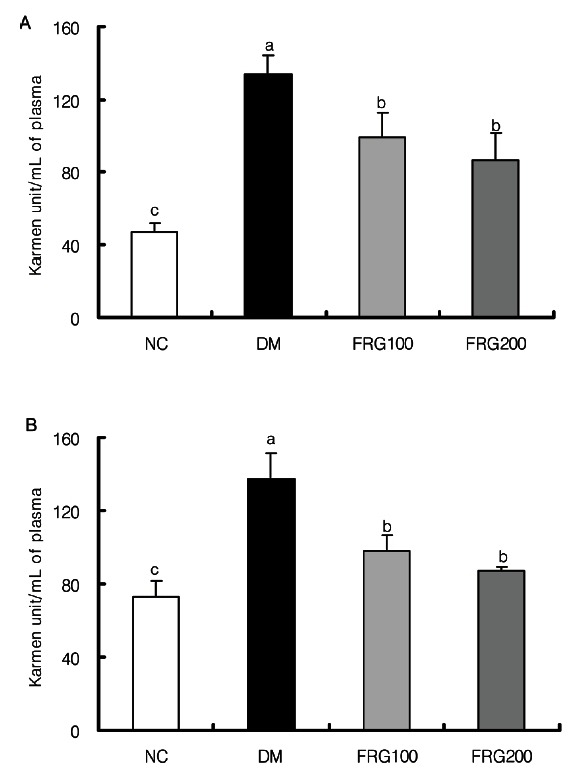
Plasma GGT activity increased significantly in the DM group compared to the NC group as shown in Fig. 3; however, it decreased upon administration of the FRG extracts. A significant decrease was observed in the FRG 100 mg/kg administration group compared to the DM group, but no significant difference was observed between FRG 200 mg/kg and the DM group. The significant decrease in GGT activity upon administration of FRG 100 mg/kg was similar to a report where a group receiving FRG 100 mg/kg had superior blood glucose lowering effects and insulin secretion stimulation effects than a group receiving FRG 200 mg/kg [24], thus leading to the suggestion that the optimal concentration of FRG extract metabolized in the liver is 100 mg/kg.
Fig. 3. Effect of fermented red ginseng (FRG) extracts on the activities of plasma γ -glutamyltransferase in streptozotocin-induced rats. NC (n= 6), normal control group; DM (n= 6), diabetic mellitus group; FRG100 (n= 6), diabetic group fed with 100 mg/ kg of FRG; FRG200 (n= 6), diabetic group fed with 200 mg/ kg of FRG. a,bMean values with different superscripts in the same column are significantly different (p˂ 0.05) by Duncan’ s multiple range test.
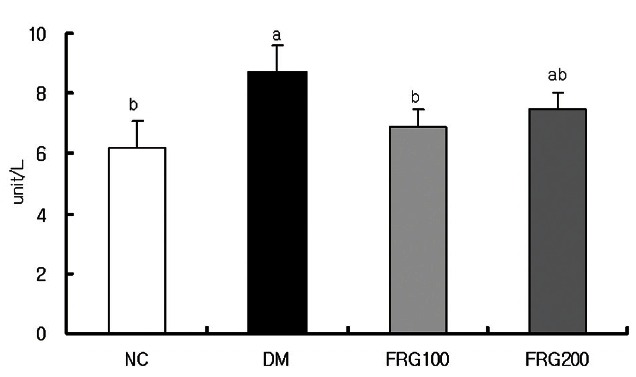
Increases in GGT are reported to be due to oxidative stress-related mechanisms in diseases like diabetes, aging, obesity, and hypertension [41,42]. STZ-induced diabetic mice had increases in GGT activity that were decreased by the administration of FRG. This is thought to be due to high levels of total polyphenols, flavonoids, and ginsenosides in FRG having antioxidant activity. A similar report showed that as concentrations of vitamin A, vitamin C, lutein, and lycopene (which all have antioxidant activity) increased, plasma GGT activity decreased [43].
Hepatic glutathione and lipid peroxide contents
In terms of changes in hepatic glutathione content, the DM group had significant decreases compared to the NC group, and a significant increase in glutathione content was shown in both the FRG 100 and FRG 200 groups regardless of the concentration of FRG extract administered. This is thought to be due to STZ’s absorption into the body, inducing an oxidative stress environment and hence glutathione is used up to buffer the oxidative effects leading to decreased glutathione content. However, with the administration of the FRG extracts, this reduced the need for glutathione, leading to an increase in its content.
Furthermore, a significant increase was shown in hepatic lipid peroxide levels in the DM group compared to the NC group. In the FRG extract groups, hepatic lipid peroxide showed a significant decrease compared to only the STZ-administered group, and there was no significant difference according to FRG extract concentration (Table 2). Because observed increases in hepatic lipid peroxide and MDA contents upon diabetes induction and increased oxidative stress have been reported [44], decreases in lipid oxidants within liver tissue upon administration of the FRG extracts were thought to be due to the extracts aiding the removal of free radicals in vivo, leading to antioxidant defense effects.
Table 2.
Effect of FRG extracts on the contents of GSH and lipid peroxide in liver of streptozotocin-induced rats
| Group (n=6) | GSH content (μmoles/g of tissue) | Lipid peroxide content (MDA μmoles/g of tissue) |
|---|---|---|
|
| ||
| NC | 64.49±7.57a | 118.67±5.56b |
| DM | 46.15±3.05b | 141.19±6.64a |
| FRG100 | 58.81±4.05a | 122.80±6.85b |
| FRG200 | 53.37±2.34a | 110.27±7.03b |
FRG, fermented red ginseng; GSH, glutathione; MDA, malondialdehyde; NC, normal control group; DM, diabetic mellitus group; FRG100, diabetic group fed with 100 mg/kg of FRG; FRG200, diabetic group fed with 200 mg/kg of FRG. a,bMean values with different superscripts in the same column are significantly different (p˂0.05) by Duncan's multiple range test.
This is further supported by a report stating that plasma TBARS concentrations were lowered upon administration of ginseng leaf extracts to diabetic rats, due to the radical scavenging effects of polyphenols that are present in ginseng leaves [45].
Anti-oxidant enzyme activities
SOD is an enzyme that primarily contributes to cellular defenses against oxidative stress, and plays a role in the conversion of superoxide anion to hydrogen peroxide. SOD enzyme activity was not significantly different between the DM and NC groups as shown in Fig. 4A, but the FRG extract groups showed a significant increase in SOD activity compared to the DM group, leading to the suggestion that free radical production was inhibited due to increased SOD activity secondary to FRG extract administration.
Fig. 4. Effect of fermented red ginseng (FRG) extracts on the activities of superoxide dismutase (A) and catalase (B) in streptozotocin-induced rats. NC (n= 6), normal control group; DM (n= 6), diabetic mellitus group; FRG100 (n= 6), diabetic group fed with 100 mg/ kg of FRG; FRG200 (n= 6), diabetic group fed with 200 mg/ kg of FRG. 1)1 unit of superoxide dismutase activity was defined as which inhibited the oxidation of pyrogallol by 50% . a,bMean values with different superscripts in the same column are significantly different (p˂0.05) by Duncan’ s multiple range test.
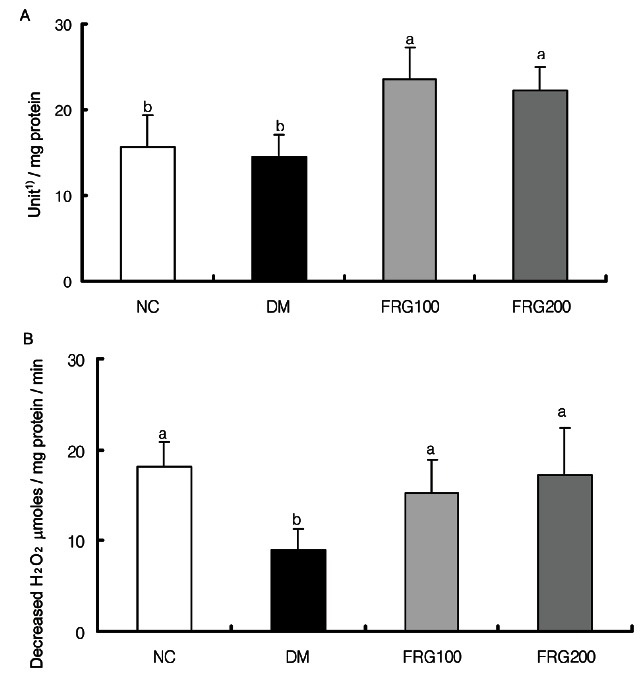
CAT, which is an enzyme that protects the body from active oxygen-induced oxidative damage by converting endogenous H2O2 to H2O, was significantly decreased in the DM group compared to the NC group, followed by a significant increase compared to the DM group upon FRG extract administration (Fig. 4B). It is thought that the increase in CAT activity was induced in order to degrade hydrogen peroxide produced by SOD activity. However, there were no differences observed between SOD and CAT activities secondary to the different concentrations of FRG extract.
GSH-Px activity was significantly decreased in the DM group compared to the NC group as shown in Fig. 5A, and had a significant increase upon FRG extract administration. There was a significant increase in GSH -Px activity by administration of the 100 mg/kg FRG extract but not the 200 mg/kg FRG extract. The activity of GR, which is an enzyme that indirectly contributes to cellular protection and maintenance of homeostasis by maintaining a reduced state of the glutathione pool in cells using oxidation-reduction reactions [46], showed a greater decrease in the DM group compared to the NC group as shown in Fig. 5B, and GR activity was significantly increased in the FRG administered groups compared to the DM group. Therefore, the data demonstrate that FRG contributed to increased activities of GSH-Px and GR, which were previously significantly decreased due to STZ-administration.
Fig. 5. Effect of fermented red ginseng (FRG) extracts on the activities of glutathione peroxidase (A) and glutathione reductase (B) in streptozotocin-induced rats. NC (n=6), normal control group; DM (n=6), diabetic mellitus group; FRG100 (n=6), diabetic group fed with 100 mg/kg of FRG; FRG200 (n=6), diabetic group fed with 200 mg/kg of FRG. a,b,cMean values with different superscripts in the same column are significantly different (p˂0.05) by Duncan's multiple range test.
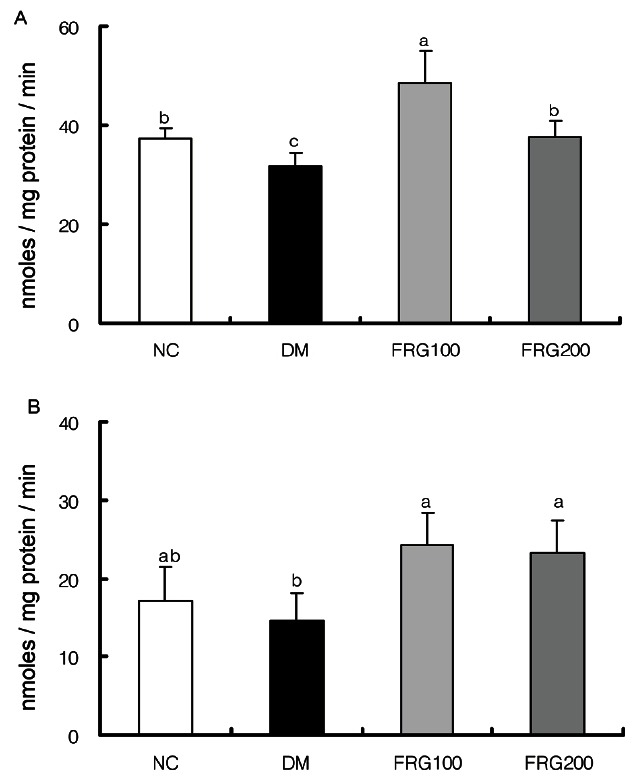
Similar to a report showing a decrease in antioxidant enzymatic activity due to the direct attack of STZ-administration-produced ROS against endogenous antioxidant enzymes [25], the DM group had a consistent decrease in all SOD, CAT, GSH-Px, and GR activities. Upon administration of the FRG extract, there was an increase in the activities of these antioxidant enzymes, and this is supported by a report where increases in the activity levels of SOD, CAT and GSH-Px were observed when ginseng leaf extract was administered to diabetic rats [45]. In particular, it is thought that ginsenosides such as Rh2 that are present in FRG, induce the expression of antioxi-dant enzymes and activate antioxidant enzyme activity, thus leading to potent antioxidant ability [26,40]. Further comprehensive research on ginsenoside types present in FRG is required.
In the case of SOD and GSH-Px activity, there was a greater increase in their activity levels upon administration of 100 mg/kg FRG extract, rather than 200 mg/kg FRG extract. The FRG 100 mg/kg administered group showed much more superior blood glucose lowering and insulin secretion increasing effects compared to the FRG 200 mg/kg administered group [24], leading to the suggestion that 100 mg/kg was the optimal administration concentration metabolized in the rat's liver.
An increase in a combination of different eliminating enzymes, rather than an increase in any single enzyme, is generally more effective in the inhibition of radical-induced cellular damage [47]. It is thought that FRG extracts augment the activity of SOD, CAT, GSH-Px, and GR enzymes to eliminate harmful radical oxygen species produced by STZ administration, resulting in protecting the body from cellular damage. Therefore, FRG had greater antioxidant effects than red ginseng as well as augmented antioxidant enzymatic activity to eliminate STZ administration-induced harmful reactive oxygen species and increased glutathione content that had previously been decreased, all leading to protection of the body from cellular damage.
Acknowledgments
This work was supported by Regional Industry Technology Development Project in year 2008 (project no. 70002701), and by Center for Traditional Microorganism Resources, Keimyung University under the support of Ministry of Knowledge and Economy.
References
- 1.Korea National Statistical Office. Available from: http://kostat.go.kr/portal/korea/index.action The cause of death statistics 2008: 2009 annual report on the cause of death statics.
- 2.Park YM, Sohn CM, Jang HC. A study on status and subjective recognition of functional foods among diabetic patients. J Korean Diet Assoc. 2005;11:216–222. [Google Scholar]
- 3.Georg P, Ludvik B. Lipids and diabetes. J Clin Basic Cardiol. 2000;3:159–162. [Google Scholar]
- 4.Sakurai T, Tsuchiya S. Superoxide production from nonenzymatically glycated protein. FEBS Lett. 2006;236:406–410. doi: 10.1016/0014-5793(88)80066-8. [DOI] [PubMed] [Google Scholar]
- 5.Lones TJ. Oxidized low density lipoproteins: a role in the pathogenesis of atherosclerosis in diabets. Diabetes Med. 1991;8:411–419. doi: 10.1111/j.1464-5491.1991.tb01624.x. [DOI] [PubMed] [Google Scholar]
- 6.Tai ES, Lim SC, Tan BY, Chew SK, Heng D, Tan CE. Screening for diabetes mellitus: a two-step approach in individuals with impaired fasting glucose improves in detection of those at risk of complications. Diabetes Med. 2000;17:771–775. doi: 10.1046/j.1464-5491.2000.00382.x. [DOI] [PubMed] [Google Scholar]
- 7.Adeghate E, Parvez SH. Nitric oxide and neuronal and pancreatic beta cell death. Toxicology. 2000;153:143–156. doi: 10.1016/S0300-483X(00)00310-3. [DOI] [PubMed] [Google Scholar]
- 8.Laybutt DR, Kaneto H, Hasenkamp W, Grey S, Jonas JC, Sgroi DC, Groff A, Ferran C, Bonner-Weir S, Sharma A, et al. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to beta-cell survival during chronic hyperglycemia. Diabetes. 2002;51:413–423. doi: 10.2337/diabetes.51.2.413. [DOI] [PubMed] [Google Scholar]
- 9.Mexwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 10.West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–180. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 11.Lalla E, Lamster IB, Drury S, Fu C, Schmidt AM. Hyperglycemia glycoxidation and receptor for advanced glycation endproducts: potential mechanisms underlying diabetic complications, including diabetes-associated periodontitis. Periodontol. 2000;23:50–62. doi: 10.1034/j.1600-0757.2000.2230104.x. [DOI] [PubMed] [Google Scholar]
- 12.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 13.Yun SH, Joo CN. Study on the preventive effect of ginsenosides against hypercholesterolemia and its mechanism. Korean J Ginseng Sci. 1993;17:1–12. [Google Scholar]
- 14.Ko SK, Lee CR, Choi YE, Im BO, Sung JH, Yoon KR. Analysis of ginsenosides of white and red ginseng concentrates. Korean J Food Sci Technol. 2003;35:536–539. [Google Scholar]
- 15.Kim ND. Pharmacological effects of red ginseng. J Ginseng Res. 2001;25:2–10. [Google Scholar]
- 16.Lee B, Heo H, Oh S, Lew J. Comparison study of Korean and Chinese ginsengs on the regulation of lymphocyte proliferation and cytokine production. J Ginseng Res. 2008;32:250–256. doi: 10.5142/JGR.2008.32.3.250. [DOI] [Google Scholar]
- 17.Kim US, Koh HK, Kang SK. Study of the effects of different products of ginseng radix aqua-acupuncture on the alloxan-induced diabetic rats. J Korean Acupunct Moxibustion Soc. 1989;6:1–13. [Google Scholar]
- 18.Yun TK, Lee YS, Lee YH, Yun HY. Cancer chemopreventive compounds of red ginseng produced from Panax ginseng C.A. Meyer. J Ginseng Res. 2001;25:107–111. [Google Scholar]
- 19.Kim DJ, Seong KS, Kim DW, Ko SR, Chang CC. Antioxidative effects of red ginseng saponins on paraquat-induced oxidative stress. J Ginseng Res. 2004;28:5–10. doi: 10.5142/JGR.2004.28.1.005. [DOI] [Google Scholar]
- 20.Lee CK, Choi JW, Kim H, Han YN. Biological activities of acidic polysaccharide of Korean red ginseng. II. Effects on hyperlipidemia induced by alcohol. J Ginseng Res. 1999;23:8–12. [Google Scholar]
- 21.Trinh HT, Han SJ, Kim SW, Lee YC, Kim DH. Bifidus fermentation increases hypolipidemic and hypoglycemic effects of red ginseng. J Microbial Biotechnol. 2007;17:1127–1133. [PubMed] [Google Scholar]
- 22.Kim DH. Metabolism of ginsenosides to bioactive compounds by intestinal microflora and its industrial application. J Ginseng Res. 2009;33:165–176. doi: 10.5142/JGR.2009.33.3.165. [DOI] [Google Scholar]
- 23.Bae EA, Han MJ, Kim EJ, Kim DH. Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res. 2004;27:61–67. doi: 10.1007/BF02980048. [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, Chae IG, Lee SG, Jeong HJ, Lee EJ, Lee IS. Effects of fermented red ginseng extracts on hyperglycemia in streptozotocin-induced diabetic rats. J Ginseng Res. 2010;34:104–112. doi: 10.5142/jgr.2010.34.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirwaikar A, Rajendran K, Kumar D, Bodla R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in dtreptozotocin-nicotineamide type 2 diabetic rats. J Ethnopharmacol. 2004;91:152–178. doi: 10.1016/j.jep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Lai DM, Tu YK, Liu IM, Chen PF, Cheng JT. Mediation of beta-endorphin by ginsenoside Rh2 to lower plasma glucose in sterptozotocin-induced diabetic rats. Planta Med. 2006;72:9–13. doi: 10.1055/s-2005-916177. [DOI] [PubMed] [Google Scholar]
- 27.Folin O, Denis W. On phosphotungastic- phosopho- molybdic compounds as color reagents. J Biol Chem. 1912;12:239–249. [Google Scholar]
- 28.Moreno MI, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000;71:109–114. doi: 10.1016/S0378-8741(99)00189-0. [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Ellman GL. Tissue sulfhydryl group. Arch Biochem Biophys. 1959;82:70–72. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 31.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 32.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 33.Szasz G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin Chem. 1969;15:124–136. [PubMed] [Google Scholar]
- 34.Aebi H. Catalase. In: Bergmeyer HU, ed. Methods of enzymatic analysis. Academic Press; New York: 1974. pp. 673–698. [Google Scholar]
- 35.Marklund S, Marklund CT. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 36.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 37.Mize CE, Langdon RG. Hepatic glutathione reductase: I. Purification and general kinetic properties. J Biol Chem. 1962;237:1589–1592. [PubMed] [Google Scholar]
- 38.Joung EM, Hwang IG, Lee MK, Cho SK, Chung BH, Jo SJ, Lee SH, Lee JS, Jeong HS. Ginsenoside compositions and antioxidant activity of cultured and mountain ginseng. J Agric Life Sci. 2010;44:61–67. [Google Scholar]
- 39.Kim SI, Ko SH, Lee YJ, Choi HY, Han YS. Antioxidant activity of yogurt supplemented with red ginseng extract. Korean J Food Cookery Sci. 2008;24:358–366. [Google Scholar]
- 40.Kim JS, Kim KW, Choi KJ, Kwak YK, Im KS, Lee KH, Chung HY. Screening of antioxidative components from red ginseng saponin. Korean J Ginseng Sci. 1996;20:173–178. [Google Scholar]
- 41.Drozdz R, Parmentier C, Hachad H, Leroy P, Siest G, Wellman M. Gamma-glutamyltransferase dependent generation of reactive oxygen species from a glutsthione/ transferrin system. Free Radic Biol Med. 1998;25:786–792. doi: 10.1016/S0891-5849(98)00127-0. [DOI] [PubMed] [Google Scholar]
- 42.Cho HC. The association between serum GGT concentration and diabetic peripheral polyneuropathy in type 2 diabetic patients. Korean Diabetes J. 2010;34:111–118. doi: 10.4093/kdj.2010.34.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR Jr, Lee DH. Is serum gamma-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic Biol Med. 2004;37:1018–1023. doi: 10.1016/j.freeradbiomed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 44.Latha M, Pari L. Modulatory effect of Scoparia dulcis in oxidative stress-induced lipid peroxidation in streptozotocin diabetic rats. J Med Food. 2003;6:379–386. doi: 10.1089/109662003772519958. [DOI] [PubMed] [Google Scholar]
- 45.Park SN, Choi SW, Boo YC, Kim CK, Lee TY. Effects of flavonoids of ginseng leaves on erythrocyte membranes against singlet oxygen caused damage. Korean J Ginseng Sci. 1990;14:191–199. [Google Scholar]
- 46.Kitamura JF, Yamazaki T, Camba EA, Sato K. Change in activities of glutathione reductase during chemical hepatocarcinogenesis. Gunn. 1983;74:649–655. [PubMed] [Google Scholar]
- 47.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-B. [DOI] [PubMed] [Google Scholar]


