Abstract
A 24-week randomized open-label study with Korean red ginseng (KRG) showed cognitive benefits in patients with Alzheimer’s disease. To further determine long-term effect of KRG, the subjects were recruited to be followed up to 2 yr. Cognitive function was evaluated every 12 wk using the Alzheimer’s Disease Assessment Scale (ADAS) and the Korean version of the Mini Mental Status Examination (K-MMSE) with the maintaining dose of 4.5 g or 9.0 g KRG per d. At 24 wk, there had been a significant improvement in KRG-treated groups. In the long-term evaluation of the efficacy of KRG after 24 wk, the improved MMSE score remained without significant decline at the 48th and 96th wk. ADAS-cog showed similar findings. Maximum improvement was found around week 24. In conclusion, the effect of KRG on cognitive functions was sustained for 2 yr follow-up, indicating feasible efficacies of long-term follow-up for Alzheimer’s disease.
Keywords: Panax ginseng, Korean red ginseng, Alzheimer disease, Long term effect
INTRODUCTION
In the recent decade, the prevalence of dementia increased as the proportion of elderly population was expanding. The number of subjects with dementia has been doubling every 20 yr and will reach 81.1 million by 2040 [1]. In spite of enormous research efforts, only a few symptomatic treatment options currently exist. Anticholinesterases and N-methyl-D-aspartate (NMDA) receptor blockers are clinically in use as anti-dementia medications, but they cannot prevent the disease cascade, and provide modest symptomatic benefits. Moreover, the active Amyloid beta immunization treatment for Alzheimer’s disease (AD), which had been suggested to modify the disease process, was halted due to critical adverse events [2]. Clinical trials with herbal medicines have been actively investigated, warranting discovery of new drug [3]. Ginseng is one of the most popular herbal medicines used, and has been proven to have effects on cognitive functions, such as attention, working memory performance, processing and auditory reaction time in healthy individuals [4,5].
However, only a few studies have shown effects of ginseng on dementia [6,7]. These studies investigated the effects of total powder extract of Panax ginseng or Korean red ginseng (KRG) in patients with AD, followed-up for either three or six mo. Although neuropsychological assessment with the Alzheimer's Disease Assessment Scale (ADAS) or the Korean version of the Mini-Mental Status Examination (K-MMSE) showed cognitive improvements, whether the effect of ginseng was transient or not remained unknown, due to limitation of study duration.
In this study, we attempted to test the long-term efficacy of ginseng by extending follow-up duration. The goals of this study were: 1) to further clarify the long-term efficacy of KRG as an adjuvant therapy to conventional anti-dementia medications in patients with AD and 2) to monitor the cognitive changes during the extended follow-up duration.
MATERIALS AND METHODS
Patients
The patients were included according to previously described inclusion and exclusion criteria [7]. The inclusion criteria were, as follows: 1) probable AD consistent with the criteria of the National Institute of Neurologic and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association [8], 2) older than 50 yr, and 3) baseline Mini-Mental State Examination score of ≥10 and ≤26. Participants treated with conventional anti-dementia medication were allowed at least for 6 mo before the study. The exclusion criteria were: 1) history of psychiatric disorder, seizure disorder, or medical condition that would limit the completeness of the study, and 2) cognitive impairment due to stroke, hypoxic brain damage, cerebral neoplasia, infection, and medications, such as antidepressants or psychoactive drugs. Sixty-one patients, aged 50 to 80 yr, had been enrolled. Informed consent to participation in this study was obtained from all subjects and the study was approved by the Institutional Review Board of Seoul National University Hospital.
Korean red ginseng treatment
The patients had been grouped according to the doses of KRG, as previously described [7]. In brief, using a 1:1:2 randomization ratio, the patients were allocated in three treatment groups: low-dose KRG (4.5 g/d), high-dose KRG (9 g/d), and control. KRG (total powder capsule, 6-year-old root; Korea Ginseng Corporation, Daejeon, Korea) was administered in a divided dose twice or three times a day. Ginsenosides, the active constituent of KRG, are composed of Rb1 (1.96%), Rb2 (2.18%), Rc (1.47%), Rd (0.72%), Re (1.11%), Rf (0.24%), Rg1 (0.49%), Rg2 (0.13%), Rg3 (0.12%), Rh1 (0.12%), and Rh2 (0.003%), and account for 8.54% of the herb [9]. Conventional anti-dementia medications (acetylcholinesterase inhibitors, i.e., donepezil, galantamine, and rivastigmine) were continued during the study period. For the initial 24 wk, there were 15 subjects in the low-dose KRG group and 15 subjects in the high-dose KRG group. The control group consisted of 31 patients who did not receive KRG and were compared to those in the KRG groups.
After 24 wk, the same doses and formula KRG powder capsules were provided continuously to the patients in the KRG groups for 2 yr. In this extended period, we continued the study with the KRG groups, but not with the control group.
Neuropsychological assessments
A neurologist or a supervised technician administered the neuropsychological tests. Neuropsychological assessments included the ADAS-cognitive subscale (ADAS-cog) and ADAS-non-cognitive subscale (ADAS-noncog), the K-MMSE and Clinical Dementia Rating (CDR) scale. The tests were performed before taking the KRG, and at 12 and 24 wk after KRG administration. In the KRG groups, neuropsychological evaluation for the long-term follow up (i.e., between 24 wk and 2 yr) was performed at 12 wk intervals.
Statistical analysis
We compared the results of neuropsychological tests between KRG groups and the control group using the SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). The data are expressed as means±SD or as numbers. Inter-group comparisons of changes in ADAS and MMSE score were performed using the Student’s t-test, and the frequencies of side effects and withdrawal from the study were compared using Fisher’s exact test. All p-values are two-tailed, and statistical significance was accepted for p-values <0.05. The neuropsychological scores from the extended follow-up period (24 wk to 2 yr) of the KRG groups were compared to baseline scores.
RESULTS
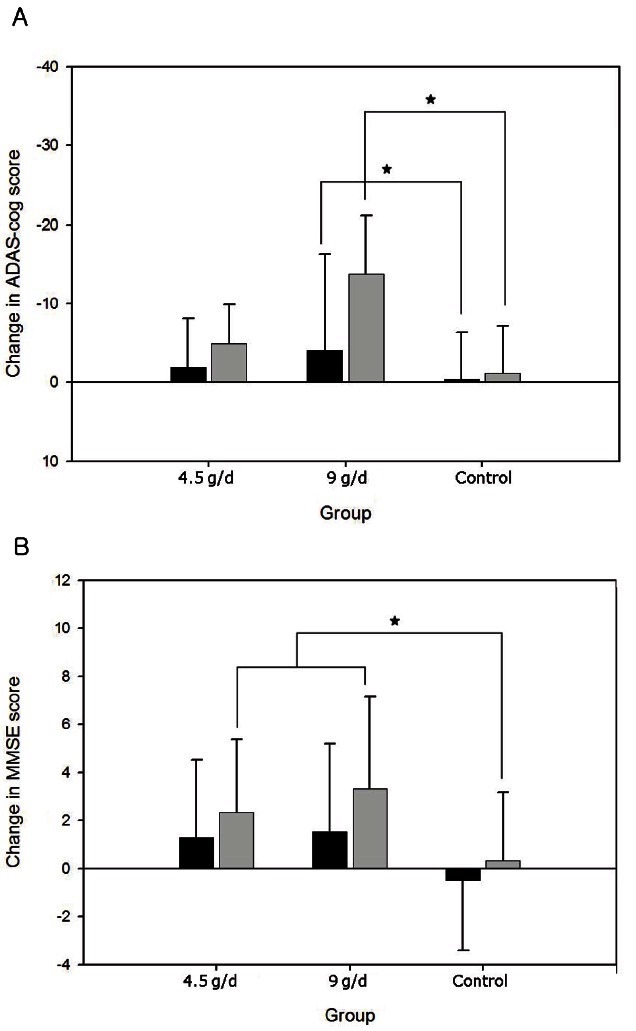
There were no differences in baseline characteristics between the groups, including age (p=0.804), gender (p=0.390), ADAS-cog (p=0.561), ADAS-noncog (p=0.060), MMSE (p=0.925), and CDR (p=0.450) scores, as well as in composition of conventional anti-dementia drugs (p=0.308) (Table 1). The performance on the ADAS-cog showed significant difference between the high-dose KRG and control. At 12 wk, the mean changes in the high-dose KRG and control groups were -4.0±12.3 and -0.4±5.9, respectively, and at 24 wk the changes were -13.8±7.4 and -1.2±6.0, respectively.
Table 1.
Baseline characteristics of the Alzheimer’s disease patients
| Korean red ginseng | Control | |||
|---|---|---|---|---|
|
| ||||
| 4.5 g | 9 g | Total | ||
|
| ||||
| No. | 15 | 15 | 30 | 31 |
| M:F ratio | 6:9 | 8:7 | 14:16 | 10:21 |
| Age | 66.1 (6.7) | 67.7 (11.8) | 66.9 (9.5) | 66.7 (7.5) |
| ADAS | 23.6 (8.4) | 27.1 (9.4) | 25.3 (8.9) | 27.3 (12.6) |
| -cog | 19.7 (6.8) | 23.0 (8.7) | 21.3 (7.8) | 20.5 (9.1) |
| -noncog | 3.9 (2.6) | 4.1 (3.3) | 4.0 (2.9) | 6.7 (5.1) |
| K-MMSE | 22.1 (4.0) | 21.4 (6.6) | 21.8 (5.3) | 21.5 (4.4) |
| CDR | 1.0 (0.5) | 1.4 (1.1) | 1.2 (0.9) | 1.1 (0.3) |
Values are given as mean (SD) scores.
ADAS, Alzheimer’s Disease Assessment Scale; ADAS-cog, ADAS-cognitive subscale; ADAS-noncog, ADAS-non-cognitive subscale; K-MMSE, Korean version of the Mini-Mental Status Examination; CDR, Clinical Dementia Rating.
The group taking 4.5 g of KRG showed no significant differences compared to control at both 12 wk (-1.9±6.2) and 24 wk (-4.9±5.0) (Fig. 1A). Similarly, there was a significant difference in the K-MMSE scores in the KRG group (4.5 g/d plus 9 g/d) compared to control at both 12 wk (1.4±3.4 vs. -0.5±2.9) and 24 wk (2.8±3.4 vs. 0.3±2.9) (Fig. 1B). However, the differences in K-MMSE scores between each KRG group and control were not significant at both follow-ups. In ADAS-non-cog score, KRG failed to show any statistical significance. No significant difference in adverse events was found between the treatment and control groups (p=0.853). Five patients in the low-dose KRG group, six patients in the high-dose KRG group, and twelve patients in the control group reported adverse effects and withdrew from the study during the first 24 wk. Participants reported mild symptoms of heat sensation, nausea, diarrhea, and headache, and all subjects who reported adverse events withdrew from the study.
Fig. 1. Changes in neuropsychological tests over 24 wk. Korean red ginseng improved Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) (A) in the high-dose ginseng group, compared with control, at both 12-week and 24-week follow-up evaluations. Korean version of the Mini-Mental Status Examination (K-MMSE) score was also improved at 24-week follow up in the ginseng group, compared to control (B). Black bar, 12 wk; grey bar, 24 wk. *p<0.05 compared to control, independent t-test.
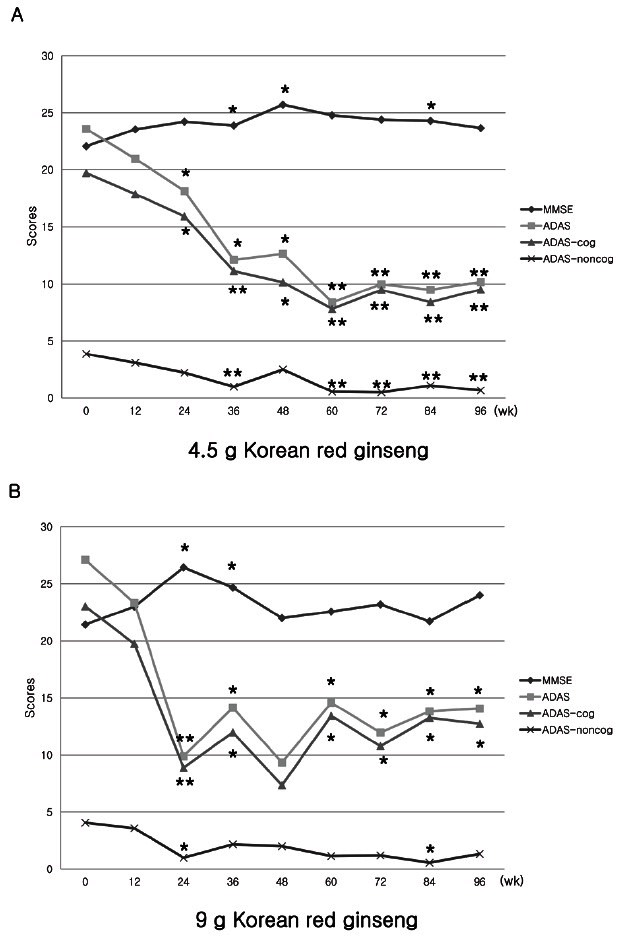
In the long-term evaluation of the efficacy of KRG after 24 wk, the ADAS-cog and MMSE scores sustained without apparent declining at each visit for 2 yr (Fig. 2A, B). In the low-dose group, the initial MMSE was 22.0±4.0, at the 48th wk was 25.7±3.0, and 23.7±4.8 at the 96th wk. In the high-dose group, the initial MMSE was 21.4±6.6, 22.0±5.7 at the 48th wk, and 24.0±3.7 at the 96th wk, showing slight improvement or similar to the initial status. There was no tendency to decline. ADAS-cog showed similar findings of improvement from 19.7±6.8 (initial) to 9.5±6.3 (96th wk) in 4.5 g group, and from 23.0±8.7 (initial) to 12.8±6.8 (96th wk) in 9.0 g group. In this study, maximum improvement was found around 24 wk; however, there was no definite evidence of declining course thereafter. Taken together, the cognitive performance measured at 2-year follow-up visit was not different from initial evaluation.
Fig. 2. The mean scores of Alzheimer’s Disease Assessment Scale (ADAS), ADAS-cognitive subscale (ADAS-cog), ADAS-non-cognitive subscale (ADAS-noncog), and Korean version of the Mini-Mental Status Examination (K-MMSE) in the Korean red ginseng group (A, 4.5 g/d group; B, 9 g/d group) at each followed time for 2 yr (96 wk). *p<0.05 compared to baseline, **p<0.01 compared to baseline, paired t-test.
DISCUSSION
The purpose of this study was to determine the efficacy of KRG with the extended period up to 2 yr. Based on the previous 12-week randomized open-label study with KRG, maintaining of KRG did not show declining of scores measured by ADAS-cog and K-MMSE after this period. Therefore, the effect of KRG on cognitive functions can be sustained at least for 2 yr, suggesting feasible long-term efficacy for AD.
Due to the explosive growth of the aged population, there’s a growing concern for dementia, particularly AD, an aging-related major degenerative disorder. Because it’s becoming an issue for the whole society, there have been many clinical trials to develop drugs to arrest the progressive cognitive decline of the dementia patients. Approved acetylcholinesterase inhibitors, such as donepezil, galantamine or rivastigmine, have been tested for their efficacy at least for 6 mo and for up to 5 yr. However, since these drugs are targeted for the reduced acetylcholine due to cholinergic neuronal loss, none of them can protect the degenerative process of AD [10,11]. In this study, we found KRG-related improvements in cognitive function and sustainability of the clinical efficacy in patients with AD. Previously, data on long-term outcomes of KRG in AD patients are limited, not exceeding several months [6,7]. Therefore, these studies were unable to determine whether the effect of ginseng was only temporary or could last with inhibiting the progressive course of AD. Our findings show that the efficacy of KRG on cognitive function in AD can be maintained for at least 2 yr.
In acetylcholinesterase inhibitors, several long-term (over 2 yr) extension trials have demonstrated that, although the efficacy of medications was apparent, they could not block or reverse the cognitive decline [10,11]. The decline in the MMSE score over 2 yr was usually 2.5 to 4.0 points, compared to initial evaluation [12,13]. In patients taking conventional medication, the usual pattern of cognitive decline shows initial improvement for 3 or 6 mo and then decrease to the initial level at around 1 yr. Then, they progressed thereafter. In contrast, in our study, the ADAS-cog and K-MMSE scores have been sustained without apparent declining at each visit time for 2 yr. This clinical course may be attributed to the difference of underlying mechanisms in ginseng from anti-cholinesterase.
The effects of ginseng on the cognitive function with underlying mechanisms have been proposed [5,14,15]. Ginsenoside Rb1 is known to improve learning and memory in hippocampus-dependent tasks and increased cell survival in the dentate gyrus and hippocampal subregion CA3 [14]. Ginsenoside Rb1 attenuated Aβ1-42-induced neurotoxicity and tau hyperphosphorylation at multiple AD-related sites in a dose-dependent manner [15]. In addition, it has been suggested that ginsenoside Rg1 and Rb1 potentiated the cholinergic pathways in the central nervous system. Thus, both Rg1 and Rb1 enhanced choline acetyltransferase activity and inhibited acetylcholine -esterase activity, thereby enhancing the function of cholinergic system [16]. Administration of Rg1 and Rb1 in weaning mice increased the cortex thickness and density of synapses in the hippocampal CA3 region, which is suggested to modulate synaptic plasticity, regarded as one of the most essential mechanism in learning and memory [17].
Our study was limited because no placebo was used in the control group and we could not prove whether KRG is a cognitive enhancer like acetylcholinesterase inhibitors or disease-modifying drugs. Additional biomarker study is required to clarify this. Nevertheless, our results suggest that KRG treatment is both safe and feasible, and is effective for the long-term management of AD patients.
Acknowledgments
This work was supported by the grants from the Korean Society of Ginseng 2009 and 2010, funded by Korea Ginseng Corporation.
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wisniewski T. Is amyloid-β-peptide immunization clinically effective in patients with Alzheimer's disease? Nat Clin Pract Neurol. 2005;1:84–85. doi: 10.1038/ncpneuro0019. [DOI] [Google Scholar]
- 3.Akhondzadeh S, Abbasi SH. Herbal medicine in the treatment of Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2006;21:113–118. doi: 10.1177/153331750602100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorensen H, Sonne J. A double-masked study of the effects of ginseng on cognitive functions. Curr Ther Res. 1996;57:959–968. doi: 10.1016/S0011-393X(96)80114-7. [DOI] [Google Scholar]
- 5.Scholey A, Ossoukhova A, Owen L, Ibarra A, Pipingas A, He K, Roller M, Stough C. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: an acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology (Berl) 2010;212:345–356. doi: 10.1007/s00213-010-1964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee ST, Chu K, Sim JY, Heo JH, Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 7.Heo JH, Lee ST, Chu K, Oh MJ, Park HJ, Shim JY, Kim M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer's disease. Eur J Neurol. 2008;15:865–868. doi: 10.1111/j.1468-1331.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 9.Nam KY. The comparative understanding between red ginseng and white ginsengs, processed ginsengs (Panax ginseng C.A. Meyer). J Ginseng Res. 2005;29:1–18. doi: 10.5142/JGR.2005.29.1.001. [DOI] [Google Scholar]
- 10.Burns A, Gauthier S, Perdomo C. Efficacy and safety of donepezil over 3 years: an open-label, multicentre study in patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2007;22:806–812. doi: 10.1002/gps.1746. [DOI] [PubMed] [Google Scholar]
- 11.Minthon L, Wallin AK, Eriksson S, Wattmo C, Andreasen N. Long-term rivastigmine treatment in a routine clinical setting. Acta Neurol Scand. 2009;119:180–185. doi: 10.1111/j.1600-0404.2008.01086.x. [DOI] [PubMed] [Google Scholar]
- 12.Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, Edwards S, Hardyman W, Raftery J, Crome P, et al. Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double-blind trial. Lancet. 2004;363:2105–2115. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- 13.Kanaya K, Abe S, Sakai M, Fujii H, Iwamoto T. Changes in cognitive functions of patients with dementia of the Alzheimer type following long-term administration of donepezil hydrochloride: relating to changes attributable to differences in apolipoprotein E phenotype. Geriatr Gerontol Int. 2010;10:25–31. doi: 10.1111/j.1447-0594.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Hoang-Gia T, Wu H, Lee MR, Gu L, Wang C, Yun BS, Wang Q, Ye S, Sung CK. Ginsenoside Rb1 improves spatial learning and memory by regulation of cell genesis in the hippocampal subregions of rats. Brain Res. 2011;1382:147–154. doi: 10.1016/j.brainres.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 15.Zhao R, Zhang Z, Song Y, Wang D, Qi J, Wen S. Implication of phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase-3β pathway in ginsenoside Rb1’s attenuation of beta-amyloid-induced neurotoxicity and tau phosphorylation. J Ethnopharmacol. 2011;133:1109–1116. doi: 10.1016/j.jep.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JT, Liu Y, Qu ZW, Zhang XL, Xiao HL. Influence of ginsenoside Rb1 and Rg1 on some central neurotransmitter receptors and protein biosynthesis in the mouse brain. Yao Xue Xue Bao. 1988;23:12–16. [PubMed] [Google Scholar]
- 17.Ying Y, Zhang JT, Shi CZ, Qu ZW, Liu Y. Study on the nootropic mechanism of ginsenoside Rb1 and Rg1: influence on mouse brain development. Yao Xue Xue Bao. 1994;29:241–245. [PubMed] [Google Scholar]


