Abstract
Red ginseng (RG, the extract of Panax ginseng Meyer) has various biological and psychological activities and may also alleviate fatigue-related disorders. The present study was undertaken to evaluate what kind of fatigue red ginseng alleviate. Animals were orally administered with 50, 100, 200, 400 mg/kg of RG for 7 days. Before experiments were performed. Physiological stress (swimming, rotarod, and wire test) are behavioral parameters used to represent physical fatigue. Restraint stress and electric field test to a certain degree, induce psychological fatigue in animals. Plasma concentration of lactate and corticosterone (CORT) were also measured after these behavioral assays. RG supplementation (100 mg/kg) increased movement duration and rearing frequency of restrainted mice in comparison with control. 100 and 200 mg/kg of RG increased swimming time in cold water (8±4℃) while at 100 mg/kg, RG increased electric field crossing over frequencies. 50, 100 and 200 mg/kg RG prolonged running time on the rotarod and at 100 mg/kg, it increased balancing time on the wire. RG at those doses also reduced falling frequencies. RG supplementation decreased plasma CORT levels, which was increased by stress. Lactate levels were not significantly altered. These results suggest that RG supplementation can alleviate more the damages induced by psychological than physical fatigue.
Keywords: Panax ginseng, Red ginseng water extract, Restraint stress, Swimming test, Behavior
INTRODUCTION
Ginseng (Panax ginseng Meyer) which contains a series of tetracyclic triterpenoid saponins (ginsenosides) is one of the most popular medicinal plants throughout the world because of its beneficial effects [1]. Red ginseng (RG) is prepared by steaming the root prior to drying. RG is widely known to contain more pharmacologically active effects than white ginseng [2,3]. Ginsenosides can be classified into three groups on the basis of the chemical structure of their sapogenins (aglycones): the panaxadiol group (e.g., Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh1); the panaxatriol group (e.g., Re, Rf, Rg1, Rg2, Rh1); and the oleanolic acid group (e.g., Ro) [4,5]. Ginsenosides Rg3, Rg5, and Rk1 were the most abundant ginsenosides in the ginseng steamed at higher temperature [6]. Jin et al. [7] suggested that the ratio of ginsenosides may be an important factor in the pharmacological effects of ginseng extracts [5]. Pharmacological effects of ginseng have been demonstrated in the central nervous system and in cardiovascular, endocrine, and immune systems. In addition, ginseng and its constituents have been ascribed antineoplastic, anti-stress, and antioxidant activity [8]. It enhances resistance to temperature stress, physical exercise, and increases swimming time in rats [9]. It has also long been used traditionally for the treatment of psychiatric diseases such as anxiety and depression [6]. It has long been considered to act as an adaptogen, but the mechanisms underlying its effect are still unclear. It is also unclear which kind of fatigue it counteracts more effectively.
Adaptogens have been defined as “natural metabolic regulators that increase the ability of organism to adapt to environmental factors and to avoid damage from such factors” [10,11]. The mode of action of adaptogens is not completely understood, but modulation of catecholamines and other stress mediators (e.g., cortisol and nitric oxide) has been proposed [11,12]. Tonic and adaptogenic effects of ginseng are believed to enhance physical performance and general vitality in healthy individuals, to increase the body’s ability to fight stress in stressful circumstances and to support resistance to diseases by strengthening normal body function as well as to reduce the detrimental effects of the aging processes [13]. Bhattacharya and Muruganandam [14] suggested that P. ginseng had significant antistress adaptogenic activity. The antistress activities of ginseng may account for its observed clinical efficacy in stress-related disorders like depression and anxiety [15,16]. Some of the ‘adaptogenic’ effects of ginseng are attributed to its actions on the hypothalamic-pituitary-adrenal axis, resulting in elevated plasma corticotrophin and corticosteroids levels [9].
In previous studies, we reported that RG and sun ginseng attenuated the response to stress through physiological effects and enhancement of physical capacity [17,18]. The role of RG in stressed animals remains to be determined, although there are a few reports stating that RG reduces the behavioral signs of fatigue-related disorders.
Our experiments were performed to identity what kind of fatigue RG supplementation alleviates. To measure the ability of RG to counteract physiological fatigue, mice were subjected to physiological stress (swimming, rotarod, and wire test), on the other hand restraint stress and electric field test represent psychological fatigue.
MATERIALS AND METHODS
Materials
RG water extract standard preparation was obtained from Korea Ginseng Corporation (Seoul, Korea). Corticosterone (CORT) were obtained from Sigma (St. Louis, MO, USA). Other unstated chemicals and reagents were of analytical grade.
Animals and treatments
The male ICR mice (17-20 g) were supplied by Hanlim Laboratory Animals Co. (Hwaseong, Korea). They were housed in animal room which was maintained at temperature (22±2℃) and humidity (55±5%) under a 12/12-h light/dark cycle with lights on from 7:00 AM. Food and water were available ad libitum. After this stabilization period, animals were orally administered with 50, 100, 200 and 400 mg/kg of RG for 1, 3 or 7 days. Enduring activities after restraint, hanging on the wire, running on the rotarod, swimming in the cold water and crossing frequency over an electric field were noted. Animal treatment and maintenance were carried out in accordance with the Principles of Laboratory Animal Care (National Institutes of Health publication no. 85- 23, revised 1985), Korea and the Animal Care and Use Guidelines of Sahmyook University, Korea.
Psychological stress
Overcoming electroshock
The electroshock test was performed in a Plexiglas box (42×27×15 cm) with a stainless steel grid floor. The box was composed of two parts: an electric stimulator and a glass operation box. At the top of the box, there was a water bottle with its mouth 5 cm away from the stainless steel floor. Mice were deprived of water for 24 h and were then individually placed in the test chamber for 20 min at 0.5 mA with free access to the drinking bottle. RG was given 30 min prior to the test. Mice were put into the box and the frequency crossing over an electroshock was recorded.
Induction of stress
Mice were exposed to restraint stress (RS) after having been orally administered with RG 30 min before the tests. Mice were subjected to RS by being placed once in a translucent, well ventilated, conical propylene holder (3 cm in diameter and 7 cm in length) for 120 min [18]. After loading stress, stress-related behavioral changes of the animals were recorded and plasma CORT was measured.
Physical stress
Cold swimming test
The mice were forced to swim in cold water maintained at 8±2℃. The apparatus is a circular water tank (diameter, 150 cm; height, 50 cm) made of stainless steel. The pool was filled with water to a depth of 15 cm. The mice were allowed to swim in the pool until they're exhausted to the point of drowning. After the test, the mice were gently dried by patting their bodies with paper towel. The time spent swimming in the water was recorded manually using a stopwatch [19].
Rotarod test
The previous day of the experiment, all mice were habituated to running on a rotarod at a speed of 36 rpm for 120 s. Next morning, the latency time to fall and falling frequency for 20 min were recorded.
Balanced wire test
Like the rotarod test, mice were habituated to grasp horizontal wires (5 mm diameter, 150 cm length) with their forepaws and tails elevated 80 cm above the floor. The balanced wire test was carried out for 20 min, and the latency time to fall and falling frequency were recorded.
Locomotor activity
Computerized EthoVision system (Noldus Information Technology, Wageningen, The Netherlands) was used to evaluate changes in locomotor activity. The observation apparatus consisted of nine plastic boxes (42×42) with a field bordered by 42-cm-high sidewalls. The total distance moved and total movement times were monitored for 10 min after stress [20].
Elevated plus-maze test
The plus maze was elevated to a height of 50 cm and consisted of a black plastic area in the shape of a plus, with two open arms (50×10 cm for rats) and two closed arms (50×10×30 cm for rats) alternating at right angles. The central crossing point (10×10 cm) of the arms was not enclosed. Mice were placed in the central square facing the open arm and allowed to explore the maze freely for 5 min. The time spent in the open arms and the closed areas were automatically recorded by the computerized EthoVision system [20].
Stress-related rearing behavior
Rearing was defined as rearing around the wall. Rearing frequency was recorded automatically by EthoVision system for 10 min.
Plasma corticosterone assay
After monitoring behavioral assays, the mice were sacrificed immediately. Blood samples (1.5 mL) were collected through heart puncture. The plasma CORT level was measured by a modified method [19] using HPLC system composed of SI-2 3001 pum, SI-2 3002 UVVisible detector, SI-2 3004 column oven, separation (Shiseido, Tokyo, Japan), and column Capcell Pak C8 MG 120 (5 μm, 1.5 mm∅×250 mm). CORT was used as the internal standard. Twenty microliters of treated sample solution were injected into the HPLC column using acetonitrile: 50 mM NaH2PO4 (33:67) as the mobile phase at a flow rate of 500 μL/min. CORT level was determined from the absorbance at 240 nm using the dsCHROM-computing program (Shiseido, Tokyo, Japan).
Plasma lactate analysis
Levels of lactate in plasma were determined by double antibody technique using a commercial kit (BioVision, Mountain View, CA, USA). Enzymeimmuno assay was performed according to the manufacturer’s instructions.
Statistical analysis
Data were expressed as the mean±SEM. For statistical evaluation of data, one-way ANOVA was used. When statistically significant differences were found, Newman- Keul's test was used as a post-hoc test to determine the statistical differences between groups. Differences were considered statistically significant when p<0.05.
RESULTS AND DISCUSSION
Effects of red ginseng on psychological fatigue-resistance activities
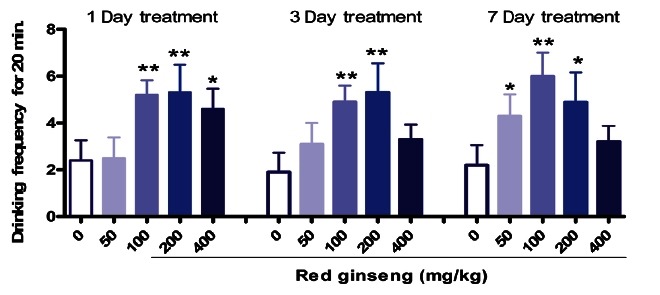
The electric field test was conducted to evaluate overcoming of psychological stress. RG supplementation significantly increased crossing frequency over an electric field (Fig. 1) most especially at the dosages of 100 and 200 mg/kg. Some studies have demonstrated a reduction of stress or its physiological accompaniments in animals. Nguyen et al. [21] suggested that Vietnamese ginseng crude saponin suppresses psychological and electroshock stress (ES)-induced antinociception. RG and white ginseng reduced conflict behavior in thirsty and ES-induced fighting in paired mice [22].
Fig. 1. Effect of red ginseng on psychological fatigue-resistant activities in mice (n=10). Each bar represents the mean±SEM of crossing frequency of electric field for 20 min. Control group vs. red ginseng extract. *p<0.05, **p<0.01.
Effects of red ginseng extract on locomotor activity
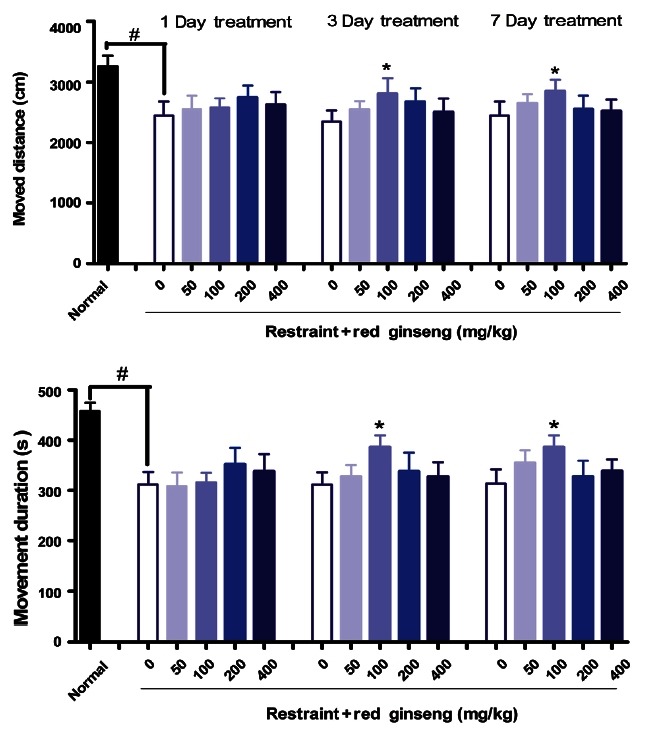
As shown in Fig. 2, stress significantly decreased locomotor activity in the open-field. RG supplementation (100 mg/kg for 3 or 7 d) significantly increased locomotor activity (p<0.05). Without stress, the water extract of RG (100 mg/kg) did not alter the locomotor activity in the open-field [6]. Pilot studies indicated that single-dose administration of ginseng had little to no acute behavior effects [22]. In our study, long term rather than single administration of RG had significant effects on locomotor activities in mice.
Fig. 2. Effects of red ginseng extracts on locomotor activity after loading restraint for 2 hours in mice (n=9-10). Each bar represents the mean±SEM of moved distance and movement duration for 10 minutes (#p<0.05, *p<0.05 vs. control group).
Effects of red ginseng extract on plasma corticosterone levels
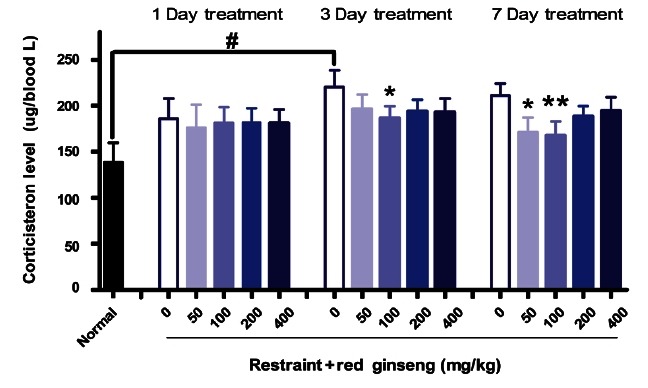
Psychological fatigue comes with depression, anxiety, and other psychological conditions. Corticosteroids play extremely important roles in fear and anxiety. Glucocorticoid secretion serves both to alert the organism to environment or physiologic changes and to maintain homeostasis under stressful conditions [23]. RG has diverse effects on the central nervous system, promotes stimulation as well as inhibits cortical activity [6]. Total ginseng Saponin (TGS) administered intraperioneally increased plasma CORT and adrenocorticotropic hormone (ACTH) level in basal state, but pretreatment of animals with TGS (5 and 20 mg/kg) significantly attenuated the immobilization stress-induced increase in plasma CORT levels [23]. Such effects of TGS in rodents indicate that the inhibitory effects of TGS administered on stress-induced plasma CORT level appear to be mediated by blocking of ACTH action peripherally in adrenal gland [16]. As shown in Fig. 3, stress increased CORT release but RG supplementation (100 mg/kg) for 3 (p<0.05) or 7 d (p<0.01), significantly suppressed the production of CORT after loading RS.
Fig. 3. Effects of red ginseng extracts on plasma corticosterone level after restraint for 2 h in mice (n=9-10). Each bar represents the mean±SEM of the corticosterone levels (#p<0.05, *p<0.05, **p<0.01 vs. control group).
Effects of RG extract on exploratory activity in the elevated plus maze and rearing behavior
Behaviors on the elevated plus maze (EPM) reflect anxiety or curiosity in mice. Animals exposed to stress spend less time in the open arms, exhibiting anxiogenic-like effects [24]. Ginseng has long been used traditionally for the treatment of psychiatric diseases such as anxiety and depression. Park et al. [6] showed that the water extract of RG did not influence activities on the EPM. However, Carr et al. [25] found that crude saponin ginseng fraction and ginsenoside RB1 significantly increased the percentage time of open area in EPM in a dose-dependent manner. Wei et al. [26], also demonstrated that American ginseng had anxiolytic-like effect, which increased the percentage of time in open arms. Our results showed that RG had a partial anxiolytic-like activity. As shown in Fig. 4, stress decreased the staying percentage in the open arms of the EPM. RG supplementation (100 mg/kg) increased staying times in the open arms of the EPM after 3 or 7 d of treatment.
Fig. 4. Effects of red ginseng extracts on stress-related specific behaviors induced by restraint stress in mice (n=8-10). Each bar represents the mean±SEM of rearing frequency for 10 min (A), % of time spent in each area over 5 min (B) (#p<0.05, *p<0.05 vs. control group).
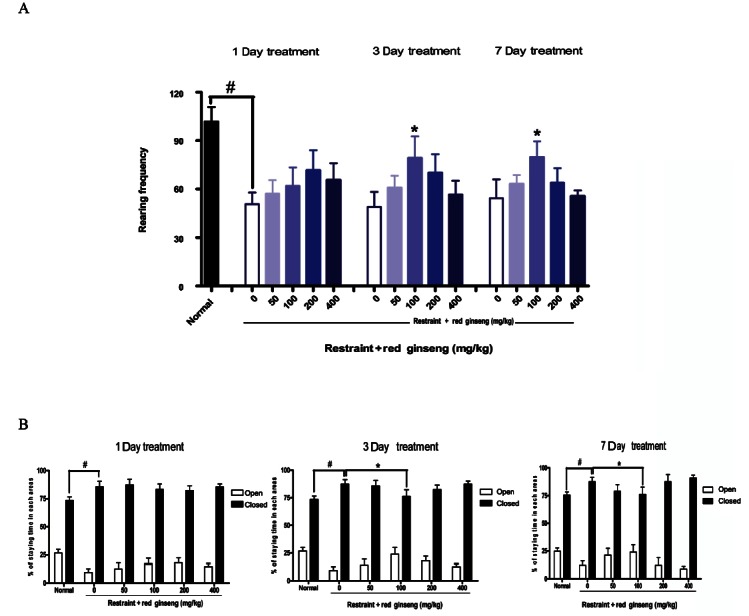
“Normal” behaviors of an animal (e.g., rearing) are affected with anxiety [27]. In our previous study, we confirmed that rearing behavior was decreased by stress but RG supplementation alleviated it [18]. As shown in Fig. 4, stress significantly decreased rearing frequency but RG (100 mg/kg) significantly increased it after 3 or 7 d of treatment. RG treatment may counteract stress-induced anxiety-related behaviors. The anxiolytic-like effects of ginseng may involve GABAergic mechanisms [6]. Our results support that ginseng may be useful for treatment of stress-related disorders.
Effects of red ginseng extract on activities on the rotating rod and the balanced wire
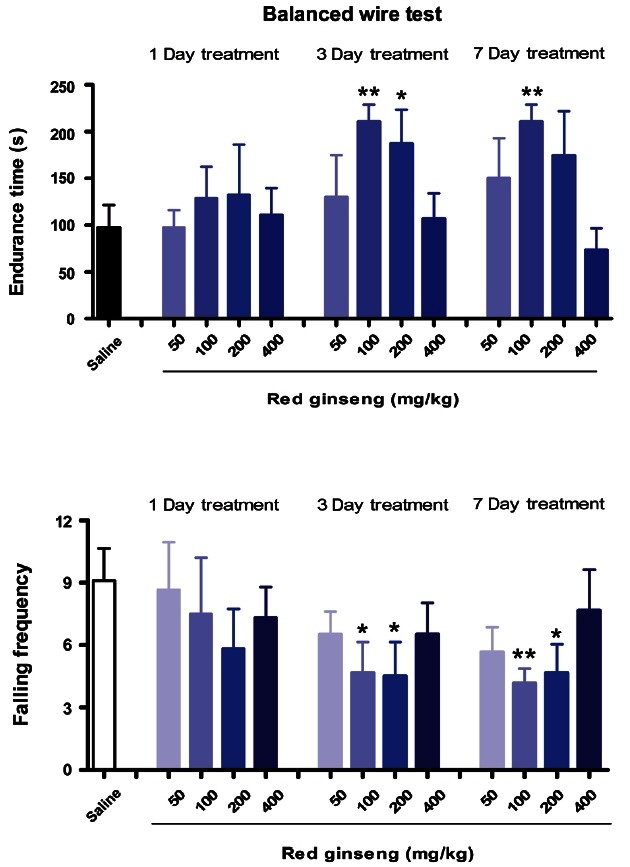
As shown in Fig. 5, a single dose of RG (200 mg/kg) significantly increased endurance time on the rotating rod. RG supplementation (100 mg/kg) significantly increased endurance time and decreased falling frequency after 3 or 7 d of treatment. Endurance times on the rotarod were also increased by RG treatment at these doses and corresponding durations: 50 mg/kg for 7 d and 200 mg/kg for 3 d. As shown in Fig. 6, the 100 mg/kg RG dose significantly increased endurance time and decreased falling frequency on the wire after 3 or 7 d of treatment. RG (200 mg/kg) also significantly increased endurance times after 3 d of treatment and decreased falling frequency after 3 or 7 d of supplementation. Filaretov et al. [28] showed that a single administration of ginseng leads to significant increases of working capacity up to 132% in a treadmill running test, and up to 179% with 7 d administration.
Fig. 5. Effects of red ginseng extract on activity on the rotating rod in mice (n=9-10). Each bar represents the mean±SEM of endurance time on the rotating rod (*p<0.05, **p<0.01 vs. control group).
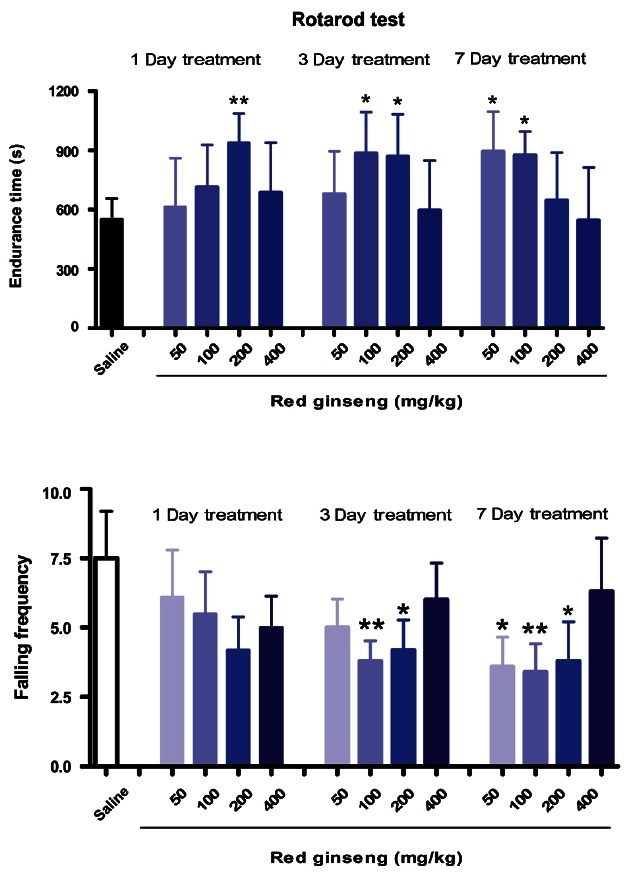
Fig. 6. Effects of red ginseng extract on activity on the balanced wire in mice (n=9-10). Each bar represents the mean±SEM of endurance time on the balanced wire (*p<0.05, **p<0.01 vs. control group).
The effects of red ginseng extract on activity in the swimming pool in mice
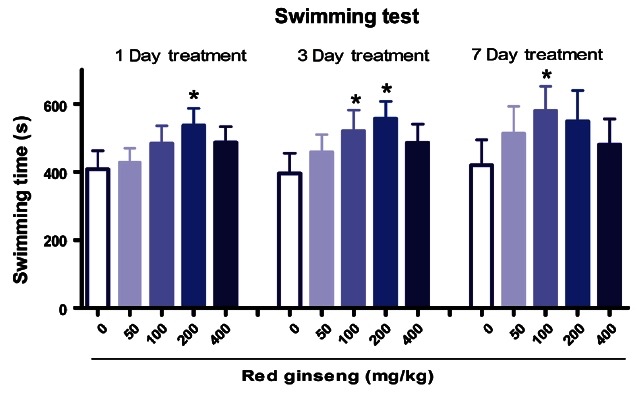
Fig. 7 showed that RG (200 mg/kg) significantly increased swimming time in cold pool after treatment for 1 or 3 d, whereas at the dosage of 100 mg/kg, swimming times were increased after 3 or 7 d of treatment. Wang et al. [29] found that ginseng polysaccharides had anti-fatigue activities. The physiological effect of fatigue is on energy metabolism of muscular activity, and the improvement of exercise endurance is the most powerful representation of anti-fatigue enhancement [30]. Grandhi et al. [31] reported a significant increase in mice swimming time after ginseng administration.
Fig. 7. Effects of red ginseng extract on activity in the swimming pool in mice (n=10). Each bar represents the mean±SEM of endurance time in the swimming pool (*p<0.05, **p<0.01 vs. saline treated group).
Effects of red ginseng extract on plasma lactate levels
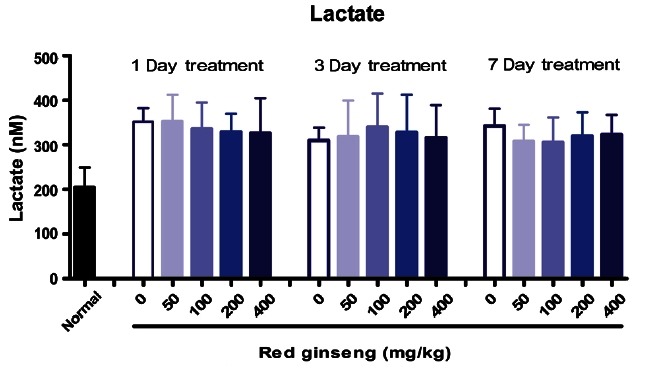
Lactic acid levels represent blood biochemical parameters related to fatigue. Physical fatigue can come from muscle weakness. The muscle produces some amount of lactic acid when doing high-intensity exercise. The increase of lactic acid level will induce many side effects in various physiological processes. Therefore, rapid removal of lactic acid is beneficial in relieving fatigue. Mice administered 20(R)-ginsenoside Rg3 (0.05 mg/kg) showed reduction in blood lactic acid levels after swimming [30]. Avakian et al. [32] demonstrated that ginseng saponin-treated animals had markedly lower concentrations of lactic acid after swimming for 30 or 60 min. In our experiments, however, RG treatment did not change blood lactate levels (Fig. 8).
Fig. 8. Effects of red ginseng on blood lactate concentration after swimming in the pool in mice (n=10). Each bar represents the mean±SEM of mM of lactate.
In summary, we found that psychological fatigue-related responses were attenuated by RG supplementation. We also found that RG had effects on physiological fatigue (swimming, rotarod, and wire test). All these effects were produced by high (200 mg/kg) and low RG doses (100 mg/kg) in short and long-term treatment, respectively. We propose that the anti-fatigue effects of RG are caused by indirect effect that results in an enhancement of physical capacity or stamina.
The effect of RG on psychological fatigue is attributed to its actions on the hypothalamic-pituitary-adrenal axis, resulting in a decrease of plasma corticosteroids levels. Our results indicate that RG might enhance resistance to fatigue via a psychological rather than physical action. We suggest that present findings have potential implications for the clinical use of red ginseng in the treatment of stress-related disorders.
Acknowledgments
This work was supported by the 2009 grant from the Korean Society of Ginseng funded by Korea Ginseng Corporation.
References
- 1.Kim YH, Kim GH, Shin JH, Kim KS, Lim JS. Effect of Korean red ginseng on testicular tissue injury after torsion and detorsion. Korean J Urol. 2010;51:794–799. doi: 10.4111/kju.2010.51.11.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh CH, Kang PS, Kim JW, Kwon J, Oh SH. Water extracts of cultured mountain ginseng stimulate immune cells and inhibit cancer cell proliferation. Food Sci Biotechnol. 2006;15:369–373. [Google Scholar]
- 3.Kim KT, Yoo KM, Lee JW, Eom SH, Hwang IK, Lee CY. Protective effect of steamed American ginseng (Panax quinquefolius L.) on V79-4 cells induced by oxidative stress. J Ethnopharmacol. 2007;111:443–450. doi: 10.1016/j.jep.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Tachikawa E, Kudo K, Harada K, Kashimoto T, Miyate Y, Kakizaki A, Takahashi E. Effects of ginseng saponins on responses induced by various receptor stimuli. Eur J Pharmacol. 1999;369:23–32. doi: 10.1016/S0014-2999(99)00043-6. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy DO, Scholey AB. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75:687–700. doi: 10.1016/S0091-3057(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Cha HY, Seo JJ, Hong JT, Han K, Oh KW. Anxiolytic-like effects of ginseng in the elevated plus-maze model: comparison of red ginseng and sun ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:895–900. doi: 10.1016/j.pnpbp.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Jin SH, Park JK, Nam KY, Park SN, Jung NP. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J Ethnopharmacol. 1999;66:123–129. doi: 10.1016/S0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]
- 8.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/S0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]
- 9.Nocerino E, Amato M, Izzo AA. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 2000;(71 Suppl 1):S1–S5. doi: 10.1016/S0367-326X(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 10.Panossian A, Wikman G, Wagner H. Plant adaptogens. III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine. 1999;6:287–300. doi: 10.1016/S0944-7113(99)80023-3. [DOI] [PubMed] [Google Scholar]
- 11.Piato AL, Detanico BC, Linck VM, Herrmann AP, Nunes DS, Elisabetsky E. Anti-stress effects of the “tonic” Ptychopetalum olacoides (Marapuama) in mice. Phytomedicine. 2010;17:248–253. doi: 10.1016/j.phymed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Panossian AG, Oganessian AS, Ambartsumian M, Gabrielian ES, Wagner H, Wikman G. Effects of heavy physical exercise and adaptogens on nitric oxide content in human saliva. Phytomedicine. 1999;6:17–26. doi: 10.1016/S0944-7113(99)80030-0. [DOI] [PubMed] [Google Scholar]
- 13.Radad K, Gille G, Rausch WD. Use of ginseng in medicine: perspectives on CNS disorders. Iran J Pharmacol Ther. 2004;3:30–40. [Google Scholar]
- 14.Bhattacharya SK, Muruganandam AV. Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacol Biochem Behav. 2003;75:547–555. doi: 10.1016/S0091-3057(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 15.Geller SE, Studee L. Botanical and dietary supplements for mood and anxiety in menopausal women. Menopause. 2007;14(3 Pt 1):541–549. doi: 10.1097/01.gme.0000236934.43701.c5. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Eun JS, Oh KW. Therapeutic effects of ginseng on psychotic disorders. J Ginseng Res. 2007;31:117–126. doi: 10.5142/JGR.2007.31.3.117. [DOI] [Google Scholar]
- 17.Lee GS, Tan-Lee BS, Kim MK, Dong KU, Kim JY, Yu GY, Han JS, Ko HS, Park IH, Cheong JH. Stress related activities of sun-ginseng in SD rats and ICR mice. J Appl Pharmacol. 2004;12:242–249. [Google Scholar]
- 18.Lee GS, Choi JY, Ko HS, Tan-Lee BS, Yu GY, Jeong CW, Park HG, Kim MK, Ryu JH, Jung IK, et al. Stressreducing effects of brown rice Koji. Food Sci Biotechnol. 2006;15:63–69. [Google Scholar]
- 19.Choi JY, Choi YJ, Dela Pena IC, Yoon SY, Lee GS, Shin CY, Ryu JH, Yu GY, Cheong JH. Vitamin C supplementation alleviates electroshock stress but not restraint stress in ICR mice. Food Sci Biotechnol. 2010;19:137–144. doi: 10.1007/s10068-010-0019-9. [DOI] [Google Scholar]
- 20.Noldus LP, Spink AJ, Tegelenbosch RA. EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav Res Methods Instrum Comput. 2001;33:398–414. doi: 10.3758/BF03195394. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen TT, Matsumoto K, Yamasaki K, Nguyen MD, Nguyen TN, Watanabe H. Crude saponin extracted from Vietnamese ginseng and its major constituent majonoside-R2 attenuate the psychological stress- and foot-shock stress-induced antinociception in mice. Pharmacol Biochem Behav. 1995;52:427–432. doi: 10.1016/0091-3057(95)00133-H. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharya SK, Mitra SK. Anxiolytic activity of Panax ginseng roots: an experimental study. J Ethnopharmacol. 1991;34:87–92. doi: 10.1016/0378-8741(91)90193-H. [DOI] [PubMed] [Google Scholar]
- 23.Kim DH, Moon YS, Jung JS, Min SK, Son BK, Suh HW, Song DK. Effects of ginseng saponin administered intraperitoneally on the hypothalamo-pituitary-adrenal axis in mice. Neurosci Lett. 2003;343:62–66. doi: 10.1016/S0304-3940(03)00300-8. [DOI] [PubMed] [Google Scholar]
- 24.Kim CS, Jo YJ, Park SH, Kim HJ, Han JY, Hong JT, Cheong JH, Oh KW. Anti-stress effects of ginsenoside RG3-standardized ginseng extract in restraint stressed animals. Biomol Ther. 2010;18:219–225. doi: 10.4062/biomolther.2010.18.2.219. [DOI] [Google Scholar]
- 25.Carr MN, Bekku N, Yoshimura H. Identification of anxiolytic ingredients in ginseng root using the elevated plusmaze test in mice. Eur J Pharmacol. 2006;531:160–165. doi: 10.1016/j.ejphar.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Wei XY, Yang JY, Wang JH, Wu CF. Anxiolytic effect of saponins from Panax quinquefolium in mice. J Ethnopharmacol. 2007;111:613–618. doi: 10.1016/j.jep.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Bhattamisra SK, Singh PN, Singh SK, Kumar V. Anxiolyic activity of Marsilea minuta linn in rodents. J Herb Med Toxicol. 2007;1:15–20. [Google Scholar]
- 28.Filaretov AA, Bogdanova TS, Podvigina TT, Bodganov AI. Role of pituitary-adrenocortical system in body adaptation abilities. Exp Clin Endocrinol. 1988;92:129–136. doi: 10.1055/s-0029-1210793. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Li S, Fan Y, Chen Y, Liu D, Cheng H, Gao X, Zhou Y. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. J Ethnopharmacol. 2010;130:421–423. doi: 10.1016/j.jep.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Tang W, Zhang Y, Gao J, Ding X, Gao S. The anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by intranasally administration. Biol Pharm Bull. 2008;31:2024–2027. doi: 10.1248/bpb.31.2024. [DOI] [PubMed] [Google Scholar]
- 31.Grandhi A, Mujumdar AM, Patwardhan B. A comparative pharmacological investigation of ashwagandha and ginseng. J Ethnopharmacol. 1994;44:131–135. doi: 10.1016/0378-8741(94)01119-2. [DOI] [PubMed] [Google Scholar]
- 32.Avakian EV, Sugimoto RB, Taguchi S, Horvath SM. Effect of Panax ginseng extract on energy metabolism during exercise in rats. Planta Med. 1984;50:151–154. doi: 10.1055/s-2007-969657. [DOI] [PubMed] [Google Scholar]


