Abstract
To evaluate the difference in expressing pharmacological effects of ginseng by intestinal microflora between Koreans, metabolic activities of ginseng, ginsenoside Rb1 and Rg1 by 100 fecal specimens were measured. The β-glucosidase activity for p-nitrophenyl- β-D-glucopyranoside was 0 to 0.42 μmol/min/mg and its average activity (mean±SD) was 0.10±0.07 μmol/min/mg. The metabolic activities of ginsenosides Rb1 and Rg1 were 0.01 to 0.42 and 0.01 to 0.38 pmol/min/mg, respectively. Their average activities were 0.25±0.08 and 0.15±0.09 pmol/min/mg, respectively. The compound K-forming activities from ginsenoside Rb1 and ginseng extract were 0 to 0.11 and 0 to 0.02 pmol/min/mg, respectively. Their average compound K-forming activities were 0.24±0.09 pmol/min/ mg and 2.14±3.66 fmol/min/mg, respectively. These activities all were not different between males and females, or between ages. Although compound K-forming activity from the aqueous extract of ginseng was low compared to that from ginenoside Rb1, their profiles were similar to those of isolated compounds. Based on these findings, we believe that the intestinal bacterial metabolic activities of ginseng components are variable in individuals and may be used as selection markers for responders to ginseng.
Keywords: Intestinal microflora, Metabolism, Ginsenoside Rb1, Ginsenoside Rg1, Pharmacological effect
INTRODUCTION
Most herbal medicines, which have been used in China, Japan, and Korea, are orally administered to human. Therefore, their components are inevitably contacted with intestinal microflora in gastrointestinal tract and may be metabolized by intestinal microflora, before absorption from the gastrointestinal tract to the blood [1,2]. For example, when ginsenoside Rb1, which is a major component of ginseng, is administered to humans or rats, it is transformed to 20-O-β-D-glucopyranosyl-20(S)- protopanaxadiol (compound K) by intestinal microflora and absorbed to the blood [3-5]. However, ginsenoside Rb1 was not detected in blood. Compound K exhibits the potent antitumor and antiallergic actions more than ginsenoside Rb1 [6-9]. Therefore, intestinal bacteria may play the important role in expressing the pharmacological activities of herbal medicines, such as ginseng.
In relation to the role of intestinal microflora on the pharmacological actions of herbal medicines, Kobashi et al. [10] reported that some drug-metabolic enzyme activities of intestinal bacteria were significantly different between Jitsu-syo and Kyo-syo Japanese, although the intestinal bacteria between Jitsu-syo and Kyo-syo Japaneses were not different. We have reported that some fecal bacterial enzymatic activities related to the pharmacological actions of herbal medicines including ginseng were variable among individuals [11-14]. The intra- and inter-individual variations of these intestinal bacteria are not significant, but their enzyme activities were affected by dietary change and physiological factors [15-17]. Nevertheless, Ikeda et al. [18] reported that these are rebound if diet or supplements were stopped for short term. Therefore, to understand the pharmacological actions of herbal medicines, the variations of these enzymatic activities between individuals are of a great importance. Because these enzymes should activate the pharmacological actions of herbal medicines. Nevertheless, studies on the metabolic activities of herbal medicine components, particularly ginseng, by human intestinal microflora are not sufficient. Therefore, we determined the metabolic activities of ginseng and its major component ginsenosides Rb1 and Rg1 by human fecal microflora to understand their pharmacological effects and metabolic fates.
MATERIALS AND METHODS
Materials
p-Nitrophenyl-β-D-glucopyranoside was purchased from Sigma (St. Louis, MO, USA). The ginseng extract was prepared based on the previously described procedure [9,19]. Fresh ginseng (1 kg) was extracted with water at 70℃ for 4 h and freeze-dried (water extract, 49 g). The freeze-dried powder was suspended in water, defatted with n-hexane, extracted with n-BuOH (1 L) two times, and evaporated (dried extract, 5.2 g). Ginsenoside Rb1 (purity, >92%), ginsenoside Rg1 (purity, >02%) and compound K (purity, 95%) were isolated using the previously published method of Bae et al. [9] and Bae et al. [19].
Subjects
The subjects were 100 healthy Korean persons (average, 40.74±13.87 years; range, 20-72 years; 54 males, 46 females). Exclusion criteria included smoking and current medication, especially regular or current use of antibiotics. The recruit of subjects and collection of their stools were approved by the Committee for the Care and Use of Clinical Study in the Medical School, Kyung Hee University.
Specimen peparation
The human fecal specimens (about 1 g) prepared according to a previous method [13], were collected in plastic cups 9 h after fasting, and then carefully mixed with a spatula and suspended with cold 9 mL saline. Preparation A-Fecal suspension was sonicated for 10 min (Ultrasonic processor; Heat System Inc., Farmingdale, NY, USA) and then centrifuged at 10,000 ×g for 20 min. The resulting supernatant was used for the assay of enzyme activity. Preparation B-Fecal bacterial suspension was centrifuged at 500 ×g for 5 min. The resulting supernatant was sonicated for 10 min and then centrifuged at 10,000 ×g for 20 min. The resulting supernatant was used for the assay of enzyme activity. Preparation C-Suspended specimen was sonicated for 10 min. The sonicated fraction was used for the assay of enzyme activity. The fecal suspension was centrifuged at 500 ×g for 5 min. The supernatant was then centrifuged at 10,000 ×g for 20 min. The resulting precipitates were used as a metabolic enzyme source for the assay of enzyme activity. The preparation and assay of the enzyme source were performed within 24 h.
β-D-Glucosidase activity assay
For the assay of β-D-glucosidase activity, the reaction mixture (total volume of 0.5 mL) contained 0.2 mL of 1 mM p-nitrophenyl-β-D-glucopyranoside, 0.2 ml of 0.1 M phosphate buffer, pH 7.0, 0.1 mL of the fecal enzyme fraction. The reaction mixture was incubated at 37℃ for 20 min. The reaction was stopped by the addition of 0.5 ml of 0.5 N NaOH, centrifuged at 3,000 ×g for 10 min and measured the absorbance at 405 nm (UV-vis spectrophotometer, Shimadzu UV-1201; Shimadzu Co. Ltd., Tokyo, Japan).
Assay of intestinal bacterial enzyme activity metabolizing ginsenosides Rb1 and Rg1
For the fecal enzyme activity for ginsenosides Rb1 and Rg1, the reaction mixture (0.5 mL) containing 0.125 mL of the human fecal suspension and 0.1 mM ginsenoside Rb1 or Rg1 was incubated at 37℃ for 4 h, and 1.5 ml of MeOH was added to stop the reaction. The reaction mixture was centrifuged at 3,000 ×g for 10 min and the levels of ginsenoside Rb1 and Rg1 in the resulting supernatant were analyzed by HPLC.
Assay of intestinal bacteria enzyme activity transforming ginsenoside Rb1 and ginseng extract to compound K
For the fecal enzyme activity for ginseng extract, the reaction mixture (0.5 mL) containing 0.125 mL of the human fecal suspension and 0.5 mg of ginseng extract was incubated at 37℃ for 4 h, and 1.5 mL of MeOH was added to stop the reaction. The reaction mixture was centrifuged at 3,000 ×g for 10 min and the compound K level in the resulting supernatant was analyzed by HPLC
HPLC analysis
The reaction mixture was analyzed by Hewlett Packard Series 1050 HPLC system. The instrument was controlled and the data were processed by a HP Chemstation (Rev. A. 09.03). The analytical column was an Agilent Hypersil ODS (100×4.6 mm i.d., 5 μm; Agilent Technologies, Palo Alto, CA, USA) protected by a C18 Security Guard Cartridge (Phenomenex, Torrance, CA, USA). The elution solvent was acentonitrile and distilled and deionized water (DDW). Separations were performed with a linear gradient 0% to 70% acetonitrile (ACN) in DDW including 0.05% formic acid for 15 min and an isocratic for 5 min in 70% ACN at a flow rate of 1.0 mL/ min and detection at 203 nm. A sample volume of 20 μL was used for injection. The retention times of Rg1, Rb1, and compound K were 10.0, 10.5, and 15.6 min, respectively.
Statistics
The SPSS ver. 8.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of the data. The differences in fecal enzyme activities between males and females and between ages were assessed by ANOVA.
RESULTS
To investigate the difference of fecal enzyme activities according to the preparation methods of bacterial enzyme fractions from stool specimens, five fecal specimens of healthy human were randomly selected, treated according to Materials and Methods, and β-glucosidase activity was assessed (Table 1). The recovery of β-glucosidase activity by preparation B was best among them. Its recovery by preparation A was lower than by preparation B. Preparation C could not usable for enzyme activity assay due to the thick color of stools.
Table 1.
Comparison of β-glucosidase activity prepared by three methods
| Preparation method | Enzyme activity (%) | |
|---|---|---|
|
| ||
| Supernatant fraction | Precipitate fraction | |
|
| ||
| A | 55±7 | 45±5 |
| B | 80±9 | 20±6 |
| C | ND | ND |
Values are mean±SD.
ND, not detectable.
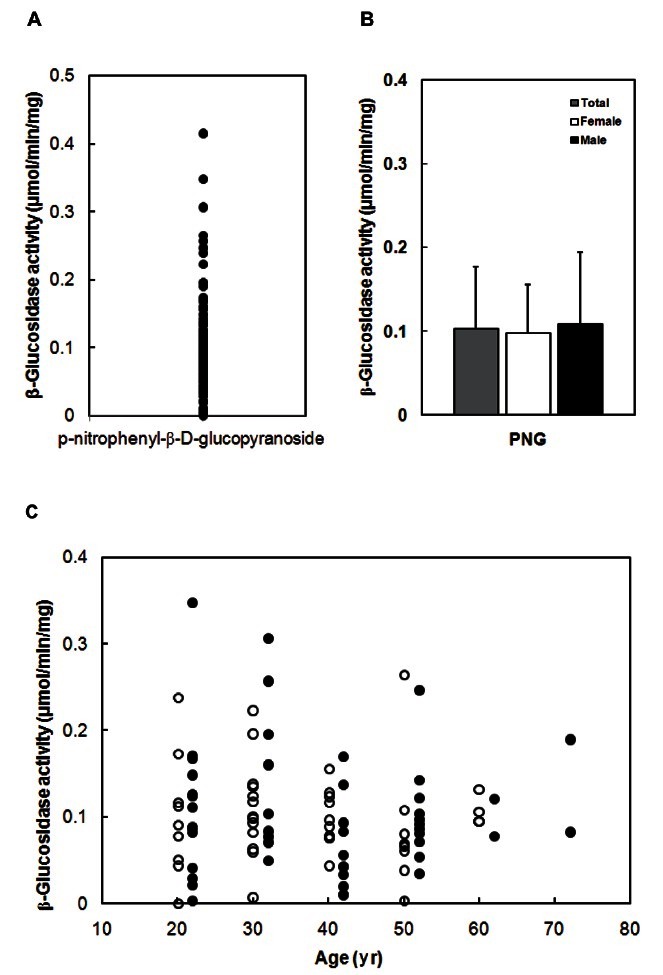
Next, we measured β-glucosidase activity for pnitrophenyl- β-D-glucopyranoside using 100 fecal specimens prepared by the method B (Table 1). The β- glucosidase activity was 0 to 0.42 μmol/min/mg, respectively (Fig. 1). The average activities (mean±SD) of total, female and male specimens were 0.10±0.07, 0.10±0.06, and 0.11±0.09 μmol/min/mg. The activity was not different between males and females, or between ages.
Fig. 1. Fecal β-glucosidase activity of 100 Koreans. (A) Distribution of β-glucosidase activity. (B) Average β-glucosidase activity. (C) Comparison of β-glucosidase activity between male (closed circle) and female (open circle), or between ages. The β-glucosidase activity was measured using p-nitrophenyl-β-D-glucopyranoside (PNG) as a subtrate.
Next we determined the metabolic activities of ginsenoside Rb1 and Rg1 by these fecal specimens (Fig. 2). The metabolic activities of these compounds were 0.01 to 0.42 and 0.01 to 0.38 pmol/min/mg, respectively. The average metabolic activities of total, female and male specimens were 0.25±0.08, 0.27±0.07, 0.24±0.09 pmol/min/mg for ginsenoside Rb1 and 0.15±0.09, 0.16±0.09, 0.13±0.09 pmol/min/mg for ginsenoside Rg1, respectively. These activities were also not different between males and females, or between ages.
Fig. 2. Fecal metabolic activities of ginsenoside Rb1 and Rg1 of 100 Koreans. (A) Distribution of ginsenosides Rb1 and Rg1-metabolic activities. (B) Average metabolic activities of ginsenoside Rb1 and Rg1. (C) Comparison of ginsenoside Rb1-metabolic activity between male (closed circle) and female (open circle), or between ages. (D) Comparison of ginsenoisde Rg1-metabolic activity between male (closed circle) and female (open circle), or between ages.

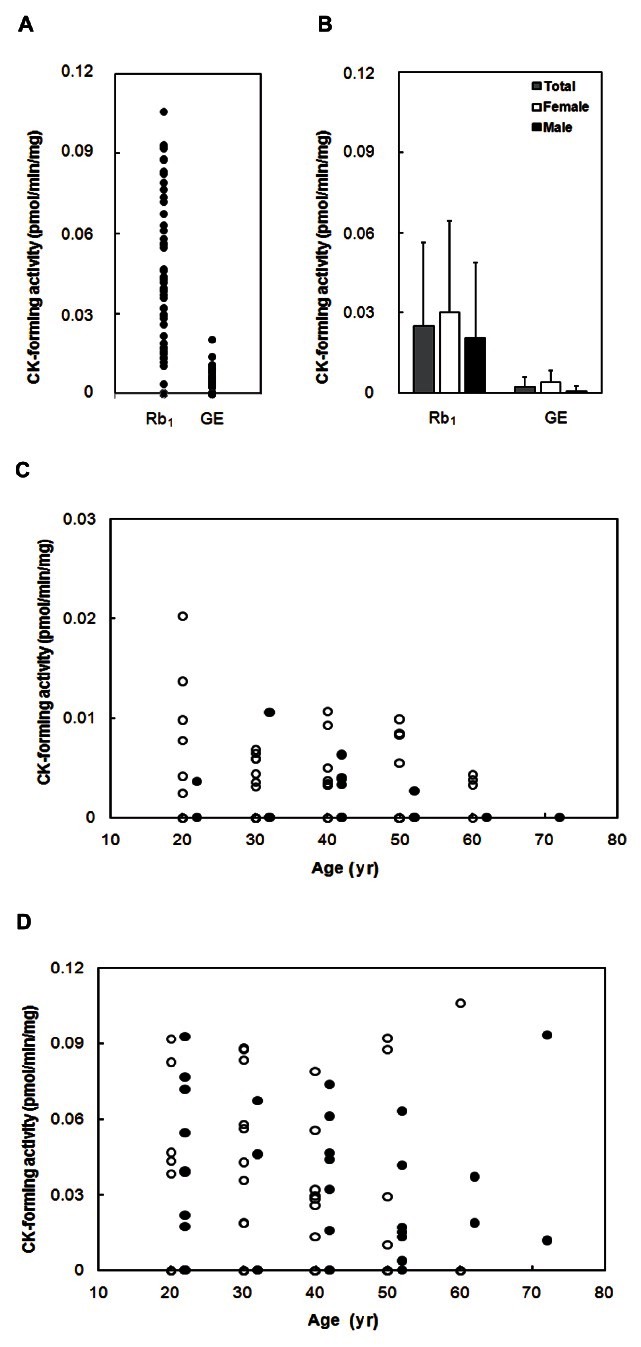
The compound K-forming activities from ginsenoside Rb1 or ginseng extract were measured using fecal specimens (Fig. 3). The compound K-forming activities from ginsenoside Rb1 and ginseng extract to compound K were 0 to 0.11 and 0 to 0.02 pmol/min/mg, respectively. The average compound K-forming activities of total, female and male specimens were 0.24±0.09, 0.26±0.08, 0.23±0.10 pmol/min/mg from ginsenoside Rb1 and 2.14±3.66, 3.91±4.40, 0.63±0.19 fmol/min/mg from ginseng extract, respectively. The activities were not different between males and females, or between ages.
Fig. 3. Fecal compound K (CK)-forming activities from ginsenoside Rb1 and ginseng extract (GE) of 100 Koreans. (A) Distribution of CK-forming activities from ginsenoside Rb1 and GE. (B) Average CK-forming activities from ginsenoside Rb1 and GE extract. (C) Comparison of CK-forming activity from ginsenoside Rb1 between male (closed circle) and female (open circle), or between ages. (D) Comparison of CK-forming activity from GE between male (closed circle) and female (open circle), or between ages.
DISCUSSION
All individuals possess their own characteristic indigenous strain of intestinal bacteria [20,21]. This is due to the affinity between the intestinal lumen of the individual and the bacteria. Newly ingested bacteria cannot necessarily colonize and proliferate in the intestine. These results are supported by previous reports that intestinal microflora in feces are thought to be rather stable over time within individuals in the absence of disease and antimicrobial therapy [10,15,21]. In addition, Kobashi et al. [10] reported that some enzymes of intestinal bacteria were significantly different between Jitsu-syo and Kyo-syo Japanese, although intestinal bacteria between Jitsu-syo and Kyo-syo Japanese were not different. Ikeda et al. [18] reported that some intestinal bacterial enzyme activities did not appear to be associated with specific populations. However, these fecal enzyme activities are affected by diet [16,17,22], but rebound if diet or supplements were stopped for short term [16,22,23]. These results suggest that the intestinal bacterial enzyme activities of each individual are indigenous, although they can be altered by other factors as well as diet.
Most herbal medicine components glycosides are activated by intestinal microflora are prodrugs [1-3]. Therefore, to understand the pharmacological actions of herbal medicines including ginseng, the bioactive components that are absorbable from the human intestine should be investigated. The enzymes, β-glucosidase and α -rhamnosidase, produced by intestinal microflora may play an important role in the pharmacological actions of ginseng and its components, such as ginsenoside Rb1 and Rg1. Of them, the representative ginsenosides-transforming enzyme is a β -glucosidase.
First we prepared fecal β-glucosidase fraction and measured its activity. For the preparation of fecal β -glucosidase fraction, we measured the difference among three fecal sample preparation methods: sonicated fecal fraction (A), sonicated bacterial fraction (B) and fecal suspension fraction (C). Of them, the preparation method B was best in the recovery of β -glucosidase activity. Therefore, we measured its activity of 100 specimens prepared by preparation B.
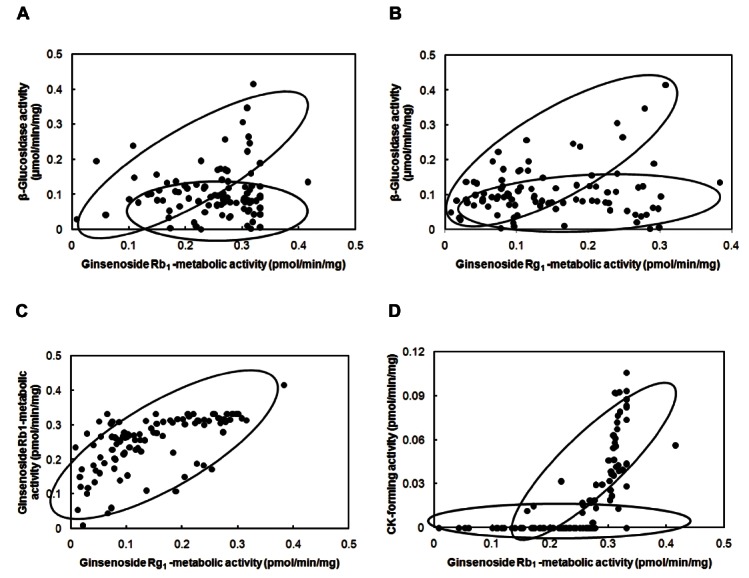
The β-glucosidase activity between individuals varied significantly. However, the activity was not different between males and females, or between ages. If an individual potently metabolizes ginsenoside Rb1 or ginseng extract to compound K, an antitumor agent that originates from ginseng [6,9], it may also transform its main constituents, such as ginsenosides Rb1 and Rg1. Therefore, we evaluated the relationship between the metabolic activities of these ginsenosides and β -glucosidase activity (Fig. 4). The potencies of ginsenoside Rb1-metabolic individuals are significantly in proportion to those of ginsenoside Rg1-metabolic individuals, but are out of proportion to that of p-nitrophenyl-β -D-glucopyranosidemetabolic activity (Fig. 4). Both ginsenoside Rb1 and p-nitrophenyl-β -D-glucopyranoside may be transformed to their bioactive compounds by β-D-glucosidases. These metabolic activities between individuals varied significantly. However, these activities were also not different between males and females, or between ages. Thus, intestinal microflora produce many kinds of β -glucosidase(s), and among them, a part of β -glucosidase(s) may transform ginsenosides to the active compound(s), such as compound K. Although compound K-forming activity from the aqueous extract of ginseng was low compared to that from ginenoside Rb1 isolated from ginseng, their profiles were similar to those of isolated compounds, like the previously reported [24]. Based on these findings, we believe that the intestinal bacterial metabolic activities of ginseng components are variable in individuals and may be used as selection markers for responders to ginseng.
Fig. 4. Profiles of the relationship between fecal enzyme activities. (A) Between ginsenoside Rb1-metabolic activity and β -glucosidase activity. (B) Between ginsenoside Rg1-metabolic activity and β -glucosidase activity. (C) Between metabolic activities of ginsenoside Rb1 and Rg1. (D) Between compound K (CK)-forming activities of ginsenoside Rb1 and ginseng extract.
Acknowledgments
This research was supported by a grant (09172 KFDA996) from the Korean Food and Drug Administration in 2010.
References
- 1.Kobashi K, Akao T. Relation of intestinal bacteria to pharmacological effect of glycosides. Biosci Microflora. 1997;16:1–7. [Google Scholar]
- 2.Kim DH. Intestinal microflora activate the pharmacological effects of herbal medicines. Nat Prod Sci. 2002;8:35–43. [Google Scholar]
- 3.Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1155–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 4.Akao T, Kanaoka M, Kobashi K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration-measurement of compound K by enzyme immunoassay. Biol Pharm Bull. 1998;21:245–249. doi: 10.1248/bpb.21.245. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Lee E, Kim D, Lee J, Yoo J, Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009;122:143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 1998;246:725–730. doi: 10.1006/bbrc.1998.8690. [DOI] [PubMed] [Google Scholar]
- 7.Choo MK, Park EK, Han MJ, Kim DH. Antiallergic activity of ginseng and its ginsenosides. Planta Med. 2003;69:518–522. doi: 10.1055/s-2003-40653. [DOI] [PubMed] [Google Scholar]
- 8.Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH. Metabolism of ginsenoside R(c) by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull. 2002;25:743–747. doi: 10.1248/bpb.25.743. [DOI] [PubMed] [Google Scholar]
- 9.Bae EA, Kim NY, Han MJ, Choo MK, Kim DH. Transformation of ginsenosides to compound K (IH-901) by lactic acid bacteria of human intestine. J Microbiol Biotechnol. 2003;13:9–14. [Google Scholar]
- 10.Kobashi K, Nakata H, Takebe H, Terasawa K. Relation of intestinal microflora to Syo. Wakan-iyaku-kaishi. 1984;1:166–167. [Google Scholar]
- 11.Bae EA, Han MJ, Kim EJ, Kim DH. Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res. 2004;27:61–67. doi: 10.1007/BF02980048. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, Kim JJ, Cho KH, Jung WS, Moon SK, Park EK, Kim DH. Biotransformation of ginsenoside Rb1, crocin, amygdalin, geniposide, puerarin, ginsenoside Re, hesperidin, poncirin, glycyrrhizin, and baicalin by human fecal microflora and its relation to cytotoxicity against tumor cells. J Microbiol Biotechnol. 2008;18:1109–1114. [PubMed] [Google Scholar]
- 13.Lee DS, Kim YS, Ko CN, Cho KH, Bae HS, Lee KS, Kim JJ, Park EK, Kim DH. Fecal metabolic activities of herbal components to bioactive compounds. Arch Pharm Res. 2002;25:165–169. doi: 10.1007/BF02976558. [DOI] [PubMed] [Google Scholar]
- 14.Yim JS, Kim YS, Moon SK, Cho KH, Bae HS, Kim JJ, Park EK, Kim DH. Metabolic activities of ginsenoside Rb1, baicalin, glycyrrhizin and geniposide to their bioactive compounds by human intestinal microflora. Biol Pharm Bull. 2004;27:1580–1583. doi: 10.1248/bpb.27.1580. [DOI] [PubMed] [Google Scholar]
- 15.Mallet AK, Rowland IR, Bearne CA, Flynn JC, Fehilly BT, Udeen YS, Farthing MJ. Effect of dietary supplements of apple pectin, wheat bran or fat on the enzyme activity of the human faecal flora. Microb Ecol Health Dis. 1988;1:23–39. doi: 10.3109/08910608809140175. [DOI] [Google Scholar]
- 16.Reddy BS, Hanson D, Mangat S, Mathews L, Sbaschnig M, Sharma C, Simi B. Effect of high-fat, high-beef diet and of mode of cooking of beef in the diet on fecal bacterial enzymes and fecal bile acids and neutral sterols. J Nutr. 1980;110:1880–1887. doi: 10.1093/jn/110.9.1880. [DOI] [PubMed] [Google Scholar]
- 17.Mykkanen H, Laiho K, Salminen S. Variations in faecal bacterial enzyme activities and associations with bowel function and diet in elderly subjects. J Appl Microbiol. 1998;85:37–41. doi: 10.1046/j.1365-2672.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda N, Saito Y, Shimizu J, Ochi A, Mizutani J, Watabe J. Variations in concentrations of bacterial metabolites, enzyme activities, moisture, pH and bacterial composition between and within individuals in faeces of seven healthy adults. J Appl Bacteriol. 1994;77:185–194. doi: 10.1111/j.1365-2672.1994.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 19.Bae EA, Park SY, Kim DH. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 20.Rumney CJ, Rowland IR. In vivo and in vitro models of the human colonic flora. Crit Rev Food Sci Nutr. 1992;31:299–331. doi: 10.1080/10408399209527575. [DOI] [PubMed] [Google Scholar]
- 21.Simon GL, Gorbach SL. The human intestinal microflora. Dig Dis Sci. 1986;31(9 Suppl):147S–162S. doi: 10.1007/BF01295996. [DOI] [PubMed] [Google Scholar]
- 22.Goldin BR, Swenson L, Dwyer J, Sexton M, Gorbach SL. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzymes. J Natl Cancer Inst. 1980;64:255–261. doi: 10.1093/jnci/64.2.255. [DOI] [PubMed] [Google Scholar]
- 23.Ling WH, Korpela R, Mykkänen H, Salminen S, Hanninen O. Lactobacillus strain GG supplementation decreases colonic hydrolytic and reductive enzyme activities in healthy female adults. J Nutr. 1994;124:18–23. doi: 10.1093/jn/124.1.18. [DOI] [PubMed] [Google Scholar]
- 24.Kim DH. The possible role of intestinal microflora in pharmacological activities of ginseng. Int J Biomed Pharm Sci. 2011 ;in press.


