Abstract
This study investigated the effect of red ginseng extract on metastasis of colon cancer cells in vitro and in vivo. Wound healing migration, cell motility, invasion, and activity, protein expression, and mRNA expression of matrix metalloproteinases (MMPs) were examined in SW480 human colon cancer cells. SW480 cells were cultured with or without 100 μg/L PMA in the absence or presence of various concentrations (100, 200, or 300 μg/mL) of red ginseng extract. Red ginseng extract treatment caused significant suppression of cell motility and invasion (p<0.05) in SW480 cells. Red ginseng extract inhibited MMP-2 and MMP-9 activity and their protein and mRNA expression in a dose-dependent manner (p<0.05) in SW480 cells. For experimental metastasis, BALB/c mice were injected intravenously with CT-26 mouse colon cancer cells in the tail vein, and were orally administered various concentrations (0, 75, 150, or 300 mg/kg body weight) of red ginseng extract for 3 weeks. Numbers of pulmonary nodules were significantly decreased in mice that were fed red ginseng extract (p<0.05). Plasma MMP-2 and MMP-9 activity significantly decreased in response to treatment with red ginseng extract in mice (p<0.05). These data suggest that red ginseng extract may be useful for prevention of cancer invasion and metastasis through inhibition of MMP-2 and MMP-9 pathways.
Keywords: Panax ginseng, Red ginseng extract, Neoplasm metastasis, SW480 cells, CT-26 cells, BALB/c mice
INTRODUCTION
Human colorectal cancer is the leading cause of colon-related death in developed countries, and its incidence has increased both worldwide and in Korea [1,2]. Due to fact that patients with metastatic colorectal cancer die within 5 years of diagnosis, tumor metastasis is the major cause of death for colorectal cancer patients [1].
Onset of tumor metastasis begins with invasion of tumor cells. Attachment of tumor cells to basement membrane causes contraction of endothelial cells [3] and secretion of proteinases, such as matrix metalloproteinase (MMP) and serine proteinase, by tumor cells, in order to initiate degradation of the extracellular-matrix (ECM) [4,5]. In particular, MMPs are secreted from endothelial cells and tumor cells, and degrade basement membrane type IV collagen and other matrix proteins [6-8]. As MMPs can be regarded as a biomarker of tumor invasion and metastasis, suppressing MMPs activity is expected to inhibit invasion and metastasis as well.
Ginseng is oriental herb that has been used as both food and medicine for more than 2000 years [9]. There are many types of ginseng including Ginseng (Panax ginseng Meyer), American ginseng (P. quinquefolium L.), and Sanchi ginseng (P. notoginseng, Yunnam China) [10]. Ginseng is composed of 60% to 70% carbohydrates (including starch) and other unique components such as ginseng saponin (ginsenoside), polyacetylene, aromatic compounds, acidic peptide, etc. [11].
Natural-dried ginseng is known as white ginseng, and red ginseng is made by steaming unpeeled white ginseng. As it is processed into red ginseng, white ginseng will lose malonyl groups (malonyl-Rb1, malonyl-Rb2, malonyl-Rc, and malonyl-Rd) and glycosyl groups at the C-20 position. Also, the hydroxyl group at the C-20 position can undergo isomerization and change into 20(S)- ginsenoside Rg3, 20(R)-ginsenoside Rh2, and 20(R)- ginsenoside Rh1, which are characteristic components of red ginseng only [12]. When ginseng is taken, its protopanaxadiol- type ginsenosides will be changed into 20-O-D-glucopyranosyl-20(S)-protopanaxadiol (compound K) in the intestine by human intestinal bacteria [13].
Various physiological and pharmacological effects of ginseng have been reported: general ‘tonic’, anti-fatigue, anti-stress, immune-modulator [14-16], antioxidant, recovery from inflammatory diseases, anti-aging, activation of brain function [17,18], improvement of mental performance [19], anti-cancer [10,20], etc.
According to an epidemiologic study of a prospective cohort of 4,634 residents (over age 40) of major ginseng-producing area in Korea, the relative risk (RR) of cancer development for ginseng takers compared to non-takers was shown to be 0.48 (95% confidence interval, 0.34- 0.67), and RR seemed to decrease as the frequency or duration of ginseng intake increased [9]. Also, ginseng takers are reported to have higher non organic-specific anti-carcinogenic effects than non-takers [21]. When 43 patients who required chemotherapy after stomach cancer surgery and 7 patients for whom surgery was impossible due to metastasis took red ginseng extract (3 g/ d) for 3 mo, red ginseng extract takers showed increased interleukin (IL)-2 content in the blood, while the content of decreased IL-10, a cytokine that suppresses anticancer immunity of the host, decreased, implying that red ginseng enabled recovery of immunity during anticancer chemotherapy [22].
Red ginseng showed higher cytotoxicity against RAW264.7 than white ginseng, and tumor cell growth suppression was explained by the arrest of G2/M phase in the cell cycle [11]. Wang et al. [1] reported that the content of ginsenoside Rg3 and ginsenoside Rh2 was very low (0.06%) in unsteamed ginseng extract, however, after 4 h steaming, their content increased to 7.8% and 1.2%, respectively, and Rg3 had a higher anti-cancer effect than Rh2.
Red ginseng and its extract have been reported to affect suppression of tumor angiogenesis, invasion, and metastasis [23-26]. However, few studies to determine the underlying mechanism of suppression of tumor cell metastasis of red ginseng have been conducted.
In this study, we verified a hypothesis that red ginseng can suppress metastasis of colon cancer cells by regulation of MMPs activity in vitro and in vivo.
MATERIALS AND METHODS
Materials and reagents
Red ginseng extract was provided from the Korea Ginseng Corporation (Seoul, Korea) which was manufactured from ginseng (P. ginseng). Red ginseng extract contained 0.519 mg/g ginsenoside Rg1 and 4.028 mg/g ginsenoside Rb1. Red ginseng extract was diluted in cell culture medium for treatment. The following reagents and chemicals were obtained from the respective suppliers: Dulbecco’s modified Eagle’s medium/Ham’s F12 nutrient mixture (DMEM/F12) and streptomycin and penicillin were obtained from Gibco/BRL (Grand island, NY, USA). In addition, antibodies for MMPs were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). RIA-grade bovine serum albumin, transferrin, and other reagents were purchased from Sigma (St. Louis, MO, USA).
Cell culture
SW480 human colon cancer cells were purchased from the American Type Culture Collection (Rockville, MD, USA) and CT-26 mouse colon cancer cells were purchased from the Korean Cell Line Bank (Seoul, Korea). SW480 cells and CT-26 cells were maintained in DMEM/F12 containing 100 mL/L of fetal bovine serum (FBS) with 100,000 U/L of penicillin and 100 mg/L of streptomycin. The medium was replaced every 2-3 d For examination of red ginseng extract for cell proliferation, SW480 cells were plated in 24 well plates at a density of 2.5×104 cells/mL in DMEM/F12 supplemented with 10% FBS. Following 48 h incubation period, monolayers were serum-starved with DMEM/F12 supplemented with 5 μg/mL transferrin, 5 ng/mL selenium, and 1 mg/ mL bovine serum albumin for 24 h. Following serum starvation, monolayers were incubated in serum free medium (SFM) with 0, 100, 200, or 300 μg/mL red ginseng extract. Using the 3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide assay, viable cell numbers were estimated at 24, 48, and 96 h after exposure of cells to red ginseng extract, as previously described [27]. Experiments were performed independently three times.
Boyden chamber motility assay
The Boyden chamber motility assay was performed as previously described [28]. PVPF filters (8 μm diameter pore size) were coated with a 0.01% gelatin solution for 16 h at room temperature. SW480 cells (2×106 cell/mL), resuspended in SFM with or without 100 μg/L PMA in the absence or presence of various concentrations (100, 200, or 300 μg/mL) of red ginseng extract, were carefully transferred into the upper chambers. The lower chamber was filled with 10% FBS medium in order to attract the cells. The Boyden chamber was incubated at 37℃ with 5% CO2 for 16 h. After gentle removal of the filter from the chamber, cells on the upper chamber side of the filter were removed by wiping the filter with paper. The filter was stained with Diff-Quick stain solution (Dade Behring, Network, NJ, USA) and cells on the lower surface of the filter, were fixed onto a glass slide. Cells in five randomly selected microscopic fields (×400) of the lower slide were then counted. Experiments were performed independently three times.
Matrigel invasion assay
The Matrigel invasion assay was performed as previously described [28]. Wells of a Matrigel chamber (BD Bioscience, Bedford, MA, USA) were filled with SFM and adapted at room temperature. SW480 cells (1×106 cell/mL), resuspended in SFM with or without 100 μg/ L PMA in the absence or presence of various concentrations (100, 200, or 300 μg/mL) of red ginseng extract were carefully transferred into the upper chambers. The lower chambers were filled with 10% FBS medium in order to attract the cells. Matrigel chambers were incubated for 16 h at 37℃ with 5% CO2. Cells on the upper surfaces of the filters were then removed by wiping the filter with paper. Filters were stained with Diff-Quick stain solution and cells on the lower surface of the filter were fixed onto a glass slide. Cells in five randomly selected microscopic fields (×400) of the lower slide were counted. Experiments were performed independently three times.
Matrix metalloproteinase activity (gelatin zymography)
MMP activity was investigated as previously described [29]. Cells were seeded into 6 well plates at density of 1×106 cells/mL and incubated in a medium containing 10% FBS for 48 h. Then, SW480 cells incubated in the absence or presence of various concentrations (100, 200 or 300 μg/mL) for 12 h. Supernatants were collected and concentrated 10-fold in Centricon centrifugal filter devices (Millipore, Bedford, MA, USA), after which MMP activity in the supernatants was investigated using gelatin zymography. Each supernatant was mixed with 2x sample buffer (Invitrogen, Carlsbad, CA, USA), and zymography was performed using gels (10% polyacrylamide, 1% gelatin). MMP activities were visualized by staining with Coomassie blue. Experiments were performed independently three times.
Western blotting analysis
MMP protein levels in the culture medium were determined by western blotting as previously described [28]. Conditioned media were collected and concentrated by the same procedures as that used for gelatin zymography. Proteins were separated by 4% to 20% gradient SDS-polyacrylamide gel electrophoresis and transferred to an immobilonTM-P transfer membrane (Millipore). The membrane was blocked in 5% milk/TBST (20 mmolL Tris-HCl, 137 mmol/L NaCl, and 0.1% Tween 20, pH 7.4) for 1 h at room temperature, incubated with monoclonal anti-MMP-2 and MMP-9 antibody/5% milk-TBST (1:200 dilution) for 3 h at room temperature, and washed for 30 min at room temperature in TBST. The membrane was then incubated with anti-mouse IgG horseradish peroxidase/5% milk-TBST (1:1,000 dilution, Amersham, Buckinghamshire, England). The presence of bound antibody was detected by chemiluminescence using Supersignal West Dura Extended Duration Substrate (Pierce, Rockford, IL, USA), and the intensity of the immunoreactive bands was quantified using a densitometer (Sanyo, Tokyo, Japan). Experiments were performed independently three times.
Reverse transcriptase polymerase chain reaction
MMP mRNA levels in the culture medium were determined as previously described [29]. Total RNA was isolated using TRI-reagent (Sigma), and cDNA was synthesized using 2 μg of total RNA with SuperScript II reverse transcriptase (Invitrogen). For amplification of cDNA, primers for MMP-2 (upstream primer, 5’-CAGGCTCTTCTCCTTTCSCAAC- 3’; downstream primer, 5’-AAGCCACGGCTTGGTTTTCCTC-3’) and MMP-9 (upstream primer, 5’-TGGGCTACGTGACCTATGACCAT- 3’; downstream primer, 5’-GCCCAGCCCACCTCCACTCCTC- 3’; annealing at 55℃ for 1 min with 35 cycles) were used. Polymerase chain reaction (PCR) products were separated on a 1% agarose gel and stained with ethidium bromide. Bands corresponding to each specific PCR product were quantified by densitometric scanning of the exposed film using the Bio-profile Bio-IL application (Vilber-Lourmat, Eberhardzell, Germany).
Experimental metastasis
Experimental metastasis was performed as previously described [30]. Animals and diet: four-week-old male BALB/c mice were obtained from Daehan Biolink (Eumsung, Korea) and were initially fed a stock diet (Harlan Teklad Laboratory Animal Diets, Madison, WI, USA) for a 1-week adjustment period. Mice were randomly divided into five groups. Experimental diets were based on the AIN-93G diet and red ginseng extract was administered orally. Animals were maintained according to animal care guidelines from the Dankook University ethics committee. They were housed in a temperature-controlled room (at 23±2℃) with light from 06:00 to 18:00 under specific pathogen-free conditions. Humidity was automatically maintained at 50±10%.
Lung metastasis: Mice were injected intravenously in the tail with CT-26 colon cancer cells suspended in SFM at a density of 5×105 cells/mL. Following injection, mice underwent oral administration of red ginseng extract each day at doses of 0, 75, 150, or 300 mg/kg BW for 3 wk, after which they were sacrificed and their lungs were removed. Lungs were stained with Bouin’s solution and the numbers of metastatic colonies were counted.
Plasma MMP activity: 2 μL plasma was mixed with 2x sample buffer (Invitrogen), and zymography was performed using gels (10% polyacrylamide, 1% gelatin). MMP activities were visualized by staining with Coomassie blue. Experiments were performed independently three times.
Statistics
Statistical analyses were performed using SAS (SAS Institute, Cary, NC, USA). Data were expressed as means with standard deviations and analyzed via analysis of variance. Statistically significant differences among the means of groups were tested at α=0.05 using Duncan›s multiple range test.
RESULTS
Korean red ginseng extract inhibits proliferation of SW480 cells
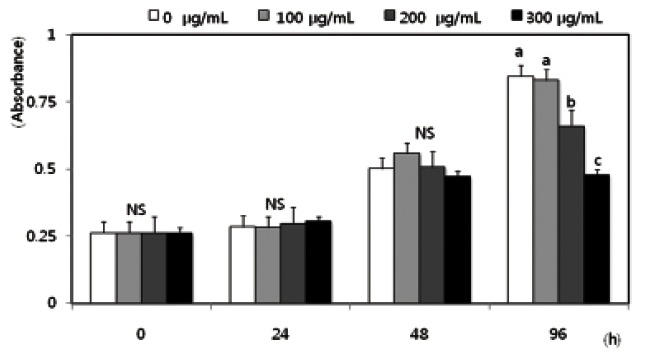
To examine the effects of red ginseng extract on SW480 colon cancer cell proliferation, cells in a monolayer culture were incubated in SFM with 0, 100, 200, or 300 μg/mL Korean red ginseng extract, and numbers of viable cells were estimated. Numbers of viable SW480 cells did not differ by red ginseng extract treatment from 0 to 300 μg/mL within 48 h. However, cell proliferation significantly decreased following a 96 h incubation with the red ginseng extract at concentrations above 200 μg/ mL (Fig. 1). In order to demonstrate that the anti- metastatic effect of red ginseng extract was independent of decreased cell proliferation, red ginseng extract treatment time in all experiments related to metastasis was never longer than 48 h in this study.
Fig. 1. Effects of Korean red ginseng extract on cell proliferation in SW 480 cells. SW 480 cells were plated in 24 well plates at a density of 2.5×104 cells/mL in Dulbecco’s modified Eagle’s medium/Ham’s F12 nutrient mixture (DMEM/F12) supplemented with 10% fetal bovine serum. After 48 h incubation, the monolayers were serum-starved with DMEM/F12 supplemented with 5 μg/mL transferrin, 5 ng/mL selenium, and 1 mg/mL bovine serum albumin for 24 h. After serum starvation, the monolayers were incubated in serum-free medium with 0, 100, 200, or 300 μg/mL red ginseng extract. Viable cell numbers were estimated by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay. Each bar represents the mean±SD calculated from three independent experiments. Comparisons among the different concentrations of red ginseng extract that yielded statistically significant differences (p<0.05) are indicated by different letters above each bar. NS, not significant.
Korean red ginseng extract decreases the motility and invasion of SW480 cells
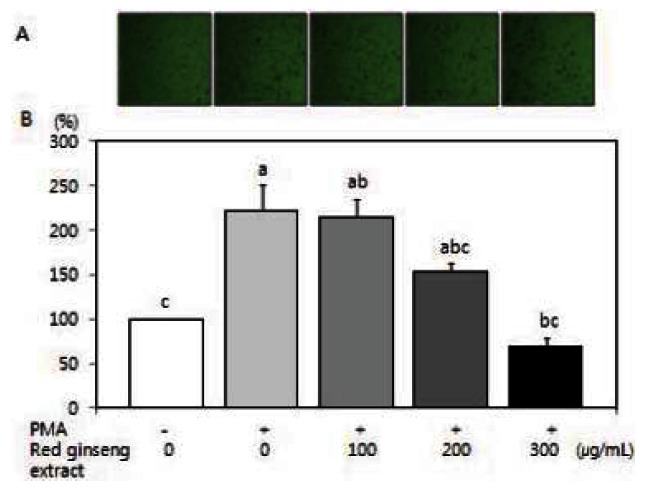
Cell migration plays an important role in the metastasis process. Because PMA has been shown to induce tumor cell migration/invasion [31,32], we examined the question of whether red ginseng extract is able to inhibit PMA-induced migration/invasion of SW480 cells. Using a Boyden chamber assay and a Matrigel invasion assay, red ginseng extract significantly decreased PMA-induced motility (Fig. 2) (p<0.05) and PMA-induced invasion (Fig. 3) (p<0.05).
Fig. 2. Effects of Korean red ginseng extract on PMA-induced motility in SW 480 cells. SW 480 cells were incubated in serum-free medium with or without 100 μg/L PMA in the absence or presence of various concentrations (100, 200, or 300 μg/mL) of red ginseng extract for 16 h in a Boyden chamber. (A) Photographs of cells treated with red ginseng extract during the cell motility assay. (B) Quantitative analysis of the cell motility assay. The number of migrated cells was expressed as a percentage of control (without PMA and red ginseng extract). Each bar represents the mean±SD of three independent experiments. Significant differences (p<0.05) among groups are indicated by different letters above each bar.
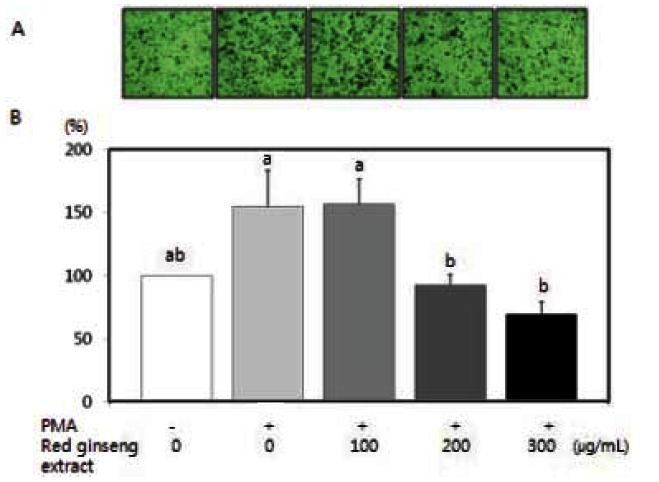
Fig. 3. Effects of red Korean ginseng extract on PMA-induced invasion in SW 480 cells. SW 480 cells were cultured in serum-free medium with or without 100 μg/L PMA in the absence or presence of various concentrations (100, 200, or 300 μg/mL) of red ginseng extract for 16 h in the Matrigel chamber. (A) Photographs of cells treated with red ginseng extract. (B) Quantitative analysis of the invasion assay. The number of cells were invaded was expressed as a percentage of control (without PMA and red ginseng extract). Each bar represents the mean±SD of three independent experiments. Significant differences (p<0.05) among groups are indicated by different letters above each bar.
Korean red ginseng extract reduces MMP-2 and MMP-9 activity in SW480 cells
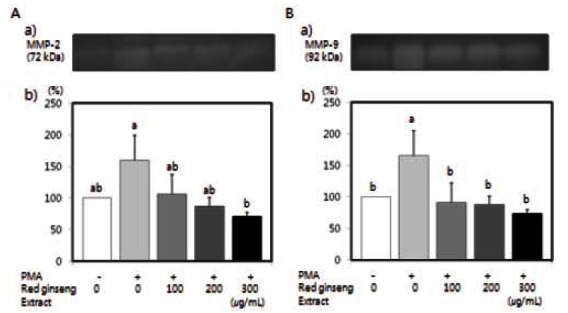
The effects of red ginseng extract on MMP-2 and MMP-9 activity were tested. PMA has been known to increase MMP activity [33,34]. Activity of MMP-2 and MMP-9 induced by PMA in SW480 cells was significantly reduced by red ginseng extract (Fig. 4) (p<0.05).
Fig. 4. Effects of Korean red ginseng extract on PMA-induced matrix metalloproteinase (MMP)-9 and MMP-2 activity in SW 480 cells. SW 480 cells were plated in 6 well plates at a density 1×106 cell/mL in Dulbecco’s modified Eagle’s medium/Ham’s F12 nutrient mixture supplemented with 10% fetal bovine serum. After 48 h incubation, the monolayers were incubated in serum-free medium with or without 100 μg/L PMA in the absence or presence of various concentrations (100, 200, or 300 μg/mL) of red ginseng extract for 16 h. Medium was collected and concentrated for zymography. (A-a) Photographs of MMP-2, (A-b) Quantitative analysis of the bands. (B-a) Photographs of MMP-9 bands. (B-b) Quantitative analysis of the bands. The enzyme activity was expressed as a percentage of control (without PMA and red ginseng extract). Each bar represents the mean±SD of three independent experiments. Significant differences (p<0.05) among groups are indicated by different letters above each bar.
Korean red ginseng extract inhibits protein and mRNA expressions of MMPs in SW480 cells
Protein expressions of MMP-2 and MMP-9 were assayed by western blot analysis. MMP-2 and MMP- 9 protein expression in SW480 cells were induced by PMA, and which were significantly decreased in a dose-dependent manner (Fig. 5) (p<0.05). We also performed RT-PCR analysis to determine the effects of red ginseng extract on MMP-2 and MMP-9 mRNA expression. As shown in Fig. 6, PMA increased MMP-2 and MMP-9 mRNA expression, and red ginseng extract significantly inhibited PMA-induced MMP-2 and MMP-9 mRNA expression in SW480 cells (p<0.05).
Fig. 5. Effects of red Korean ginseng extract on PMA-induced matrix metalloproteinase (MMP)-9 and MMP-2 protein expression in SW 480 cells. SW 480 cells were plated in 6-well plates at a density 1×106 cells/well in Dulbecco’s modified Eagle’s medium/Ham’s F12 nutrient mixture supplemented with 10% fetal bovine serum for. After 48 h incubation, the monolayers were incubated in serum-free medium in serum-free medium with or without 100 μg/L PMA in the absence or presence of various concentrations (100, 200, or 300 μg/mL) of red ginseng extract for 16 h. Medium was collected and concentrated for western blotting. (A-a) Photographs of the MMP-2 bands. (A-b) Quantitaitive analysis of the bands. (B-a) Photographs of the MMP-9 bands. (B-b) Quantitaitive analysis of the bands. The protein expression of enzyme was expressed as a percentage of control (without PMA and red ginseng extract). Each bar represents the mean±SD of three independent experiments. Significant differences (p<0.05) among groups are indicated by different letters above each bar.
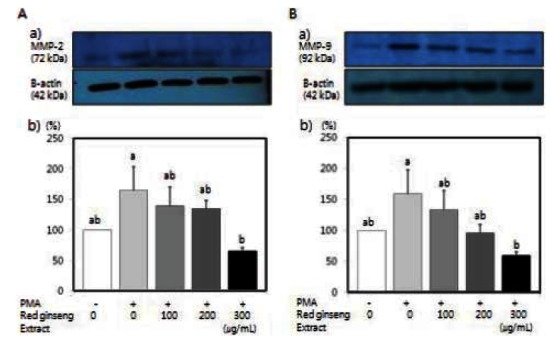
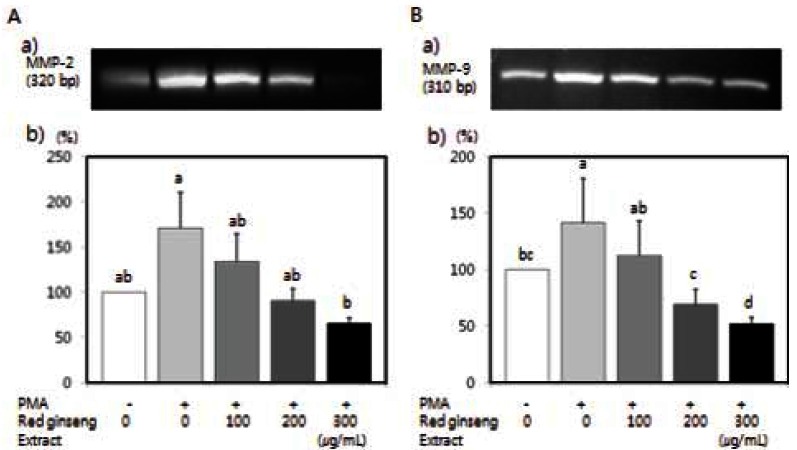
Fig. 6. Effects of Korean red ginseng extract on PMA-induced matrix metalloproteinase (MMP)-9 and MMP-2 mRNA expression in SW 480 cells. SW 480 cells were cultured in serum-free medium with or without 100 μg/L PMA in the absence or presence of various concentrations (100, 200, or 300 μg/mL) of red ginseng extract. Total RNA was isolated and reverse transcriptase polymerase chain reaction was performed. Photographs of the ethidium bromide-stained gels, which are representative of three independent experiments, are shown. (A-a) Photographs of the MMP-2 bands. (A-b) Quantitaitive analysis of the bands. (B-a) Photographs of the MMP-9 bands. (B-b) Quantitaitive analysis of the bands. The mRNA expression of enzyme was expressed as a percentage of control (without PMA and red ginseng extract). Each bar represents the mean±SD and significant differences (p<0.05) among groups are indicated by different letters above each bar.
Korean red ginseng extract decreases experimental metastasis in BALB/c mice
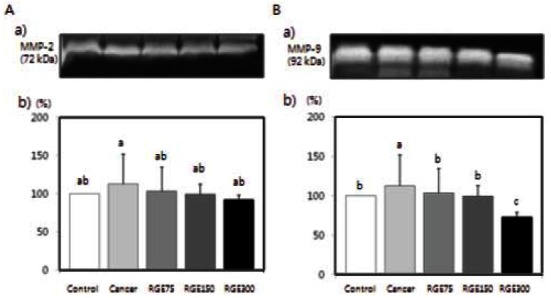
Neither cancer cells injection nor red ginseng extract intake had an influence on weights of experimental animals (Table 1). Fig. 7 shows the effects of red ginseng extract on the number of colonies of CT-26 cells in the lungs. Red ginseng treatment significantly decreased the number of colonies (p<0.05). Plasma MMP-2 and MMP- 9 activity were significantly reduced in mice that were fed red ginseng extract (Fig. 8) (p<0.05).
Table 1.
Body weight of experimental rats
| Groups1) | Initial body weight (g) | Final body weight (g) |
|---|---|---|
|
| ||
| Control | 21.7±0.32) | 21.6±0.72) |
| Cancer | 21.6±0.5 | 21.5±0.5 |
| REG75 | 21.7±0.4 | 21.9±0.6 |
| REG150 | 21.7±0.4 | 21.9±0.5 |
| REG300 | 21.7±0.3 | 21.9±0.5 |
1) Mice were injected intravenously in the tail with CT-26 colon cancer cells suspended in serum free medium at a density of 5x105 cells/ mL. Following injection, mice underwent oral administration of red ginseng extract (RGE) each dat at doses of 0 (RGE0), 75 (RGE75), 150 (RGE150), or 300 (RGE300) mg/kg body weight for 2 weeks.
2) Not significant.
Fig. 7. Red ginseng extract (RGE) inhibits lung colonization by CT- 26 cells in BALB/c mice. CT-26 cells (5×105/mL) were injected into the lateral tail vein of 40 BALB/c mice. RGE was then administered orally each day at a dose of 75 (RGE75), 150 (RGE150), or 300 (RGE300) mg/kg body weight for 3 weeks. Each bar represents the mean±SD and significant differences (p<0.05) among groups are indicated by different letters above each bar.
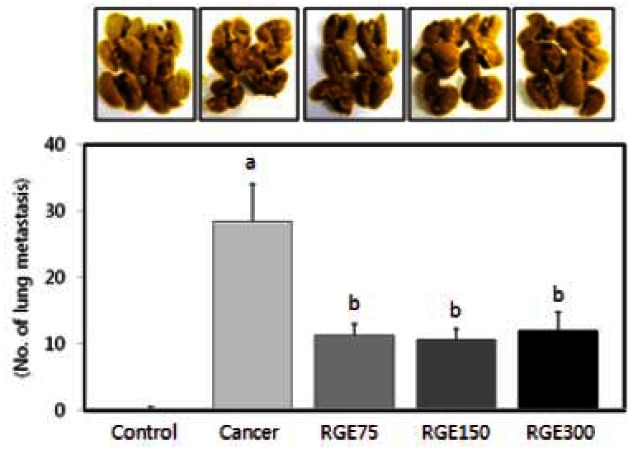
Fig. 8. Effects of red ginseng extract (RGE) on matrix metalloproteinase (MMP)-9 and MMP-2 activity in CT-26 cells tumor-induced BALB/ c mice. MMP activity in plasma of mice, which were injected colon cancer cell and then administered orally each day at a dose of 75 (RGE75), 150 (RGE150), or 300 (RGE300) mg/kg body weight for 3 weeks, was investigated by zymography. (A-a) Photographs of the MMP-2 bands. (A-b) Quantitaitive analysis of the bands. (B-a) Photographs of the MMP-9. (B-b) Quantitaitive analysis of the bands. The enzyme activity was expressed as a percentage of control (without PMA and RGE). Each bar represents the mean±SD of three independent experiments. Significant differences (p<0.05) among groups are indicated by different letters above each bar.
DISCUSSION
Tumor metastasis is one of the most important causes of death of cancer patients. Therefore, research on natural materials with an anti-metastatic effect would be very meaningful. This study is the first to show that red ginseng extract can suppress metastasis of colorectal cancer by inhibiting MMPs activity, both in vitro and in vivo.
In the present study, Korean red ginseng extract significantly decreased proliferation of SW480 cells after 96 h incubation. Wang et al. [1] reported that red ginseng extract increased pro-apoptotic gene expression (CASP5) and decreased anti-apoptotic gene expression (IGF1R) in SW480. Ginsenoside Rg3 induced apoptosis by suppressing NF-κB expression in SW620 and HCT 116 colon cancer cells [35], and LNCap, PC-3, and DU145 prostate colon cancer cells [36]. Also, ginsenoside Rd significantly changed apoptosis-related proteins, such as Rho GDP dissociation inhibitor, Tropomyosin 1, and Annexin 5 in HT 29 cells [37].
As mentioned above, Korean red ginseng extract suppressed tumor cell growth. However, in this study, in order to demonstrate that the anti-metastatic effect of the red ginseng extract is independent of suppression of tumor cell growth, we performed experiments related to metastasis under the condition that treatment time with the red ginseng extract did not last longer than 48 h. As a result, the red ginseng extract decreased PMA-induced mobility, invasion, and the activity, protein expression, and mRNA expression of MMPs of cancer cells, indicating that suppression of cancer metastasis by the red ginseng extract was independent of inhibition of tumor cell growth.
Tumor invasion is a key step in metastasis and includes tumor cell adhesion, migration, and enzymatic degradation of ECM and basement membrane [38]. Extracellular proteolysis is very important in cell migration and invasion, and is mediated by MMPs and serine proteinase [4,5]. MMP is a zinc-dependent proteinase and can be classified by structure, substrate specificity, and position within the cell into collagenase, gelatinase, stromelysins, membrane-type MMP, and other MMPs [39]. MMP is secreted as an inactive pro-enzyme, and its activation is controlled by gene transcription, proenzyme activation, and suppression of tissue inhibitor of matrix metalloproteinase, etc. [40].
Mochizuki et al. [23] reported that ginsenoside Rg3 suppressed invasion of human lung carcinoma (OG10), pancreatic adenocarcinoma (PSN1), and B16-BL6 melanoma. Ginsenoside Rg3 suppressed proliferation of human umbilical vein endothelial cells, formation of tube and invasion, activation of MMP-9 and MMP-2 in an aortic ring culture [41], and expression of MMP-9 in a human ovarian cancer SKOV-3 cell line [42].
PMA, a powerful tumor promoter, has been shown to induce tumor cell migration/invasion and increase MMPs activity [31-33]. Han et al. [34] showed that PMA increased MMP activity and affected invasion and motility properties of HM3 cells . PMA has been reported to regulate various cellular responses through the protein kinase C (PKC) pathway [43], and PKC signaling pathways may be involved in regulation of invasion and metastatic properties of colon cancer cells [44,45]. Therefore, it seems that the red ginseng extract suppresses motility/ invasion by inhibiting activation and gene expression of MMPs in SW480 cells via PKC pathway.
In the present study, oral intake of the red ginseng extract decreased the number of pulmonary nodules in experimental metastasis and the activity of MMP-2 and MMP-9 in plasma in a dose-dependent manner. Shibata reported that oral treatment with the ginsenoside Rg3 at the concentrations of 2.5 or 5 mg/kg/d for 20 and 45 wk, suppressed cancer metastasis by 78% in Wistar rat [46]. Lung metastasis of B16-F10 mouse melanoma cells was suppressed in C57BL/6 mice that received ginsenoside Rp at a concentration of 3 mg/kg for 13 days [47]. Mochizuki et al. [23] reported on significantly decreased lung metastasis of B16-BL6 melanoma in rats that received injection (100 μg/mouse) or oral administration (100-1,000 μg/mouse) of ginsenoside Rg3. Matsuoka et al. [48] also reported that MMI-166, an inhibitor of MMPs, decreased liver metastasis in vivo, therefore, MMP should play a crucial role in cancer metastasis.
In conclusion, we have shown that Korean red ginseng extract can inhibit metastasis of SW480 colon cancer cells and that oral administration of red ginseng extract inhibits the metastasis of CT-26 cells to the lungs in a BALB/c murine model of experimentally induced cancer. We suggest that Korean red ginseng extract may contribute to metastasis inhibition by decreasing activity and expression of MMP-2 and MMP-9
Acknowledgments
This work was supported by the 2009 grant from the Korean Society of Ginseng funded by Korea Ginseng Corporation.
References
- 1.Wang CZ, Li XL, Wang QF, Mehendale SR, Fishbein AB, Han AH, Sun S, Yuan CS. The mitochondrial pathway is involved in American ginseng-induced apoptosis of SW- 480 colon cancer cells. Oncol Rep. 2009;21:577–584. doi: 10.3892/or_00000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministry of Health and Welfare. Annual report of the central cancer registry in Korea. Ministry of Health and Welfare; Seoul: 2008. [Google Scholar]
- 3.Okegawa T, Pong RC, Li Y, Hsieh JT. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim Pol. 2004;51:445–457. [PubMed] [Google Scholar]
- 4.Pepper MS, Montesano R, Mandriota SJ, Orci L, Vassalli JD. Angiogenesis: a paradigm for balanced extracellular proteolysis during cell migration and morphogenesis. Enzyme Protein. 1996;49:138–162. doi: 10.1159/000468622. [DOI] [PubMed] [Google Scholar]
- 5.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 6.Zucker S, Mirza H, Conner CE, Lorenz AF, Drews MH, Bahou WF, Jesty J. Vascular endothelial growth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int J Cancer. 1998;75:780–786. doi: 10.1002/(SICI)1097-0215(19980302)75:5<780::AID-IJC19>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Fisher C, Gilbertson-Beadling S, Powers EA, Petzold G, Poorman R, Mitchell MA. Interstitial collagenase is required for angiogenesis in vitro. Dev Biol. 1994;162:499–510. doi: 10.1006/dbio.1994.1104. [DOI] [PubMed] [Google Scholar]
- 8.Zucker S, Conner C, DiMassmo BI, Ende H, Drews M, Seiki M, Bahou WF. Thrombin induces the activation of progelatinase A in vascular endothelial cells. Physiologic regulation of angiogenesis. J Biol Chem. 1995;270:23730–23738. doi: 10.1074/jbc.270.40.23730. [DOI] [PubMed] [Google Scholar]
- 9.Yun TK, Choi SY, Lee YS. A prosoective study on ginseng intake and cancer for population-preliminary report. Korean J Ginseng Sci. 1995;19:87–92. [Google Scholar]
- 10.Ko SR, Lee YH, Choi KJ, Park JD. Comparative cytotoxic activities of various ginsengs on human cell lines. Korean J Ginseng Sci. 1998;22:18–21. [Google Scholar]
- 11.Jung NP, Song SO, Choi SU. Cytotoxicity of white and red ginseng against cancer cells and their effects on the cell cycle. J Ginseng Res. 2000;24:183–187. [Google Scholar]
- 12.Park JD. Recent studies on the chemical constituents of Korean ginseng (Panax ginseng C. A. Meyer). Korean J Ginseng Sci. 1996;20:389–415. [Google Scholar]
- 13.Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1150–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-X. [DOI] [PubMed] [Google Scholar]
- 15.Hartz AJ, Bentler S, Noyes R, Hoehns J, Logemann C, Sinift S, Butani Y, Wang W, Brake K, Ernst M, et al. Randomized controlled trial of Siberian ginseng for chronic fatigue. Psychol Med. 2004;34:51–61. doi: 10.1017/S0033291703008791. [DOI] [PubMed] [Google Scholar]
- 16.Shin JY, Song JY, Yun YS, Yang HO, Rhee DK, Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002;24:469–482. doi: 10.1081/IPH-120014730. [DOI] [PubMed] [Google Scholar]
- 17.Kim ND. Phamacological effects of red ginseng. J Ginseng Res. 2001;25:2–10. [Google Scholar]
- 18.Cho YD. Clinical effects of ginseng components and biochemical mechanism. J Ginseng Res. 2001;25:19–25. [Google Scholar]
- 19.Chong SK, Oberholzer VG. Ginseng: is there a use in clinical medicine? Postgrad Med J. 1988;64:841–846. doi: 10.1136/pgmj.64.757.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surh YJ, Na HK, Lee JY, Keum YS. Molecular mechanisms underlying anti-tumor promoting activities of heatprocessed Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;(16 Suppl):S38–S41. doi: 10.3346/jkms.2001.16.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukushima S, Wanibuchi H, Li W. Inhibition by ginseng of colon carcinogenesis in rats. J Korean Med Sci. 2001;(16 Suppl):S75–S80. doi: 10.3346/jkms.2001.16.S.S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh SO, Kim J, Cho MY. Prospective study for Korean red ginseng extract as an immune modulator following a curative gastric resection in patients with advanced gastric cancer. J Ginseng Res. 2004;28:104–111. doi: 10.5142/JGR.2004.28.2.104. [DOI] [Google Scholar]
- 23.Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 24.Sato K, Mochizuki M, Saiki I, Yoo YC, Samukawa K, Azuma I. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull. 1994;17:635–639. doi: 10.1248/bpb.17.635. [DOI] [PubMed] [Google Scholar]
- 25.Iishi H, Tatsuta M, Baba M, Uehara H, Nakaizumi A, Shinkai K, Akedo H, Funai H, Ishiguro S, Kitagawa I. Inhibition by ginsenoside Rg3 of bombesin-enhanced peritoneal metastasis of intestinal adenocarcinomas induced by azoxymethane in Wistar rats. Clin Exp Metastasis. 1997;15:603–611. doi: 10.1023/A:1018491314066. [DOI] [PubMed] [Google Scholar]
- 26.Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 1998;246:725–730. doi: 10.1006/bbrc.1998.8690. [DOI] [PubMed] [Google Scholar]
- 27.Kim EJ, Kang IJ, Cho HJ, Kim WK, Ha YL, Park JH. Conjugated linoleic acid downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. J Nutr. 2003;133:2675–2681. doi: 10.1093/jn/133.8.2675. [DOI] [PubMed] [Google Scholar]
- 28.Lee HS, Seo EY, Kang NE, Kim WK. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J Nutr Biochem. 2008;19:313–319. doi: 10.1016/j.jnutbio.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Na MH, Seo EY, Kim WK. Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells. Nutr Res Pract. 2009;3:265–271. doi: 10.4162/nrp.2009.3.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soel SM, Choi OS, Bang MH, Yoon Park JH, Kim WK. Influence of conjugated linoleic acid isomers on the metastasis of colon cancer cells in vitro and in vivo. J Nutr Biochem. 2007;18:650–657. doi: 10.1016/j.jnutbio.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Huang Q, Shen HM, Ong CN. Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-kappaB. Biochem Pharmacol. 2004;68:361–371. doi: 10.1016/j.bcp.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Torricelli C, Fortino V, Capurro E, Sacchi G, Ponzo P, Pacini A, Muscettola M, Maioli E. Role of PTHrp and PTHrp-engaged pathways in MCF-7 cells migration/invasion. Matrix Biol. 2006;25:104–111. doi: 10.1016/j.matbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Meyer E, Vollmer JY, Bovey R, Stamenkovic I. Matrix metalloproteinases 9 and 10 inhibit protein kinase C-potentiated, p53-mediated apoptosis. Cancer Res. 2005;65:4261–4272. doi: 10.1158/0008-5472.CAN-04-2908. [DOI] [PubMed] [Google Scholar]
- 34.Han SY, Lee MS, Kim HR, Baek SH, Ahn DH, Chae HS, Erickson RH, Sleisenger MH, Kim YS. Phorbol 12-myristate 13-acetate induces alteration in mucin gene expression and biological properties of colon cancer cells. Int J Oncol. 2000;17:487–494. doi: 10.3892/ijo.17.3.487. [DOI] [PubMed] [Google Scholar]
- 35.Kim SM, Lee SY, Yuk DY, Moon DC, Choi SS, Kim Y, Han SB, Oh KW, Hong JT. Inhibition of NF-kappaB by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxel. Arch Pharm Res. 2009;32:755–765. doi: 10.1007/s12272-009-1515-4. [DOI] [PubMed] [Google Scholar]
- 36.Kim SM, Lee SY, Cho JS, Son SM, Choi SS, Yun YP, Yoo HS, Yoon do Y, Oh KW, Han SB, et al. Combination of ginsenoside Rg3 with docetaxel enhances the susceptibility of prostate cancer cells via inhibition of NF-kappaB. Eur J Pharmacol. 2010;631:1–9. doi: 10.1016/j.ejphar.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Lee SY, Kim GT, Roh SH, Song JS, Kim HJ, Hong SS, Kwon SW, Park JH. Proteome changes related to the anticancer activity of HT29 cells by the treatment of ginsenoside Rd. Pharmazie. 2009;64:242–247. [PubMed] [Google Scholar]
- 38.Harlozinska A. Progress in molecular mechanisms of tumor metastasis and angiogenesis. Anticancer Res. 2005;25:3327–3333. [PubMed] [Google Scholar]
- 39.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/S0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 41.Yue PY, Wong DY, Wu PK, Leung PY, Mak NK, Yeung HW, Liu L, Cai Z, Jiang ZH, Fan TP, et al. The angiosuppressive effects of 20(R)-ginsenoside Rg3. Biochem Pharmacol. 2006;72:437–445. doi: 10.1016/j.bcp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 42.Xu TM, Cui MH, Xin Y, Gu LP, Jiang X, Su MM, Wang DD, Wang WJ. Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J (Engl) 2008;121:1394–1397. [PubMed] [Google Scholar]
- 43.Lee HW, Ahn DH, Crawley SC, Li JD, Gum JR Jr, Basbaum CB, Fan NQ, Szymkowski DE, Han SY, Lee BH, et al. Phorbol 12-myristate 13-acetate up-regulates the transcription of MUC2 intestinal mucin via Ras, ERK, and NF-kappa B. J Biol Chem. 2002;277:32624–32631. doi: 10.1074/jbc.M200353200. [DOI] [PubMed] [Google Scholar]
- 44.Heider I, Schulze B, Oswald E, Henklein P, Scheele J, Kaufmann R. PAR1-type thrombin receptor stimulates migration and matrix adhesion of human colon carcinoma cells by a PKCepsilon-dependent mechanism. Oncol Res. 2004;14:475–482. doi: 10.3727/0965040042380496. [DOI] [PubMed] [Google Scholar]
- 45.Masur K, Lang K, Niggemann B, Zanker KS, Entschladen F. High PKC alpha and low E-cadherin expression contribute to high migratory activity of colon carcinoma cells. Mol Biol Cell. 2001;12:1973–1982. doi: 10.1091/mbc.12.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;(16 Suppl):S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park TY, Park MH, Shin WC, Rhee MH, Seo DW, Cho JY, Kim HM. Anti-metastatic potential of ginsenoside Rp1, a novel ginsenoside derivative. Biol Pharm Bull. 2008;31:1802–1805. doi: 10.1248/bpb.31.1802. [DOI] [PubMed] [Google Scholar]
- 48.Matsuoka T, Yashiro M, Sawada T, Ishikawa T, Ohira M, Chung KH. Inhibition of invasion and lymph node metastasis of gastrointestinal cancer cells by R-94138, a matrix metalloproteinase inhibitor. Anticancer Res. 2000;20:4331–4338. [PubMed] [Google Scholar]


